Abstract
The effects of freeze-thaw, freezing and sediment geochemistry on terminal anaerobic processes occurring in sediments taken from below cyanobacterial mats in meltwater ponds of the McMurdo Ice Shelf in Antarctica were investigated. Depending on the geochemical and physical status of the sediments (i.e., frozen or thawed), as well as passage of sediment through a freeze-thaw cycle, terminal carbon and electron flow shifted in which the proportions of hydrogen and acetate utilized for methanogenesis and sulfate reduction changed. Thus, in low-sulfate (or chloride) sediment which was thawed and incubated at 4°C, total carbon and electron flow were mediated by acetate-driven sulfate reduction and H2-driven methanogenesis. When the same sediments were incubated frozen, both methanogenesis and sulfate reduction decreased. However, under these conditions methanogenesis was favored over sulfate reduction, and carbon flow from acetate to methane increased relative to sulfate reduction; >70% of methane was contributed by acetate, and more than 80% of acetate was oxidized by pathways not coupled to sulfate reduction. In high-sulfate pond sediments, sulfate reduction was a major process mediating terminal carbon and electron flow in both unfrozen and frozen incubations. However, as with low-sulfate sediments, acetate oxidation became uncoupled from sulfate reduction with freezing. Geochemical and temperature effects could be expressed by linear models in which the log (methanogenesis to sulfate reduction) was negative log linear with respect to either temperature or the log of the sulfate (or chloride) concentration. From these relationships it was possible to predict the ratio for a given temperature (low-sulfate sediments) or sulfate (chloride) concentration. Small transitory changes, such as elevated sulfate reduction coupled to increased acetate turnover, resulted from application of a freeze-thaw cycle to low-salinity pond sediments. The results demonstrate how ecophysiological processes may change in anaerobic systems under extreme conditions (e.g., freezing) and provide new insights into microbial events occurring under these conditions.
The impacts of physical and chemical changes including climate variables (e.g., temperature) on biological processes contributing to productivity have been studied in a variety of Antarctic ecosystems (6-8), but to date the impacts on anaerobic communities of such changes have not yet been described. The purpose of this work is to determine the effects of physical and chemical changes on anaerobic community function in pond sediments of the McMurdo Ice Shelf.
The McMurdo Ice Shelf is in the northwestern corner of the Ross Ice Shelf, between Ross Island and Brown Peninsula. An area of about 1,500 km2 is known as “dirty ice,” an ablation zone covered by gravel. A large portion of this gravel originates from marine sediment, and much of the shelf ice is frozen seawater (4). The area has an undulatory surface, and during the summer melt is covered by ponds of a wide size range between hummocks with a vertical profile of up to 20 m (9). Ponds form and disappear again over decadal time scales, leading to a wave-like cycling of the shelf surface through ponds and hummocks (3). Freezing, thawing, and evaporation often lead to pronounced solute gradients and water column stratification (6, 8).
The bottoms of these ponds are covered with thick mats consisting of cyanobacteria, diatoms, and green algae (9, 10), below which is often a layer of anoxic sediment. Variations in chemical and physical conditions between the ponds leads to community differentiation within and between the mats and to differences in mat morphology, thus creating in a small area a variety of modern unlithified stromatolites unique on Earth (19). The mats harbor a small population of grazers, mainly the rotifer Philodinia gregaria, but their activity does not appear to have a key function in the ecosystem (13). Apart from the discrete ponds on the ice shelf there are also ponds in estuaries (5). These ponds are connected at high tide and also contain diverse assemblages of algae and cyanobacteria with primary production characteristics similar to discrete ponds (5).
Previous studies have shown that ponds may vary in conductivity from being highly saline to almost freshwater (10), and recently we described the anaerobic events in sediments of a low-salinity pond, unofficially known as Orange Pond. These studies showed that in sediments below the cyanobacterial mat terminal carbon and electron flow was partitioned so that fatty acid oxidation was mainly coupled to sulfate reduction, while methane was produced from H2 and CO2 (11). Apart from this study, most of the knowledge of the biological processes occurring in these ponds relates to photosynthetic activity of, and carbon flux associated with, cyanobacterial mats in relation to differing physical and chemical characteristics of the ponds and climate changes (3, 5).
In this work we describe the effects of temperature, geochemistry, and freeze-thaw on terminal anaerobic processes in sediments of six meltwater ponds on the McMurdo Ice Shelf. The shifts in carbon and electron flow in response to these physical and chemical changes are discussed in terms of substrate and electron acceptor availability, changes in free energy of terminal pathways, and in substrate affinities in these anaerobic communities.
MATERIALS AND METHODS
Location of sampling area and study pond.
The study area was immediately south of Bratina Island (78°00′S, 165°35′E) on the McMurdo Ice Shelf (Fig. 1). The area was surveyed in January 1991 by B. R. George (New Zealand Department of Survey and Land Information, K 191, plan no. 37/165A). The study ponds were discrete and were designated with the unofficial names, Fresh, Skua, P-70, Brack, Salt, and Orange. The water chemistry and algal mats in the ponds have been previously described (9, 10, 17). Underlying the cyanobacterial mat at the bottom of each pond was a layer of anaerobic sediment, which varied in depth depending on the pond, reaching a depth maximum of about 18 to 20 cm. Below this depth the sediment was frozen.
FIG. 1.
Location of study area (denoted by arrow). MIS, McMurdo Ice Shelf.
Sampling procedure.
Unless stated otherwise pond sediments were removed from a depth of 10 to 20 cm (60 cm from the shoreline) from 0 to 5 cm below the cyanobacterial mat by coring as previously described (11). Samples from the same pond were pooled and stored frozen in sealed near-filled containers. Samples were thawed and stored for short periods of time at 4°C prior to their use in incubation experiments (see below).
Incubation techniques.
For studies on methanogenesis, sediment was transferred to 70-ml serum bottles (10 cm3) under a gas stream of 70% N2-30% CO2 in all cases. Degassed pond water was added in the ratio 1 part to 2 parts sediment by volume. Bottles were sealed with butyl septum stoppers which were secured with aluminum closures and were either incubated under low- or high-resolution temperature regimens. In the former, the selected temperatures and durations were 4°C for up to 10 days (short term), −18°C for up to 8 months (seasonal winter), 4°C for up to 4 months (seasonal summer) ex vivo, and 1 year in situ (annuals). For high-resolution temperature effects, incubations (100 days) were carried out at temperature intervals in the range −18 to 4°C. Methane was determined in replicate (n = 3) short-term incubations by sampling at various intervals over a time course, and in longer incubations (>100 days) by sampling at zero time and at a fixed point at the termination of incubation.
For incubations in which the partitioning of acetate to methane and CO2 were required, [2-14C]acetate (0.2 ml, 51 mCi mmol−1, 25 μCi ml−1) was added via syringe to butyl septum-stoppered 70-ml serum bottles each containing 10 ml of sediment (taken from a depth of 0 to 5 cm) diluted with 5 ml of degassed water taken from the same pond as the sediment under a gas mixture of 70% N2-30% CO2. Sediments were incubated at −10 or 4°C. Bottles also contained a glass center tube for CO2 capture by NaOH. Incubation was terminated by the addition of 0.3 ml of 50% H2SO4 to the sediment slurry (after thawing in the case of frozen incubations) immediately preceded by the addition of 2.5 ml of 3 N NaOH to the center well, and bottles were stored for 2 h to allow for complete absorption of CO2 before analysis. To determine label associated with acetate remaining in the incubation, the acidified fraction was centrifuged at 7,000 × g for 20 min at 5°C and the acetate fraction in the supernatant obtained after high-performance liquid chromatography (HPLC) (see “Analysis of radioactive incubations,” below).
Studies of turnover of acetate were carried out by addition of 0.2 ml of [2-14C]acetate (51 mCi mmol−1, 25 μCi ml−1) via syringe to butyl septum-stoppered serum bottles containing 12 ml of sediment slurry made up from 2 parts pond sediment (depth, 0 to 5 cm) to 1 part degassed water taken from the same pond as the sediment, under a gas stream of 70% N2-30% CO2. Sediments were incubated at either −10 or 4°C. Either samples (0.5 to 1 ml) were withdrawn from duplicate vessels over various intervals over 10 days (4°C incubations), or the contents of duplicate bottles were taken at various intervals over 3 months and then thawed (frozen incubations). Removed or thawed samples were then spun at 7,000 × g for 20 min at 5°C, and the supernatants were stored at −18°C until required for analysis.
Studies on sulfate reduction were carried out by incubation of 2 ml of sediment slurry (2 parts sediment to 1 part degassed pond water by volume) in plastic syringes (3.0 ml), the sawn-off end of which was sealed with a butyl septum stopper (11), or in 12-ml glass tubes (Evacuret) sealed with septum stoppers secured with a screw cap. The latter was used to determine the effects of hydrogen in the gas atmosphere in which H2 ranged from 3 to 18 kPa, the balance being N2-CO2 in which the concentration of CO2 was set at 30% of the gas phase. Two microcuries of Na235SO4 (100 mCi mmol−1; 10 μCi ml−1) was injected into each sample, which was shaken to distribute the label evenly and then incubated under the same conditions as for methanogenesis (syringes) or on a gyro-rotatory shaker set at 30 rpm (glass tubes) to minimize the slow diffusion of the gas from the gas phase to the sediment.
Analysis of radioactive incubations.
Analysis of radiolabeled gases from [2-14C]acetate incubations was performed after addition of nitrogen gas to bottles to relieve the negative pressure as a result of CO2 absorption and counting 14CH4 after injection of gas samples into sealed scintillation vials and 14CO2 trapped in NaOH as previously described (11). Label from 35SO42− incubations was determined as previously described (11), but in the case of tubes containing 35SO42−, 2 ml of 6 N HCl was injected to evolve [35S]H2S, which was carried to the zinc acetate trapping solution by sparging the contents of the tube with a nitrogen gas stream. Specific radioactivity of [2-14C]acetate in supernatants from turnover studies or label remaining after partition studies was determined after capture of the acetate fraction by high-performance liquid chromatography. Separation was achieved on an Alltech C18 column at 28°C with a flow rate of 0.5 ml min−1 using 0.1% (vol/vol) sulfuric acid as the mobile phase based on a previously described method (16). Label in the captured fraction was determined by liquid scintillation procedures. Label associated with the pellet from partition studies was determined after suspension of the pellet in water and counting an aliquot in Triton X-based scintillation fluid.
Analysis of nonradioactive incubations.
Methane and hydrogen were determined by gas chromatographic procedures as previously described (11). Short-chain fatty acids were analyzed by gas chromatography after centrifugation of sediments or sediment slurries (6,000 × g for 20 min at 2°C) and acidification of the supernatant. Separation was achieved on an SGE BP-21 column (inner diameter, 0.53 mm; length, 2 m) at 80°C with a nitrogen carrier gas flow of 3 ml min−1 in a Hewlett Packard (model 5890) gas chromatograph equipped with a flame-ionization detector. This procedure was also used to corroborate values for acetate as determined by HPLC.
Chemical analysis of sediments.
The preparation of sediment for chemical analyses and the analytical methods used were as previously described (11). Sodium was measured by flame emission spectrometry and chloride was measured by colorimetry after extraction of dried sediment with distilled water; pH was determined after filtering the sediment slurry. Total Kjeldahl N was determined by colorimetry after acid digestion of sediment followed by steam distillation. Sulfate was determined by turbidometry after phosphate extraction of sediment. Amounts of soluble reactive phosphorus (SRP), ammonia-N, nitrite-N, and nitrate-N were determined colorimetrically after sediment was extracted with 1 M KCl. Total organic matter was determined by drying sediment to 105°C for dry weight and then heating to 500°C to obtain the ash-free dry weight.
Chemicals.
All chemicals were of reagent grade and were obtained from commercial sources. The radioisotopes [2-14C]acetate (51 mCi · mmol−1) and Na235SO4 (100 mCi · mmol−1) were obtained from the Radiochemical Center, Amersham, England.
RESULTS
Physical and chemical characteristics of sediment.
The physical and chemical characteristics of sediments from the six ponds are summarized in Tables 1 and 2. Sediments from all ponds were black in color, producing a sulfidogenic odor, and ranged from gravel or gravel and decaying mat to medium sand in appearance. The density of the sediments ranged from 1.35 to 2 g cm−3, and the dry weight as a percentage of wet weight ranged from 37 to 81% (Table 1). For the different pond sediments organic matter ranged from 1.2% (Fresh Pond) to 7.7% (P-70) of the dry weight. Salts were elevated in sediments from Brack and Salt ponds (Table 2), and also of significance were the elevated levels of SO42− in these sediments. SRP was substantially higher in sediments from Orange Pond than in sediments from the other ponds. The profiles for nitrogen in its various forms in the sediments did not show any clear parallels between the ponds. Thus, while Kjeldahl nitrogen was elevated for sediments from P-70 and Brack ponds, NH4+ nitrogen was elevated in sediments from P-70 and Fresh ponds. The highest levels of NO3− and NO2− nitrogen were found in Orange Pond sediments, and with the exception of NO3− in Fresh Pond sediments, levels were substantially lower in the sediments from the other ponds. The pH of the pond sediments trended towards alkaline, with the lowest values shared between Orange and Salt ponds.
TABLE 1.
Characteristics of McMurdo Ice Shelf sedimentsa
| Pond | Description | Density (g cm−3) | Dry weight (% of wet wt) | % Organic matter (dry wt) |
|---|---|---|---|---|
| Fresh | Gravel | 1.99 | 77.6 | 1.2 |
| Orange | Gravel, decaying mat | 1.48 | 48.7 | 4.2 |
| P-70 | Gravel, decaying mat | 1.40 | 44.7 | 7.7 |
| Skua | Gravel, decaying mat | 1.35 | 36.7 | 6.4 |
| Brack | Medium sand | 1.66 | 62.1 | 3.3 |
| Salt | Medium sand | 1.96 | 81.1 | 1.3 |
Sediments were taken from a depth of 0 to 5 cm below the cyanobacterial mat at a water depth of 10 to 20 cm.
TABLE 2.
Chemical analysis of sediments from McMurdo Ice Shelf ponds
| Chemical | Concn (μg · [cm3 of sediment]−1)a
|
|||||
|---|---|---|---|---|---|---|
| Fresh | Orange | P-70 | Skua | Brack | Salt | |
| Na+ | 301 | 815 | 442 | 116 | 902 | 4,688 |
| CI− | 104 | 215 | 377 | 64 | 532 | 4,688 |
| SO42− | 416 | 365 | 279 | 75 | 2,088 | 7,922 |
| SRP | 0.24 | 1.46 | 0.61 | 0.09 | 0.24 | 0.31 |
| Kjeldahl nitrogen | 305 | 292 | 786 | 377 | 617 | 396 |
| NH4+ nitrogen | 97 | 22 | 85 | 21 | 12 | 12 |
| NO3− nitrogen | 0.62 | 1.72 | 0.15 | 0.08 | <0.09 | 0.03 |
| NO2− nitrogen | 0.05 | 0.73 | 0.05 | 0.03 | <0.01 | 0.02 |
| pH | 9.1 | 7.7 | 8.9 | 8.4 | 8.5 | 7.7 |
Values are for sediments taken from 0 to 5 cm below the cyanobacterial mat at a water depth of 10 to 20 cm. All sediments contain iron at >4.6% (dry weight).
Geochemical influence on pathways of carbon and electron flow mediated by methanogenesis and sulfate reduction.
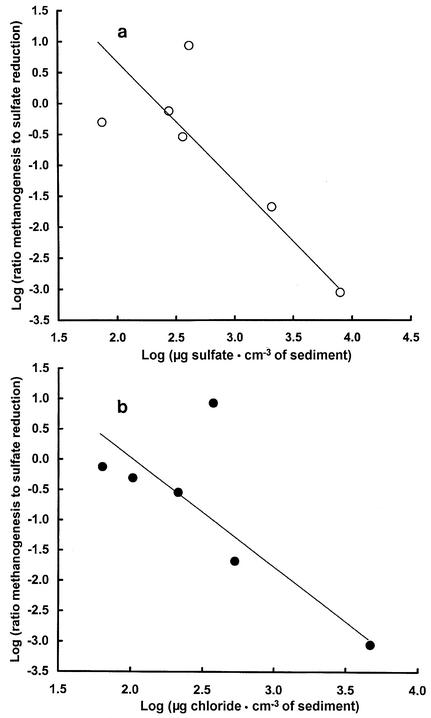
Data for methanogenesis and sulfate reduction during short-term incubation at 4°C in six pond sediments are shown in Table 3. When the logs of the ratios of methanogenesis to sulfate reduction were plotted for the six pond sediments against the logs of sulfate and chloride concentrations, a negative log-linear response was obtained (Fig. 2). From the slope k of the plots (k = −Δlog r/Δlog [SO42−] or −Δlog r/Δlog [Cl−]) in which r0 is the y axis intercept of the regression line at log [SO42−] or [Cl−] = 0, it is possible to predict at any given concentration of SO42− or Cl− the ratio of methanogenesis to sulfate reduction. In Table 4 the equation above has been rearranged so that the ratio of methanogenesis to sulfate reduction can be predicted at the limits of the experimentally determined range.
TABLE 3.
Rates of major processes occurring in sediments below the cyanobacterial mat for six ponds of the McMurdo Ice Shelf
| Pond | Incubation typea | Mean rate (nmol · cm−3 day−1) ± SD ofb:
|
|
|---|---|---|---|
| Methanogenesis | Sulfate reduction | ||
| Orange | ST | 100.9 ± 18.9 | 351.2 ± 63.7 |
| S1 | 14.2 ± 1.9 | 14.6 ± 1.3 | |
| S2 | 2.4 ± 0.5 | 0.02 ± 0.01 | |
| A | 3.6 ± 0.8 | 6.8 ± 0.4 | |
| Skua | ST | 156.6 ± 32.5 | 314.2 ± 39.6 |
| S1 | 31.6 ± 1.9 | 21.8 ± 1.0 | |
| S2 | 7.7 ± 4.1 | 0.14 ± 0.03 | |
| A | 21.8 ± 0.6 | 15.9 ± 6.7 | |
| P-70 | ST | 108.1 ± 6.2 | 143.3 ± 33.6 |
| S1 | 70.3 ± 18.0 | 22.1 ± 7.1 | |
| S2 | 12.5 ± 7.6 | 0.22 ± 0.01 | |
| A | 8.9 ± 0.5 | 11.2 ± 2.6 | |
| Fresh | ST | 99.9 ± 13.2 | 11.6 ± 1.2 |
| S1 | 1.25 ± 0.3 | 0.3 ± 0.02 | |
| S2 | 0.06 ± 0.02 | 0.006 ± 0.002 | |
| A | 0.02 ± 0.01 | <0.01 | |
| Brack | ST | 3.38 ± 0.06 | 124.1 ± 20.9 |
| S1 | 0.15 ± 0.03 | 42.9 ± 2.9 | |
| S2 | 0.09 ± 0.01 | 0.18 ± 0.018 | |
| A | 0.06 ± 0.01 | 27.7 ± 9.2 | |
| Salt | ST | 1.11 ± 0.03 | 160.8 ± 1.2 |
| S1 | 0.27 ± 0.10 | 9.3 ± 4.6 | |
| S2 | <0.001 | 0.23 ± 0.17 | |
| A | 0.04 ± 0.01 | 12.1 ± 2.3 | |
Abbreviations: ST, short-term incubation at 4°C for up to 10 days; S1, seasonal summer incubation (4°C) up to 4 months; S2, seasonal winter incubation (−18°C) up to 8 months; A, 1-year in situ incubation.
Values for ST were determined on sediments collected in the field in January 1997 in the linear range for [35S]sulfide and methane production. Values for S1 and S2 were determined for sediments collected in January 1998. Values for A were determined from in situ incubations from January 1997 to January 1998. Values are means of at least triplicate determinations ± 1 SD.
FIG. 2.
Plots of log methanogenesis sulfate reduction rate ratio versus log sulfate concentration (a) and chloride concentration (b) for sediments of six ponds. Rates were obtained using values (Table 3) for short-term incubations at 4°C.
TABLE 4.
Predictions of methanogenesis-to-sulfate reduction ratio (r) as a function of sulfate or chloride in sediments
| Ion | μg · cm−3 | Log μg · cm−3 | k | Log r0a | Log rb | r |
|---|---|---|---|---|---|---|
| Sulfate | 200 | 2.31 | 1.57 | 3.85 | 0 | 1.00 |
| 9,000 | 3.95 | 1.57 | 3.85 | −2.35 | <0.01 | |
| Chloride | 150 | 2.17 | 1.59 | 3.30 | −0.10 | 1.25 |
| 8,500 | 3.93 | 1.59 | 3.30 | −2.94 | <0.01 |
Determined from the expression log r (log μg of SO42− or Cl−·cm−3)·k using values of log [SO4−] or [Cl−] and log r in Fig. 2.
Determined from the expression log r0 − (log μg of SO42− or Cl−·cm−3) ·k.
We determined whether the decline in the ratio of methanogenesis to sulfate reduction at elevated sulfate and chloride concentrations could be explained on the basis of a shift in the partitioning of label between CO2 and methane from 2-14C-labeled acetate. Rates of incorporation of label into methane and CO2 from 2-14C-labeled acetate were measured for sediments from all six ponds incubated at 4°C, and the partition index p, expressed by the term dpm 14CO2 day−1/(dpm 14CO2 day−1 + dpm 14CH4 day−1). For all sediments p was ≥0.99, indicating that labeled acetate methyl group was mainly converted to CO2 (90% of label from degraded acetate recovered in this product). Thus, partitioning of label cannot be used to explain the change in the ratio.
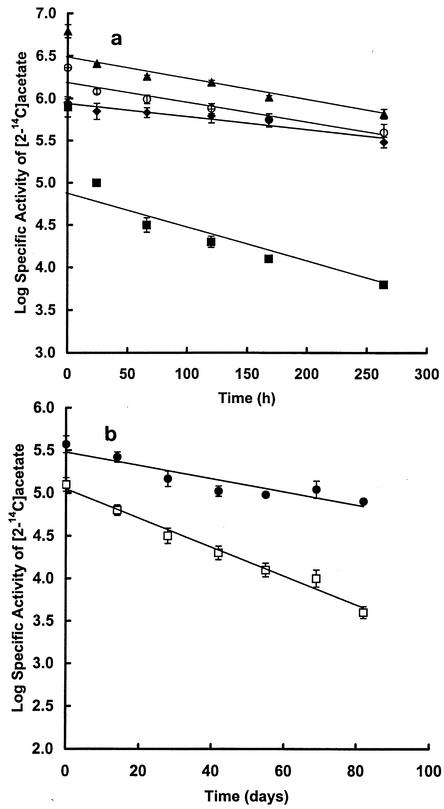
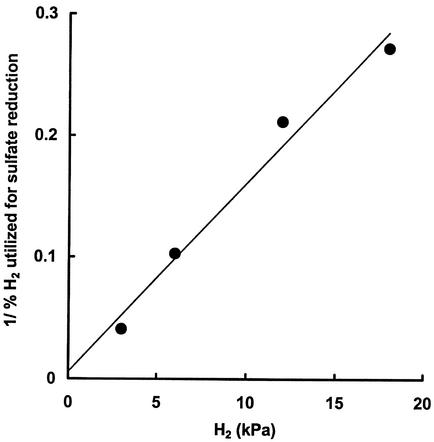
In order to resolve the terminal pathways in high- and low-sulfate (or chloride) systems, acetate dissimilation was measured in low-sulfate (or chloride) sediments (Orange Pond), or in high-salt (or -chloride) sediments (Brack and Salt ponds). Rates determined from the slope of the plots of log of specific radioactivity of acetate versus time (Fig. 3a) multiplied by the pool size for 4°C incubations are shown in Table 5. The percentage methane derived from acetate cleavage or sulfate reduction coupled to acetate oxidation can be determined by comparing the turnover rates with the rates of methanogenesis and sulfate reduction, amended for p. On this basis about 70% of sulfate reduction was accounted for by acetate in all three pond sediments, and of the methane produced in the low sulfate (or chloride sediment) only about 2% was contributed by acetate. Our previous investigation demonstrated that in these sediments methane was almost exclusively produced from H2 and CO2 (11). In high-sulfate (or chloride) sediments very little methane was produced (Table 3), and in experiments in which sediments were incubated in the presence of added hydrogen while there was no stimulation of methanogenesis, sulfate reduction was enhanced (Table 6), accounting for 1 to 25% of the hydrogen utilized at initial levels ranging from 3 to 18 kPa. Transformation of the results in Table 6 plotting 1/percent H2 utilized for sulfate reduction versus initial H2 (in kilopascals) (Fig. 4) predicts that under physiological conditions (H2 < 5 Pa) for high-sulfate ponds, sulfate reduction would account for all of the hydrogen produced. The percentages of carbon flow via acetate (or H2 or CO2) flow to methane or to CO2 via sulfate reduction for high- and low-sulfate ponds are shown in Table 7. The results demonstrate that in low-sulfate sediments the passage of carbon flow is to CO2 via acetate and to methane via H2CO2. However in high-sulfate sediments terminal carbon flow is almost exclusively mediated by sulfate reduction.
FIG. 3.
Plots of decrease in the log specific radioactivity of acetate after addition of [2-14C]acetate to sediments which were incubated at 4°C (a) and below freezing (b). (a) Average pool sizes were 0.76 and 0.78 (Orange with and without freeze-thaw, respectively), 0.36 (Brack), and 0.82 (Salt) μmol cm−3 of sediment. Symbols: ♦, Orange; ○, Orange after freeze-thaw cycle; ▪, Brack; ▴, Salt pond sediments. For these incubations sediments were retrieved in 1997 (Brack and Salt) and 1999 (Orange). (b) Average pool sizes were 0.86 (Orange), and 0.42 (Brack) μmol cm−3 of sediment. Symbols: •, Orange (−10°C); □, Brack (−10°C) sediments retrieved in 2001. Values are means of at least duplicate determinations ± 1 SD (error bars).
TABLE 5.
Rates of sulfate reduction, methanogenesis, and acetate dissimilation in pond sediments and calculations of the contributions of acetate and hydrogen to methanogenesis and sulfate reduction
| Pond | Yr of sampling | Temp (°C) | Rate (nmol · cm−3 day−1) of:
|
poxc | % Methane accounted for by acetated | % Sulfate reduction accounted for by acetatee | ||
|---|---|---|---|---|---|---|---|---|
| Sulfate reductiona | Methane productiona | Acetate dissimilationb | ||||||
| Salt | 1997 | 4 | 160.8 ± 1.2 | 1.1 ± 0.3 | 111.2 | 0.99 | 100 | 68 |
| Brack | 1997 | 4 | 124.0 ± 20.9 | 3.3 ± 0.6 | 87.8 | 0.99 | 27 | 70 |
| 2001 | −10 | 7.6 ± 0.5 | 0.68 ± 0.04 | 17.1 | 0.98 | 50 | 100 | |
| Orange | 1999 | 4 | 106.9 ± 2.4 | 35.5 ± 3.9 | 71.8 | 0.99 | 2 | 66 |
| 1999 | 4f | 145.6 ± 9.8 | 37.5 ± 4.0 | 102.4 | 0.99 | 2 | 70 | |
| 2001 | −10 | 0.44 ± 0.1 | 1.70 ± 0.34 | 16.4 | 0.92 | 77 | 100 | |
Values are means of at least duplicate determinations ± 1 SD.
Determined from the slopes of plots (Fig. 2) of log specific radioactivity of acetate (× 2.303) × acetate pool size as previously described (10).
Obtained by measuring the rate of 14CH4 and 14CO2 and the calculation 14CO2/(14CO2 + 14CH4). Values are means, and the SD is <0.01.
Based on the equation CH3COO− + H+ → CH4 + CO2 and determined from (nanomoles of acetate centimeter−3 day−1 × [1 − p]/nanomoles of CH4 centimeter−3 day−1) × 100.
Based on the equation CH3COO− + SO42− → 2 HCO3− + HS− and determined from (nanomoles of acetate centimeter−3 day− 1 × p/nanomoles of sulfate reduced centimeter−3 day−1) × 100.
Freeze-thaw.
TABLE 6.
Proportion of hydrogen utilized for sulfate reduction in Brack Pond sediments incubated at 4°C
| Initial H2 level (kPa) | Mean amt (μmol) ± SD ofa:
|
% H2 used for sulfate reductionb | |
|---|---|---|---|
| H2 utilized | Sulfate reduced | ||
| 0 | 0 | 0.57 ± 0.07 | 0.0 |
| 3 | 11.69 ± 0.89 | 1.29 ± 0.04 | 24.8 |
| 6 | 19.08 ± 0.39 | 1.04 ± 0.04 | 9.7 |
| 12 | 19.53 ± 2.89 | 0.80 ± 0.11 | 4.7 |
| 18 | 19.78 ± 4.33 | 0.76 ± 0.21 | 3.7 |
Determined after 136 h of incubation in sediments retrieved in 2001. Values are means of at least duplicate determinations ± 1 SD.
Determined according to the equation 4 H2 + SO42− + H− → 4 H2O + HS−.
FIG. 4.
Stimulation of methanogenesis by H2 in sediments from Brack pond: plot of the reciprocal of the percentage of H2 utilized for sulfate reduction versus initial H2 (in kilopascals). The level of H2 in the unamended system was 5 kPa.
TABLE 7.
Calculation of carbon flow via acetate or H2O, CO2 to sulfate reduction and methanogenesis in pond sediments of the McMurdo Ice Shelf incubated at 4°C
| Pond | % Carbon flowa via:
|
|||
|---|---|---|---|---|
| H2 or CO2 to CH4 | Acetate to CH4 | Acetate coupled to sulfate reduction | H2 or CO2 coupled to sulfate reduction | |
| Orange (no freeze-thaw) | 24 | 0.3 | 50 | ND |
| Orange (freeze-thaw) | NDb | 0.3 | 55 | ND |
| Brack | <1 | 0.67 | 67 | >30 |
| Salt | <1 | 0.70 | 69 | >25c |
Values were determined using the data in Tables 5 and 6. They were calculated as percent contribution of acetate or H2 to process (sulfate reduction or methanogenesis) × (rate of process)/(rate of methanogenesis + rate of sulfate reduction). For Orange Pond the previous determination of 100% H2 utilization being accounted for by methane (11) was used in the calculation, and for Brack Pond the value of 100% H2 utilization accounting for sulfate reduction (see Table 6 and Fig. 4) was used.
ND, not determined.
Based on the results for Brack Pond showing 100% H2 utilization by sulfate reduction.
Effects of freeze-thaw on methanogenesis and sulfate reduction simulating seasonal change.
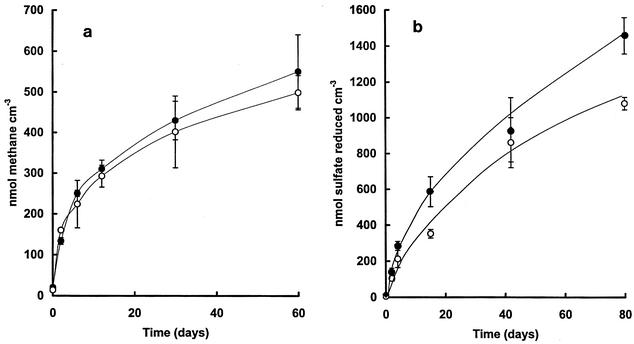
Rates of sulfate reduction and methanogenesis were measured in sediments from a low-sulfate (low salinity) pond (Orange) which had been taken through a single freeze-thaw cycle and incubated against unfrozen controls at 4°C. Time courses for methane production and sulfate reduction showed that freeze-thawing had virtually no effect on the methane production during subsequent incubation, but sulfate reduction was stimulated in the case of freeze-thawing (Fig. 5). The increase in sulfate reduction rate was accompanied by an increase in acetate turnover and in an increase in the percent acetate utilized in sulfate reduction (see Tables 5 and 7 for freeze-thaw effects). The effects of freeze-thaw on the ratio of methanogenesis to sulfate reduction in high-sulfate sediments were not investigated on the basis that since the changes in the low-sulfate sediments were small, these would be difficult to detect in sediment with low methanogenic potential.
FIG. 5.
Effects of freeze-thaw on methane production (a) and sulfate reduction (b) in sediments of Orange pond. Symbols: ○, no pretreatment by freeze-thaw; •, sediments which had been frozen over a period of 12 h and then thawed at 4°C (total time for freeze-thaw cycle, 24 h). Incubation temperature was 4°C. Values are means of triplicate determinations ± 1 SD (error bars).
Effect of temperature decrease on carbon and electron flow in sediments from low-sulfate ponds.
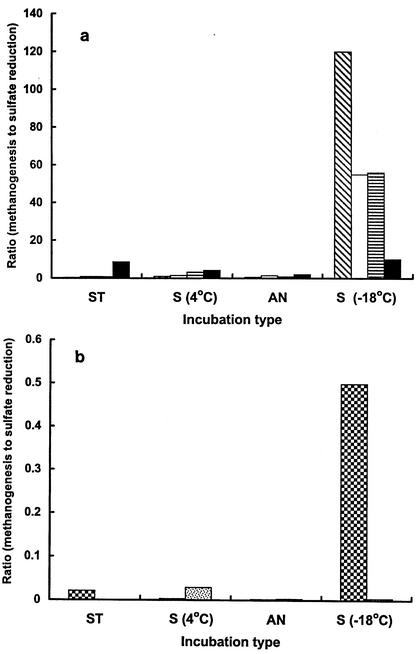
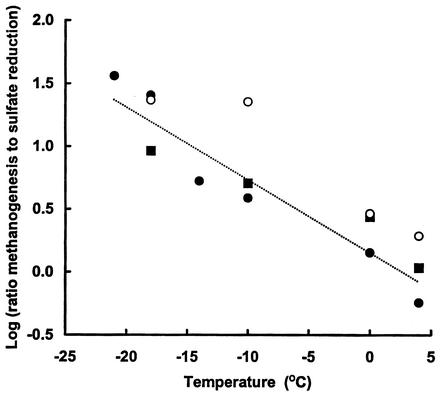
Figure 6 a shows the ratios for methanogenesis versus sulfate reduction for 4 ponds with low levels of sulfate (or chloride) in which the data were obtained from Table 3 for sediments incubated at 4°C (short term), seasonally at 4°C and −18°C, and in vivo annually. The results showed that the ratio of methanogenesis to sulfate reduction increased substantially in the −18°C incubations indicating that decreased temperature increased the ratio, although in the case of Fresh sediments measurements of sulfate reduction were near the detection limit for this method. When sediments from three ponds (Orange, P-70, and Skua) were incubated at various temperatures ranging from −21°C to 4°C, both sulfate reduction and methanogenesis decreased with decreasing temperature (Table 8), but the rate of decrease in sulfate reduction was greater. A composite plot of log ratio of methanogenesis to sulfate reduction against temperature for these sediments was negative log linear (Fig. 7). From the slope k of the plots (k = −Δlog r/ΔT) in which r0 is the y axis intercept of the regression line at T = 0, it is possible to predict at any given temperature the ratio of methanogenesis to sulfate reduction. In Table 9 the equation above has been rearranged to predict the ratio of methanogenesis to sulfate reduction at three designated temperatures in low-sulfate sediments, two of which are at the limits of the experimentally determined range.
FIG. 6.
Effect of incubation type on the ratio of methanogenesis to sulfate reduction rate for low-sulfate sediments (a) and high-sulfate sediments (b). The ratios were obtained using values from Table 3. (a) Bar notation: cross-hatched, Orange; no fill, Skua; horizontal hatch, P-70, filled, Fresh. (b) Bar notation: checkered, Brack; speckled, Salt.
TABLE 8.
Effects of temperature on rates of sulfate reduction and methanogenesis using sediments from low-sulfate ponds
| Pond | Incubation temp (°C) | Mean rate (nmol · cm−3 day−1) ± SD of a:
|
Methanogenesis/ sulfate reduction | |
|---|---|---|---|---|
| Methanogenesis | Sulfate reduction | |||
| Orange | 4 | 5.59 ± 1.20 | 9.69 ± 1.36 | 0.57 |
| 0 | 5.39 ± 0.22 | 3.79 ± 0.39 | 1.42 | |
| −10 | 1.70 ± 0.34 | 0.44 ± 0.10 | 3.86 | |
| −14 | 1.79 ± 0.59 | 0.34 ± 0.08 | 5.26 | |
| −18 | 1.78 ± 0.19 | 0.070 ± 0.006 | 25.42 | |
| −21 | 1.45 ± 0.11 | 0.040 ± 0.004 | 36.25 | |
| Skua | 4 | 17.63 ± 0.62 | 16.29 ± 1.96 | 1.08 |
| 0 | 14.69 ± 0.36 | 5.36 ± 0.16 | 2.74 | |
| −10 | 1.97 ± 0.10 | 0.39 ± 0.12 | 5.05 | |
| −18 | 1.38 ± 0.49 | 0.15 ± 0.10 | 9.20 | |
| P-70 | 4 | 33.29 ± 6.31 | 17.20 ± 3.45 | 1.93 |
| 0 | 27.85 ± 3.25 | 9.58 ± 1.78 | 2.91 | |
| −10 | 3.16 ± 0.16 | 0.14 ± 1.01 | 22.57 | |
| −18 | 1.87 ± 0.34 | 0.008 ± 0.002 | 23.30 | |
Determined after incubation of sediments for 100 days. Values are means of triplicate determinations ± 1 SD.
FIG. 7.
Plots of log ratio of methane production rate: sulfate reduction rate versus temperature for low-sulfate pond systems. Symbols: •, Orange; ▪, Skua; ○, P-70. Values were taken from Table 8.
TABLE 9.
Prediction of the ratio of methanogenesis to sulfate reduction (r) as a function of temperature in low-sulfate sediments
Determined from the expression log r (T)·k using values of T and log r in Fig. 7.
Determined from the expression log r0 − (T·k).
The increased ratio of methanogenesis to sulfate reduction at low temperature was accompanied by a decrease in p, as shown for the −10°C incubation of Orange Pond sediment (Table 5). Under these conditions over 70% of the methane produced was contributed by acetate. However, acetate turnover could only be partially accounted for by conversion to methane, and to a much lesser extent by sulfate reduction; almost 90% was oxidized via alternative pathways (Table 10). We did not determine hydrogen consumption in these sediments (due to physical barriers to diffusion in frozen sediments); however, we have assumed that this precursor accounted for the remainder of the methane produced. The fate of reducing equivalents associated with oxidation of acetate to CO2 via alternative electron acceptors was not investigated further but is discussed in more detail (see Discussion below).
TABLE 10.
Calculation of carbon flow via acetate to sulfate reduction, to methanogenesis, and in uncoupled processes in pond sediments of the McMurdo Ice Shelf incubated at temperatures below freezing
| Pond | Temp (°C) | % Carbon flow via acetate:
|
||
|---|---|---|---|---|
| To CH4a | Coupled to sulfate reductionb | To CO2 (electron acceptor undefined)c | ||
| Orange | −10 | 8.0 | 2.6 | 89.4 |
| Brack | −10 | 3.9 | 44.4 | 51.7 |
Determined from the percentage of CH4 accounted for by acetate × CH4 production/rate of acetate dissimilation (data from Table 5).
Determined from the percentage of sulfate reduction accounted for by acetate × sulfate reduction/rate of acetate dissimilation (data from Table 5).
Calculated as 100 − (% CH4 [ac] + % SO42−[ac]).
Effects of temperature decrease on carbon and electron flow in high-sulfate and -chloride sediments.
The ratios for methanogenesis versus sulfate reduction for 2 ponds with high levels of sulfate (or chloride) are shown in Fig. 6b in which the data were obtained from Table 3 for sediments incubated at 4°C (short-term), seasonally at 4°C and −18°C, and in vivo annually. The results show that the −18°C incubation resulted in an increase in the ratio of methanogenesis to sulfate reduction for sediment from one pond (Brack) but not for Salt. When sediments from Brack Pond were incubated at −10°C, there was a slight shift in carbon flow in favor of methane production from acetate (Table 5). However, as with low-sulfate sediments, acetate turnover could only be partially accounted for by sulfate reduction and methanogenesis. A substantial proportion (>50%) was oxidized via alternative pathways (Table 10).
DISCUSSION
The present study demonstrates the effects of freeze-thaw, temperature, and sediment geochemistry on terminal anaerobic processes occurring in sediments from meltwater ponds on the McMurdo Ice Shelf. Depending on the geochemical and physical status of the sediments (i.e., frozen or thawed) as well as the time after thawing, the pattern of terminal carbon and electron flow shifted. Thus, in low-sulfate (low-chloride) pond sediment (unfrozen), carbon and electron flow were mediated by acetate-driven sulfate reduction and H2-driven methanogenesis. In the same sediments after freezing, there was a shift in carbon flow towards methane production (via acetate cleavage) and sulfate reduction only partially accounted for acetate oxidation to CO2. In high-sulfate sediments, sulfate reduction was the major process mediating terminal carbon and electron flow in unfrozen sediments (Fig. 8, sulfate-reducing environment), but the same sediments upon freezing showed a shift in carbon flow (via acetate) towards methane and uncoupling of acetate oxidation from sulfate reduction. Small transitory short-term changes, such as elevated sulfate reduction coupled to increased acetate turnover, resulted from application of a freeze-thaw cycle to low-sulfate sediments.
FIG. 8.
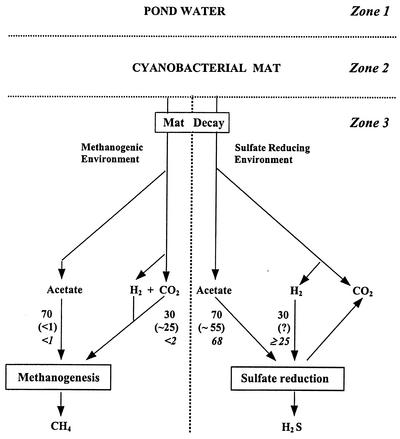
Carbon flow in methanogenic and sulfate-reducing environments below cyanobacterial mat in McMurdo pond ecosystems in unfrozen sediments. Numbers (not in italics or parentheses) refer to the percent contribution to sulfate reduction or methanogenesis from acetate or hydrogen based on the stoichiometry of known anaerobic transformations (18, 20); numbers in parentheses are from this study and Mountfort et al. (11) for low-sulfate sediments; numbers in italics are for high-sulfate sediments obtained from this study.
The results obtained with incubations with low-sulfate sediments incubated above freezing are consistent with our previous findings from Orange Pond that methanogenesis is driven mainly by H2 (11). The effects of freezing on anaerobic community function have not been described before and the uncoupling of acetate oxidation from sulfate reduction accompanied by an increased contribution of acetate to methane suggests that the methanogenic community is more tolerant of these conditions than sulfate reducers. The results also demonstrate that acetate is metabolized to CO2 in frozen sediment via as-yet-uncharacterized pathways.
One or several factors could explain the shift in the pattern of terminal and electron flow favoring both the production of methane from acetate and the uncoupling of sulfate reduction from acetate in frozen sediments. Shifts in Gibbs free energy favoring methanogenesis from acetate at low temperatures have previously been used to explain events when methanogenic ecosystems are incubated at low temperature. Thus, Conrad et al. (2) have reported that decreasing temperature in methanogenic communities results in a decrease in the Gibbs free energy for H2- or CO2-driven methanogenesis with a result that acetate plays an increased role in the process. However, the use of Gibbs free energies to explain the events occurring in frozen sediment does have limitations. In the low-sulfate sediments reported here, methanogenesis was favored over sulfate reduction upon freezing. Calculations of the Gibbs free energy changes under these conditions would slightly favor sulfate reduction over methanogenesis from acetate and hydrogen (15, 18, 20); therefore, the changes could not explain the observed shift towards methanogenesis. It appears more probable that in these sediments freezing restricts sulfate reduction to a greater degree than methanogenesis, either by providing a physical barrier preventing access of the microbes to sulfate, or by reducing the affinity of sulfate reducers for their substrates in which the physical barrier may (or may not) be a component. This results in uncoupling of acetate oxidation from sulfate reduction and the increased ratio of methanogenesis to sulfate reduction in which acetate cleavage would account for the increased proportion of the former. The demonstration by Nedwell and Rutter (12) that in psychrotrophic Antarctic bacteria the changes in the specific growth rate (μ) and the species affinity (μmax/K) for the same substrate differed during a temperature shift provides a precedent for the affinity explanation.
In a previous study we measured the uptake of hydrogen in low-sulfate pond sediment after addition of excess hydrogen, determined the methane accounted for by added hydrogen, and then extrapolated to physiological conditions (11). In this way nearly all the hydrogen produced could be accounted for by methane in these sediments. The results in this study are consistent with the earlier results, including those for sediments that have undergone freeze-thaw prior to incubation. In frozen incubations we have assumed that methane not accounted for by acetate is accounted for by hydrogen in which the pattern of carbon flow to methane would be typical of a mesophilic methanogen ecosystem (Table 5; Fig. 8). However, the fate of reducing equivalents associated with acetate oxidation to CO2 (uncoupled to sulfate reduction) is not clear. One possibility is that freezing enhances acetate oxidation (relative to sulfate reduction) utilizing oxygen at low levels, or an as-yet-undefined electron acceptor. Studies on the anaerobic metabolism of acetate in Propionibacterium freudenreichii (1) showing that hexacyanoferrate can function as an electron acceptor suggest that some forms of oxidized iron may have this capability [e.g., Fe(OH)3]. Substantial levels of iron are also present in all the pond sediments (Table 2). Nitrate is unlikely to be an alternative electron acceptor because of the very low levels occurring in the sediments (Table 2). Another potential pathway utilizing H2 in these sediments is the production of methane from dimethylsulfide (DMS). Low-sulfate sediments showed the capacity to produce DMS from dimethylsulfoniopropionate (DMSP) in the presence of the methanogenic inhibitor, bromoethanesulfonic acid (BES), and methane from DMSP with transitory accumulation of DMS in the absence of BES. However, when sediments were incubated with cyanoabacterial mat (contains < 1% dry weight DMSP), a rapid increase in methane was not preceded by DMS, and in BES-treated incubations the intermediate was not detected (11). Therefore, DMS is unlikely to be an important precursor of methane in these sediments.
There appears some scope for predicting the terminal anaerobic community function using the slope of negative log linear plots of methanogenesis to sulfate and temperature or sulfate (or chloride) concentration. The models might find particular use in situations where sediments are permanently frozen and difficult to access, making ex vivo determinations of rate problematic. For their universal application the models would be required to be tested over a range of sediment types at different locations in the Antarctic preferably along a gradient showing a range of mean annual temperatures or salt concentrations. Even then the models would not provide information on the absolute rates for terminal processes indicative of sediment productivity, and the temperature-based model would be limited to sediments low in sulfate (or chloride).
Compared to the effects produced by salt and temperature those resulting from freeze-thaw were relatively minor (Tables 5 and 7). The event produced transitory increases in acetate turnover and in sulfate reduction after which rates returned to control levels. It is unclear why methanogenesis wasn't stimulated after freeze-thaw but one possible explanation could be that the increase in hydrogen availability was insufficient to produce a measurable increase in methanogenesis. In these sediments increased acetate would not be expected to stimulate methanogenesis based on our previous studies on SCFA additions (11). The freeze-thaw mechanism probably affords a means for release of nutrients (including minerals) as has been shown in other Antarctic ecosystems for dissolved organic matter from soils (14). The freeze-thaw regimen we used consisted of only a single cycle and is based on results from annual temperature logging in the ponds in which it appears that change of season from winter to summer may be heralded by no more than a single freeze-thaw event (I. Hawes, personal communication). The small changes associated with freeze-thaw, together with the rapid return of microbial function to normal, suggests both a beneficial effect (albeit small) to the microbial community and resilience of the community to the process.
The present work provides the first example of anaerobic events occurring in a sediment which has been incubated in a temperature range spanning that typically found in the Antarctic. The effects of sediment geochemistry are also described, as are those of freeze-thaw. While the effects of sediment chemistry are not surprising based on previous studies with mesophilic sediments (18, 20) the results obtained with temperature shift are interesting. They provide some of the first insights into the function of anaerobic processes under conditions of freezing. and allow predictions to be made on anaerobic processes in the seasonal cycle. Thus, it may be predicted that sulfate reduction would predominate at higher seasonal temperatures (as in summer), while in winter, carbon flow from acetate would be mediated via pathways in which oxidation would be coupled to alternative electron acceptors which have yet to be defined. Future studies will be directed to further understanding the dynamics of anaerobic processes during freezing and their contribution to overall carbon cycling in the photosynthetic mat-sediment ecosystem.
REFERENCES
- 1.Beck, S., and B. Schink. 1995. Acetate oxidation through modified citric acid cycle in Propionibacterium freudenreichii. Arch. Microbiol. 163:182-187. [Google Scholar]
- 2.Conrad, R., H. Schultz, and M. Babbel. 1994. Temperature limitation of hydrogen turnover and methanogenesis in anoxic paddy soil. FEMS Microbiol. Ecol. 45:281-289. [Google Scholar]
- 3.deMora, S. J., R. F. Whitehead, and M. Gregory. 1994. The chemical composition of glacial meltwater ponds and streams on the McMurdo Ice Shelf, Antarctica. Antarctic Sci. 6:17-27. [Google Scholar]
- 4.Hart, C. P. 1990. Holocene megafauna in the McMurdo Ice Shelf sediments—fossilization and implications for glacial processes. Antarctic J. 25:11-14. [Google Scholar]
- 5.Hawes, I., C. Howard-Williams, A.-M. J. Schwarz, and M. T. Downes. 1995. Environment and microbial communities in a tidal lagoon at Bratina Island, McMurdo Ice Shelf, Antarctica, p. 170-177. In B. Battaglia, J. Valencia, and D. W. H. Walton (ed.), Antarctic communities. Cambridge University Press, Cambridge, United Kingdom.
- 6.Hawes, I., C. Howard-Williams, and R. D. Pridmore. 1993. Environmental control of microbial biomass in the ponds of the McMurdo Ice Shelf, Antarctica. Arch. Hydrobiol. 127:271-287. [Google Scholar]
- 7.Hawes, I., and C. Howard-Williams. 1998. Primary production processes in streams of the McMurdo Dry Valleys, Antarctica, p. 129-139. In J. C. Priscu (ed.), The McMurdo Dry Valleys, Antarctica, a cold desert ecosystem. American Geophysical Union, Washington, D.C.
- 8.Hawes, I., R. Smith, C. Howard-Williams, and A.-M. Schwarz. 1999. Environmental conditions during freezing, and response of microbial mats in ponds of the McMurdo Ice Shelf, Antarctica. Antarctic Sci. 11:198-208. [Google Scholar]
- 9.Howard-Williams, C., R. Pridmore, M. T. Downes, and W. F. Vincent. 1989. Microbial biomass, photosynthesis and chlorophyll a related pigments in the ponds of the McMurdo Ice Shelf, Antarctica. Antarctic Sci. 1:125-131. [Google Scholar]
- 10.Howard-Williams, C., R. D. Pridmore, P. A. Broady, and W. F. Vincent. 1990. Environmental and biological variability in the McMurdo Ice Shelf ecosystem, p. 32-51. In K. R. Kerry and G. Hempel (ed.), Antarctic ecosystems: ecological change and conservation. Springer-Verlag, Berlin, Germany.
- 11.Mountfort, D. O., H. F. Kaspar, M. Downes, and R. A. Asher. 1999. Partitioning during carbon and electron flow in sediments of a low-salinity meltwater pond near Bratina Island, McMurdo Ice Shelf, Antarctica. Appl. Environ. Microbiol. 65:5493-5499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nedwell, D. B., and M. Rutter. 1994. Influence of temperature on growth rate and competition between two psychrotolerant Antarctic bacteria: low temperature diminishes affinity for substrate uptake. Appl. Environ. Microbiol. 60:1984-1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suren, A. L. 1989. Microfauna associated with algal mats in melt ponds on the Ross Ice Shelf. Polar Biol. 10:329-335. [Google Scholar]
- 14.Tearle, F. V. 1987. Cryptogenic carbohydrate release and microbial response during spring freeze-thaw cycles in Antarctic fellfield fines. Soil Biol. Biochem. 19:381-390. [Google Scholar]
- 15.Thauer, R. K., K. Jungermann, and K. Decker. 1977. Energy conservation in chemotrophic anaerobic bacteria. Bacteriol. Rev. 41:100-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Titus, E., and G. A. Ahearn. 1988. Short chain fatty acid transport in the intestine of a herbivorous teleost. J. Exp. Biol. 135:77-94. [DOI] [PubMed] [Google Scholar]
- 17.Vincent, W. F., R. W. Castenholz, M. T. Downes, and C. Howard-Williams. 1993. Antarctic cyanobacteria: light, nutrients, and photosynthesis in the microbial mat environment. J. Phycol. 29:745-755. [Google Scholar]
- 18.Vogels, G. D., J. T. Keltjens, and C. van der Drift. 1988. Biochemistry of methane production, p. 707-770. In A. J. B. Zehnder (ed.), Biology of anaerobic organisms. John Wiley and Sons, New York, N.Y.
- 19.Wharton, R. A., B. C. Parker, and G. M. Simmons. 1983. Distribution, species composition and morphology of algal mats in Antarctic Dry Valley lakes. Phycologia 22:355-365. [Google Scholar]
- 20.Widdel, F. 1988. Microbiology and ecology of sulphate and sulfur reducing bacteria, p. 469-585. In A. J. B. Zehnder (ed.), Biology of anaerobic organisms. John Wiley and Sons, New York, N.Y.