Abstract
When exposed to 254-nm UV, spores of Encephalitozoon intestinalis, Encephalitozoon cuniculi, and Encephalitozoon hellem exhibited 3.2-log reductions in viability at UV fluences of 60, 140, and 190 J/m2, respectively, and demonstrated UV inactivation kinetics similar to those observed for endospores of DNA repair-defective mutant Bacillus subtilis strains used as biodosimetry surrogates. The results indicate that spores of Encephalitozoon spp. are readily inactivated at low UV fluences and that spores of UV-sensitive B. subtilis strains can be useful surrogates in evaluating UV reactor performance.
UV technologies for disinfection of municipal drinking water have been in use for several years in Europe (6), and interest in UV is rapidly gaining momentum as a choice for North American water treatment. In fact, field testing, installation, and actual operation of UV systems have already begun at a number of North American sites. UV disinfection of municipal drinking water has become increasingly attractive for the following reasons. First, it has been discovered recently that oocysts of protozoan intestinal parasites such as Cryptosporidium parvum, which are resistant to chlorination, are readily inactivated by relatively low doses of UV (2, 3). Second, UV treatment of water results in little or no formation of harmful disinfection by-products (5).
The microsporidian intestinal parasite Encephalitozoon intestinalis has recently also been placed on the first Contaminant Candidate List under the Safe Drinking Water Act (4), and two other microsporidian species, Encephalitozoon cuniculi and Encephalitozoon hellem, are known to be pathogenic for humans (15), but to date only E. intestinalis has been reported to be sensitive to UV radiation at a dose of 60 J/m2 (7). However, the sensitivities of E. cuniculi and E. hellem to UV disinfection have not been evaluated.
United States Environmental Protection Agency (EPA) regulators are currently assessing UV disinfection as a key component of the Long Term 2 Enhanced Surface Water Treatment Rule (LT2ESWTR) (13). Under the LT2ESWTR, validation tests will be necessary to prove that any given UV reactor conforms to the standards of drinking water disinfection, which will be determined by the EPA. UV reactor validation testing is used to determine conditions that can be monitored to ensure delivery in routine operation of the proper UV fluence required for adequate water disinfection. Physical monitoring of applied irradiation by using electronic UV sensors can be problematic and may not yield an accurate picture of the biologically effective UV fluence in large-scale municipal settings (for discussion, see reference 6). Therefore, to determine the biologically effective fluence delivered by a UV reactor, one must rely on more direct measures, such as biodosimetry, the inactivation of a nonpathogenic test organism whose UV dose-response characteristics have been precisely quantified (1, 6). Escherichia coli bacteriophage MS-2 and spores of Bacillus subtilis strain ATCC 6633 have been used extensively as UV biodosimetry reference organisms (biodosimeters). The 254-nm UV fluences required to cause a 4-log inactivation of the MS-2 and B. subtilis biodosimeters are approximately 800 and 600 J/m2, respectively (6, 14), which greatly exceed the 4-log inactivation fluence for C. parvum oocysts, which is only approximately 80 J/m2 (2, 3). If information is required as to how closely a UV reactor system approaches the performance of an ideal system, reactor validation should include at least two biodosimeters of widely different UV sensitivities, with one exhibiting a UV sensitivity similar to or greater than that of the target pathogen and the other exhibiting greater UV resistance than the target pathogen (1).
The present study had two objectives. The first objective was to define the sensitivities of E. intestinalis, E. cuniculi, and E. hellem to 254-nm UV radiation. The second objective was to investigate the use of spores of B. subtilis DNA repair-deficient strains as biodosimeters that would exhibit inactivation kinetics similar to those of the microsporidial spores when irradiated with 254-nm UV under identical conditions. For comparative purposes, experiments were performed in parallel at the EPA facility in Cincinnati, Ohio (EPA), and at the University of Arizona, Tucson (UA).
Spores of E. cuniculi ATCC 50502, E. hellem ATCC 50451, and a duodenal isolate of E. intestinalis, ATCC 50603, were purified weekly from stock flasks, enumerated by hemocytometry (15), and stored in phosphate-buffered saline or sterile deionized water at 4°C (UA). B. subtilis strains WN333 (trpC2 ΔsplAB::ermC1) (10) and WN626 (uvrB42 ΔsplAB::ermC1 amyE::tet) (this study) were used for biodosimetry. B. subtilis strains were routinely cultivated at 37°C on Luria-Bertani agar (9) containing the appropriate antibiotics at final concentrations of 1 μg of erythromycin/ml and 25 μg of lincomycin/ml (for WN333) and 10 μg of tetracycline/ml (for WN626). Spores were produced in Schaeffer's sporulation medium (12) and purified by lysozyme treatment and buffer washing as described previously (11) (UA). UV irradiation was performed with a standard low-pressure mercury vapor lamp (model UVGL-25; UVP, Inc., Upland, Calif.) (UA) or with a collimated beam apparatus containing two 15-W low-pressure UV lamps, model G15T8 (American UV Co., Lebanon, Ind.) as the light source and a UV reflector assembly, model XX-15S (UVP, Inc.) (EPA). Both devices emit predominantly 254-nm UV. UV fluences were measured by using either a UVX radiometer (UVP, Inc.) fitted with the appropriate calibrated probe for 254-nm UV (model UVX-25) (UA) or a model IL-1700 radiometer fitted with detector model SED240, an NS254 filter, and a wide-eye diffuser (International Light, Inc. Newburyport, Mass.) (EPA). Both UA and EPA radiometer units were calibrated by their respective manufacturers to a U.S. National Institutes of Standards and Technology standard. Furthermore, both instruments were directly compared to one another at UA by using the same UV source and were found to be in agreement. The UV irradiance distribution across the surface to be treated was predetermined by measuring the 254-nm irradiance at 0.5-cm intervals along the x-y axes of a 6-cm-diameter grid originating at the center of the UV beam. The average incident irradiance over the entire 6-cm-diameter petri dish was calculated by using UVCalc, a Microsoft Excel worksheet devised by James Bolton and kindly posted on the International UV Association website (http://www.iuva.org). B. subtilis and Encephalitozoon spores were diluted from stock suspensions into sterile deionized water to a final concentration of 1 × 107 spores per 10 ml. The absorbances at 254 nm of the spore suspensions were measured in a UV spectrophotometer and entered into UVCalc to derive the exposure time needed for each final UV dose used. Spore suspensions were pipetted into an open 6-cm-diameter petri dish set atop a rotating platform and exposed to the indicated UV doses. Serial 10-fold dilutions of B. subtilis spores were plated on Schaeffer's sporulation medium containing the appropriate antibiotics and incubated overnight at 37°C for viable colony counts. B. subtilis spore inactivation was calculated by using the formula log10 inactivation = log10 (N0/Nt), where N0 and Nt stand for the CFU of the suspension at the exposure times zero and t, respectively.
Logit dose-response relationships were developed for untreated fresh spores of the three Encephalitozoon species by titrating spore inocula by cell culture techniques as follows (8). The E. intestinalis, E. cuniculi, and E. hellem spores were inoculated onto RK-13-seeded 15-mm Thermanox coverslips of a 24-well microtiter plate and incubated for 7 days as previously described (16). Wells showing one or more infected cells were scored as positive, and those without infection were scored as negative. The proportion of infected wells (P), obtained by dividing the number of positive wells infected by the total number of wells inoculated, was used to calculate the response logit for each inoculum: response logit = ln[P/(1 − P)]. Dose-response functions for each species were determined by linear regression analysis of the response logit versus the log10 of each dose of spores producing a positive score (8). The resulting regression equations of the form response logit = m(log10 dose) + b were used to estimate the number of viable spores remaining after treatment. This number was used to calculate the log change of viability as the log10 of the estimated viable spores (D) divided by the total number of spores in each inoculum (Do) (i.e., log change + log [D/Do]) (8).
The results of 145 wells from the in vitro assay showed that the most sensitive spores of the three microsporidian species tested with low-pressure UV light were those of E. intestinalis, which exhibited 3.2-log inactivation at 60 J/m2. E. cuniculi and E. hellem spores showed 3.2-log inactivation at 140 J/m2 and 190 J/m2, respectively (Table 1). Thus, in this study E. intestinalis spores exhibited a degree of UV sensitivity comparable to that of both C. parvum oocysts (2, 3) and E. intestinalis spores from a recently published study (7). Furthermore, spores of E. cuniculi and E. hellem were observed to be approximately twice as resistant to UV as E. intestinalis spores. In all cases, a UV fluence of approximately 200 J/m2 or higher would be predicted to inactivate each of the three pathogenic microsporidian species tested.
TABLE 1.
UV inactivation kinetics of E. intestinalis, E. hellem, and E. cuniculi sporesa
| Spores | Site | UV fluence (J/m2) | No. of infected/ no. inoculated (P) | Response logit | Live spores (D) | Log applied dose (Do) | Log change (D/Do) | Temp (°C) |
|---|---|---|---|---|---|---|---|---|
| E. intestinalis | UA | 50 | 141/5 (0.93) | 2.639057 | 75 | 3.2 | −1.3 | 25 |
| UA | 60 | 1/10 (0.10) | −2.197225 | 1 | 3.2 | −3.2 | 25 | |
| EPA | 60 | 2/15 (0.13) | −1.8718 | 1 | 3.2 | −3.2 | 25 | |
| UA | 80 | 3/15 (0.20) | −1.386294 | 1 | 3.2 | −3.2 | 25 | |
| UA | 90 | 0/10 (0) | 3.2 | −3.2 | 25 | |||
| EPA | 90 | 0/10 (0) | 3.2 | −3.2 | 25 | |||
| UA | 120 | 0/5 (0) | 3.2 | −3.2 | 25 | |||
| UA | 140 | 0/5 (0) | 3.2 | −3.2 | 25 | |||
| UA | 140 | 0/5 (0) | 3.2 | −3.2 | 5 | |||
| E. hellem | UA | 120 | 8/10 (0.80) | 1.386294 | 55 | 3.2 | −1.5 | 25 |
| UA | 140 | 5/10 (0.50) | 0 | 16 | 3.2 | −2.0 | 25 | |
| UA | 170 | 2/10 (0.20) | −1.386294 | 4 | 3.2 | −2.6 | 25 | |
| UA | 190 | 0/10 (0) | 3.2 | −3.2 | 25 | |||
| E. cuniculi | UA | 80 | 6/10 (0.60) | 0.405465 | 29 | 3.2 | −1.8 | 25 |
| UA | 100 | 4/10 (0.40) | −0.405465 | 8 | 3.2 | −2.3 | 25 | |
| UA | 130 | 2/10 (0.20) | −1.386294 | 2 | 3.2 | −3.0 | 25 | |
| UA | 140 | 0/5 (0) | 3.2 | −3.2 | 25 |
Calculations performed according to Korich et al. (8). See the text for details.
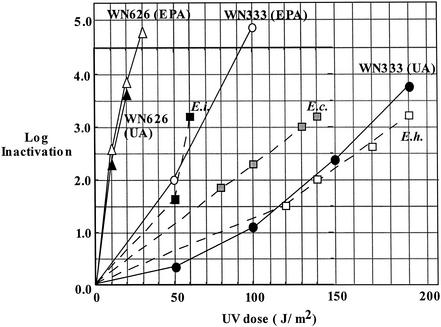
The UV inactivation kinetics of two B. subtilis biodosimetry strains, WN333 and WN626, were determined in parallel at EPA and at UA. Spores of strain WN626 were observed to be extremely sensitive to UV, exhibiting between 3.5- and 4-log reduction at only 20 J/m2 (Fig. 1). The data from the two laboratories concerning UV sensitivity of WN626 spores were in excellent agreement (Fig. 1). The UV sensitivity of spores of B. subtilis strain WN333 was found to vary by roughly a factor of 2 between the determinations at EPA versus those at UA (Fig. 1). The UV fluence required for 4-log inactivation of WN333 spores was found to be approximately 85 J/m2 at EPA and 195 J/m2 at UA (Fig. 1), which is likely due to differences in the enumeration techniques used at the two locations (membrane filters were used at EPA, and spread plates were used at UA). At both locations, however, WN333 spores exhibited UV inactivation kinetics similar to those of spores of the three Encephalitozoon species tested (Fig. 1).
FIG. 1.
Summary of spore inactivation by 254-nm UV. B. subtilis biodosimetry strains WN333 (circles) and WN626 (triangles) at EPA (open symbols) or at UA (filled symbols). For comparison, the UV inactivation kinetics from Table 1 are plotted for spores of E. intestinalis (solid squares), E. cuniculi (hatched squares), and E. hellem (open squares). The data points shown for WN333 at UA are averages of three separate determinations, which varied by ±5%.
The experiments described above were performed at room temperature (ca. 25°C). However, the water temperature in many municipal water treatment situations can be considerably lower, which we thought might affect the UV inactivation kinetics of microsporidial spores. In order to test this notion, spores of E. intestinalis were irradiated at UA to a final fluence of 140 J/m2 at either 25°C or 5°C and exhibited >3.2-log inactivation at both temperatures (Table 1). Therefore, spores irradiated at 5°C were not more resistant to UV. In addition, the UV inactivation kinetics of B. subtilis WN333 and WN626 spores at UA were found to be the same at 25°C and 5°C (data not shown).
In conclusion, the data presented here indicate that spores of the microsporidial species E. intestinalis, E. cuniculi, and E. hellem exhibit UV inactivation kinetics similar to those of C. parvum oocysts and that both microsporidian spores and oocysts would be inactivated by 254-nm UV at a fluence of 200 J/m2. Spores of B. subtilis biodosimetry strains WN333 and WN626 closely mimic the Encephalitozoon spore (Fig. 1) and C. parvum oocyst (2, 3) dose-response curves and thus provide suitable surrogates for Encephalitozoon and Cryptosporidium spp. in UV reactor validation studies. B. subtilis spores have several advantages for use as a biodosimetry surrogate. (i) They are nonpathogenic. (ii) They do not require eukaryotic cell culture or animal facilities. (iii) They afford testing results in ≤24 h (as opposed to 6 to 10 days required for the Encephalitozoon in vitro cell culture assay). (iv) They can be used in- house by any municipal water testing facility equipped to perform basic microbiology. The ease and speed of manipulation and the potential production capability make B. subtilis DNA repair-deficient spores strong potential candidates for use as biodosimetry surrogates in a broad variety of UV testing and verification applications.
Acknowledgments
We thank Belinda Galeano for excellent technical assistance, Thomas Hargy for insightful comments and Dick Korich for critical review of the manuscript.
This work was supported by U.S. EPA Cooperative Agreement No. R-82883501-0.
REFERENCES
- 1.Bolton, J. R., E. R. Blatchley III, A. Cabaj, W. Cairns, O. Hoyer, K. G. Linden, R. O. Rahn, M. Sasges, R. Sommer, and E. Whitby. 2000. Terms and definitions in ultraviolet disinfection. Proceedings of Disinfection 2000, New Orleans, La. Water Environment Federation, Alexandria, Va. [On line.]
- 2.Clancy, J. L., T. M. Hargy, M. M. Marshall, and J. Dyksen. 1998. Inactivation of oocysts of Cryptosporidium parvum in water using ultraviolet light. J. Am. Water Works Assoc. 90:92-102. [Google Scholar]
- 3.Clancy, J. L., Z. Bukhari, T. M. Hargy, J. R. Bolton, B. W. Dussert, and M. M. Marshall. 2000. Using UV to inactivate Cryptosporidium. J. Am. Water Works Assoc. 92:97-104. [Google Scholar]
- 4.Federal Register. 1998. Announcement of the Drinking Water Contaminant Candidate List. Fed. Regist. 63:10273-10287.
- 5.Haider, T., R. Sommer, S. Knasmuller, P. Eckl, W. Pribil, A. Cabaj, and M. Kundi. 2002. Genotoxic response of Austrian groundwater samples treated under standardized UV (254 nm): disinfection conditions in a combination of three different bioassays. Water Res. 36:25-32. [DOI] [PubMed] [Google Scholar]
- 6.Hoyer, O. 2000. The status of UV technology in Europe. Int. UV Assoc. News 2:22-27. [Google Scholar]
- 7.Huffman, D. E., A. Gennaccaro, J. B. Rose, and B. W. Dussert. 2002. Low- and medium-pressure UV inactivation of microsporidia Encephalitozoon intestinalis. Water Res. 36:3161-3164. [DOI] [PubMed] [Google Scholar]
- 8.Korich, D. G., M. M. Marshall, Z. Bukhari, H. V. Smith, J. O'Grady, C. R. Fricker, J. Rosen, and J. L. Clancy. 2000. Inter-laboratory comparison of the CD-1 neonatal mouse dose response model for Cryptosporidium parvum oocysts. J. Eukaryot. Microbiol. 47:294-298. [DOI] [PubMed] [Google Scholar]
- 9.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 10.Nicholson, W. L., L. Chooback, and P. Fajardo-Cavazos. 1997. Analysis of spore photoproduct lyase operon (splAB) function using targeted deletion-insertion mutations spanning the Bacillus subtilis operons ptsHI and splAB. Mol. Gen. Genet. 255:587-594. [DOI] [PubMed] [Google Scholar]
- 11.Nicholson, W. L., and P. Setlow. 1990. Sporulation, germination, and outgrowth, p. 391-450. In C. R. Harwood and S. M. Cutting (ed.), Molecular biological methods for Bacillus. John Wiley & Sons, Sussex, England.
- 12.Schaeffer, P., J. Millet, and J.-P. Aubert. 1965. Catabolic repression of bacterial sporulation. Proc. Natl. Acad. Sci. USA 54:704-711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scharfenaker, M. A. 2002. Draft LT2ESWTR out of the box. J. Am. Water Works Assoc. 94:24-37. [Google Scholar]
- 14.Sommer, R., W. Pribil, S. Appelt, P. Gehringer, J. Eschweiler, H. Leth, A. Cabaj, and T. Haider. 2001. Inactivation of bacteriophages in water by means of non-ionizing (UV-253.7 nm) and ionizing (gamma) radiation: a comparative approach. Water Res. 35:3109-3116. [DOI] [PubMed] [Google Scholar]
- 15.Wittner, M., and L. M. Weiss. 1999. The microsporidia and microsporidiosis. American Society for Microbiology, Washington, D.C.
- 16.Wolk, D. M., C. H. Johnson, E. W. Rice, M. M. Marshall, K. F. Grahn, C. B. Plummer, and C. R. Sterling. 2000. A spore counting method and cell culture model for chlorine disinfection studies of Encephalitozoon syn. Septata intestinalis. Appl. Environ. Microbiol 66:1266-1273. [DOI] [PMC free article] [PubMed] [Google Scholar]