Abstract
The effect of intracellular charged amino acids on freeze tolerance in doughs was determined by constructing homozygous diploid arginase-deficient mutants of commercial baker's yeast. An arginase mutant accumulated higher levels of arginine and/or glutamate and showed increased leavening ability during the frozen-dough baking process, suggesting that disruption of the CAR1 gene enhances freeze tolerance.
In the baking industry, frozen-dough technology has recently been accepted because of its advantages in supplying oven-fresh bakery products to consumers, as well as improving labor conditions for bakers (5, 6). Ordinary commercial baker's yeast is generally susceptible to damage during frozen storage and does not retain sufficient leavening ability after frozen storage. Freeze tolerance is a necessary characteristic of yeast used in frozen doughs because post-thaw leavening activity is essential prior to baking. In this study, our goal was to construct freeze-tolerant baker's yeast strains from commercial strains by regulating the metabolism of cryoprotectants.
Accumulation of the disaccharide trehalose in Saccharomyces cerevisiae is widely believed to be a major determinant of freeze tolerance (2, 4). A strong correlation between trehalose content and stress tolerance has been demonstrated (1, 13). On the other hand, amino acids are reportedly effective as stress protectants in a wide variety of organisms (3, 12, 15, 17). Recently, by using proline analogue-resistant mutants (17) and proline oxidase-deficient mutants (18), Takagi et al. showed that certain amino acids are effective as cryoprotectants and suggested that charged amino acids, such as arginine and glutamate, are determinants of freeze tolerance. To clarify the cryoprotective role of charged amino acids in commercial baker's yeast, in this study, we used specific mutants affected in arginine metabolism.
Arginase (encoded by the CAR1 gene) participates in the first committed step of arginine degradation (9, 16). The enzyme catalyzes the hydrolysis of l-arginine to l-ornithine and urea (19). The response of arginase expression to multiple environmental signals has been well studied and characterized (8, 14). On the basis of these studies, we expected that Car1 depletion would cause greater accumulation of arginine and related amino acids and that charged amino acids accumulated in the yeast cell should function as cryoprotectants. In this study, we examined the effects of Car1 depletion on the accumulation of charged amino acids and on freeze tolerance. We show that intracellular arginine and/or glutamate accumulated and that freeze tolerance was enhanced in car1 mutants derived from commercial baker's yeast.
Construction of car1 mutants from commercial baker's yeast.
To determine the effect of CAR1 disruption during the baking process, we constructed diploid homozygous car1 disruption mutants derived from commercial baker's yeast. Laboratory yeast strains were not suitable for assessment of freeze tolerance during the baking process because laboratory S. cerevisiae strains differ from commercial baker's yeast strains in many properties, such as leavening ability and stress tolerance. In this study, we used two haploid strains, T7 (prototroph MATa) and T18 (prototroph MATα), that were isolated from a commercial baker's yeast strain. Strains T7 and T18 were selected on the basis of transformation efficiency, growth rate, and fermentation ability. Strain T118 (prototroph MATa/α CAR1/CAR1), which was obtained by mating T7 and T18, has properties (e.g., leavening ability, flavor formation, and freeze tolerance) similar to those of the commercial baker's yeast used in Japan (13; data not shown). We isolated spontaneous ura3 mutants of strains T7 and T18 by positive selection with 5-fluoroorotic acid to enable selection of transformants with a URA3 selection marker, yielding strains T7ura and T18ura. CAR1gene disruption of strains T7ura and T18ura was done by the one-step gene disruption method, which involved double-recombination events at the homologous sites (11). PCR cloning of the internal sequence of CAR1 was achieved by using oligonucleotides designed on the basis of the sequence reported by Sumrada and Cooper (16). The URA3 gene was inserted into the CAR1 gene as a selective marker. The inactive copy of CAR1 on the plasmid was linearized and used for transformation of T7ura and T18ura. Replacement of the chromosomal CAR1 allele by the inactive copy was confirmed by Southern blot analysis (data not shown). The resulting strains, T7dC and T18dC, were used to construct diploid CAR1 mutants. Mating of T7dC and T18dC was done to generate CA118 (MATa/α car1/car1). To confirm Car1p depletion, we measured the arginase activity of stationary-phase cells and nitrogen-starved cells. Stationary-phase cells of strains T118 and CA118 were grown in YPD medium (medium containing 10 g of yeast extract [Difco] per liter, 20 g of peptone [Difco] per liter, and 20 g of glucose per liter) at 30°C for 48 h. Nitrogen-starved cells were obtained as follows. After cultivation at 30°C for 36 h in YPD medium, cells were transferred to SD-N medium (medium containing 1.7 g of yeast nitrogen base without amino acids and ammonium sulfate [Difco] per liter and 20 g of glucose per liter) and kept at 30°C for 12 h. The cells were disrupted in buffer A (buffer containing 10 mM Tris-HCl, 10 mM MnCl2, and 20 mM glycine, pH 7.0) by mixing with glass beads and then centrifuged at 10,000 × g for 10 min at 4°C. After the arginase enzyme reaction was done by the method of Whitney and Magasanik (19), the hydrolytic product, urea, in the supernatant of the reaction mixture was determined with a UREA N B kit (Wako). Arginase assays showed that the arginase activity of CA118 decreased to less than 10% of that of strain T118 under both stationary and nitrogen starvation conditions (Table 1). These results showed that arginase activity was depleted in strain CA118. Although higher-level induction of arginase in strain T118 was not observed during nitrogen starvation, this case may be similar to that reported by Middelhoven et al. (10).
TABLE 1.
Arginase activities of strains T118 and CA118
| Strain | Medium | Mean activity (μmol of urea min−1 mg of protein−1) ± SD |
|---|---|---|
| T118 | YPD | 2.52 ± 0.054 |
| T118 | SD-N | 2.26 ± 0.233 |
| CA118 | YPD | 0.02 ± 0.011 |
| CA118 | SD-N | 0.20 ± 0.068 |
Freeze tolerance in liquid culture and doughs of car1 mutants.
To determine the possibility of using the car1 mutant industrially, we measured the five characteristics important for baking, i.e., growth rate, cell yield from molasses, carbohydrate content, fermentation ability, and flavor formation. To obtain yeast cells of strains CA118 and T118, we simulated the industrial yeast production process by fed-batch culture using cane molasses and then measured the five characteristics. The measured characteristics, other than growth rate, of CA118 were identical to those of T118 (data not shown). The growth rate of CA118 was determined by measuring optical density at 600 nm. In YPD medium, the cell density of CA118 was slightly higher than that of T118 (Fig. 1A). These data suggested that CAR1 disruption did not degenerate the characteristics for industrial use of commercial baker's yeast.
FIG. 1.
Growth characteristics (A) and number of viable cells (B) of a car1 disruption mutant derived from commercial baker's yeast. The growth rates of a diploid homozygous car1 mutant (CA118) and its parent strain (T118) were monitored by measurement of optical density at 600 nm (OD600). Strains T118 and CA118 were grown in 5 ml of YPD medium at 30°C for 48 h and then subjected to three freeze-thaw cycles at 24-h intervals. Survival rates are shown relative to the initial number of viable cells. The data shown are mean values of three independent experiments.
To estimate the effect of CAR1 disruption on viability under freeze stress, we measured the survival rate in liquid culture during repeated freeze-thaw cycles by the method of Matsunami et al. (7). The cells were grown at 30°C for 48 h in YPD medium, and then the cultures (ca. 5 × 108 cells per ml) were kept at −20°C by using a 50% ethylene glycol bath (NCB-3200; EYELA). Freezing and thawing of the cultures were repeated three times at 24-h intervals without incubation for growth. Viability was measured by plating the cultures onto YPD agar plates in triplicate. Plates were incubated at 30°C for 48 h before the colonies were counted. The change in the number of viable cells of CA118 was compared to that of T118 (Fig. 1B). Although the number of viable cells of both T118 and CA118 decreased after repeated freeze-thaw cycles, the survival rate of strain CA118 was 10 to 1,000 times higher than that of strain T118. These data showed that CAR1 disruption affects the freeze tolerance of liquid cultures subjected to repeated freeze-thaw cycles.
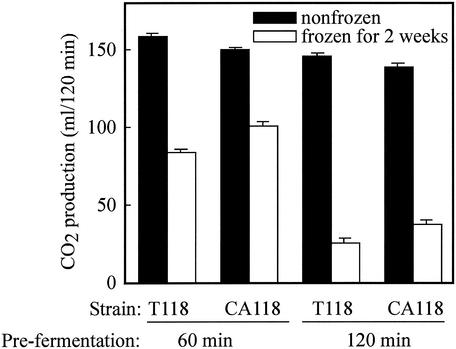
To determine the freeze tolerance of CA118 during the baking process, we assessed freeze tolerance in white bread doughs by using a method previously described (13). In brief, doughs containing CA118 or T118 were prefermented for 60 or 120 min at 30°C before being frozen at −20°C. The doughs were kept for 2 weeks at −20°C. The frozen doughs were thawed for 30 min at 30°C, and then the amount of CO2 produced in 120 min at 30°C was measured by using a Fermograph AF-1000 (Atto Co.). Strain CA118 showed approximately 20% greater leavening activity than did strain T118 after 60 and 120 min of prefermentation (Fig. 2). Although the prolonged prefermentation time decreased the gassing power of both strains, the gassing power of CA118 remained approximately 20% greater than that of T118. These data strongly suggested that CAR1 disruption of commercial baker's yeast enhanced its freeze tolerance in dough. The difference between the leavening abilities of strains CA118 and T118 after freezing and thawing was far less than the differences between their viabilities in liquid medium. This difference may be due to the cryoprotective function of dough, because dough contains possible cryoprotectants such as carbohydrates, proteins, and amino acids. The increased freeze tolerance of CA118 is a significant improvement, because an increase in freeze tolerance drastically shortens the final fermentation time after freezing.
FIG. 2.
Freeze tolerance during baking of a diploid homozygous car1 mutant (CA118) derived from commercial baker's yeast. White-bread doughs were prepared by using strain CA118 and its parent strain, T118. Doughs were prefermented for 60 or 120 min and then frozen for 2 weeks at −20°C. The remaining gassing power was measured and compared with that of nonfrozen samples. Results are shown as means ± standard deviations based on three independent experiments.
Accumulation of charged amino acids in car1 strains.
To estimate the effect of CAR1 disruption on amino acid accumulation, we evaluated the determinant of increased freeze tolerance in CA118 by measuring the amount of intracellular amino acids in stationary-phase cells obtained from the batch culture with YPD medium (Fig. 3A) and the continuously fed batch culture with molasses (simulating industrial yeast production) (Fig. 3B) (13). Aliquots of culture broth (5 ml) were used to assay the intracellular amounts of amino acids. The cells were washed twice with 10 ml of distilled water and then resuspended in 0.5 ml of distilled water. Intracellular amino acids were extracted by boiling the suspension for 20 min (17). After centrifugation (5 min at 15,000 × g), each supernatant was filtered by using a nitrocellulose membrane (MILLEX-HA; Millipore). All of the amino acids in the supernatant were subsequently measured with an amino acid analyzer (L-8500; Hitachi). The intracellular amounts of amino acids other than arginine and glutamate in strain CA118 differed only slightly (<5%) from those in strain T118 (data not shown). In the batch culture, the amount of accumulated arginine in cells of CA118 was five times greater than that in cells of the parent strain, T118. Unexpectedly, in the continuously fed batch culture, the amount of arginine in CA118 was nearly the same as that in strain T118 and the amount of glutamate was approximately 40% greater. In general, the continuously fed batch fermentation used in baker's yeast production is respiratory and often nitrogen limited. Such nitrogen limitation might have caused the greater accumulation of glutamate in CA118. Furthermore, feedback mechanisms involving arginine might have contributed to the greater glutamate content of strain CA118. Certain arginine-synthesizing enzymes (e.g., acetylglutamate synthetase, acetylglutamate kinase, and ornithine carbamoyltransferase) are regulated by feedback control of arginine (10). The results of amino acid analyses suggested that car1 mutation enhanced the intracellular accumulation of arginine and/or glutamate. The amount of proline, which reportedly is an osmoprotectant of microorganisms (3, 17), was almost the same (<5%) for CA118 and T118 (Fig. 3A and B).
FIG. 3.
Intracellular content of arginine, glutamate, and proline in a car1 mutant derived from commercial baker's yeast. A diploid homozygous car1 mutant (CA118) and its parent strain (T118) were grown in a batch culture (A) and a continuously fed batch culture (B). Intracellular arginine, glutamate, and proline contents were measured as described in the text. Intracellular glutamate content was measured during liquid fermentation (C). Results are shown as means ± standard deviations based on three independent experiments.
Figure 2 shows that the leavening ability of CA118 after freezing was greater than that of T118 and that the leavening abilities of both CA118 and T118 decreased in a prefermentation time-dependent manner. We hypothesized that the glutamate content of CA118 should decrease with increasing prefermentation time if intracellular glutamate is a determinant of freeze tolerance in CA118. We examined the changes in glutamate content during fermentation (Fig. 3C). We used a liquid fermentation method (13) instead of dough fermentation because measurement of intracellular amino acid contents in doughs was difficult because of the high background level of amino acids derived from other components of dough. The fermentation rates of commercial baker's yeast strains in liquid fermentation medium (162 ml of CO2/60 min/g of yeast for T118, 161 ml of CO2/60 min/g of yeast for CA118) were approximately four times higher than those in white-dough fermentation (44 ml of CO2/60 min/g of yeast for T118 and CA118) under our experimental conditions. As shown in Fig. 3C, the glutamate accumulated in strain CA118 decreased depending on prefermentation time in liquid fermentation medium. Although the experimental conditions for the data shown in Fig. 2 differ from those for the data shown in Fig. 3C, it should be noted that both freeze tolerance and glutamate content decrease in a prefermentation-dependent manner. We estimated that the greater glutamate content of CA118 should exert a cryoprotective effect even after 120 min of prefermentation (Fig. 2 and 3C) because the intracellular glutamate content of CA118 may be still greater that that of T118 on the basis of the difference in the fermentation rates between liquid fermentation and dough fermentation. After 30 min of fermentation in liquid fermentation medium (equivalent to approximately 120 min of fermentation in dough), the glutamate content of CA118 was approximately 10% greater than that of T118. Our data on the freeze tolerance and amino acid accumulation of CA118 suggest that charged amino acids, including glutamate, may play the role of a cryoprotectant and that arginase gene disruption can enhance the freeze tolerance of baker's yeast. Although we do not know what mechanism is responsible for the glutamate decrease that occurs during fermentation, it can be speculated that glutamate was utilized as a nitrogen source because of nitrogen limitation in dough.
We also measured the intracellular trehalose content of CA118 (data not shown) and found no difference in trehalose content between T118 and CA118. This suggests that the increased freeze tolerance of CA118 should not be due to intracellular trehalose content.
In this study, we showed that CAR1 gene disruption enhances the freeze tolerance of commercial baker's yeast. Although the molecular mechanism of freeze tolerance by the accumulation of amino acids remains unclear, the tolerance was strongly correlated with the accumulation of higher levels of arginine and glutamate. Further research at the molecular level is planned to determine the role of these amino acids in freeze tolerance.
Acknowledgments
This study was supported partly by a grant-in-aid (project for utilizing advanced technologies in agriculture, forestry and fisheries; 1418) from the Ministry of Agriculture, Forestry, and Fisheries, Japan.
We thank Tadanao Suzuki (National Food Research Institute) for skillful technical assistance in the amino acid analyses and Chise Suzuki (National Food Research Institute) for critical comments on the manuscript.
REFERENCES
- 1.Attfield, P. V., A. Raman, and C. J. Northcott. 1992. Construction of Saccharomyces cerevisiae strains that accumulate relatively low concentrations of trehalose and their application in testing the contribution of the disaccharide to stress tolerance. FEMS Microbiol. Lett. 94:271-276. [DOI] [PubMed] [Google Scholar]
- 2.Crowe, J. H., L. M. Crowe, and D. Chapman. 1984. Preservation of membranes in anhydrobiotic organisms: the role of trehalose. Science 223:701-703. [DOI] [PubMed] [Google Scholar]
- 3.Csona, L. N. 1981. Proline over-production results in enhanced osmotolerance in Salmonella typhimurium. Mol. Gen. Genet. 182:82-86. [DOI] [PubMed] [Google Scholar]
- 4.Hino, A., K. Mihara, K. Nakashima, and H. Takano. 1990. Trehalose levels and survival ratio of freeze-tolerant versus freeze-sensitive yeasts. Appl. Environ. Microbiol. 56:1386-1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hsu, K. H., R. C. Hoseney, and P. A. Sib. 1979. Frozen dough. I. Factors affecting stability of yeasted doughs. Cereal Chem. 56:419-424. [Google Scholar]
- 6.Hsu, K. H., R. C. Hoseney, and P. A. Sib. 1979. Frozen dough. II. Effects of freezing and storing conditions on stability of yeasted dough. Cereal Chem. 56:424-426. [Google Scholar]
- 7.Matsutani, K., Y. Fukuda, K. Murata, A. Kimura, I. Nakamura, and N. Yajima. 1990. Physical and biochemical properties of freeze-tolerant mutants of a yeast Saccharomyces cerevisiae. J. Ferment. Bioeng. 70:275-276. [Google Scholar]
- 8.Messenguy, F., F. Vierendeels, B. Scherens, and E. Dubois. 2000. In Saccharomyces cerevisiae, expression of arginine catabolic genes CAR1 and CAR2 in response to exogenous nitrogen availability is mediated by the Ume6 (CargRI)-Sin3 (CargRII)-Rpd3 (CargRIII) complex. J. Bacteriol. 182:3158-3164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Middelhoven, W. J. 1964. The pathway of arginine breakdown in Saccharomyces cerevisiae. Biochim. Biophys. Acta 93:650-652. [DOI] [PubMed]
- 10.Middelhoven, W. J., and G. J. Arkesteyn. 1981. Induction and derepression of arginase and ornithine transaminase in different strains of Saccharomyces cerevisiae. Antonie Van Leeuwenhoek 47:121-131. [DOI] [PubMed] [Google Scholar]
- 11.Rothstein, R. J. 1983. One-step gene disruption in yeast. Methods Enzymol. 101:202-211. [DOI] [PubMed] [Google Scholar]
- 12.Rudolph, A. S., and J. H. Crowe. 1985. Membrane stabilization during freezing: the role of two natural cryoprotectants, trehalose and proline. Cryobiology 22:367-377. [DOI] [PubMed] [Google Scholar]
- 13.Shima, J., A. Hino, C. Yamada-Iyo, Y. Suzuki, R. Nakajima, H. Watanabe, K. Mori, and H. Takano. 1999. Stress tolerance in doughs of Saccharomyces cerevisiae trehalase mutants derived from commercial baker's yeast. Appl. Environ. Microbiol. 65:2841-2846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smart, W. C., J. A. Coffman, and T. G. Cooper. 1996. Combinatorial regulation of the Saccharomyces cerevisiae CAR1 (arginase) promoter in response to multiple environmental signals. Mol. Cell. Biol. 16:5876-5887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stewart, G. R., and J. A. Lee. 1974. The role of proline accumulation in halophytes. Planta 120:279-289. [DOI] [PubMed] [Google Scholar]
- 16.Sumrada, R. A., and T. G. Cooper. 1984. Nucleotide sequence of the Saccharomyces cerevisiae arginase gene (CAR1) and its transcription under various physiological conditions. J. Bacteriol. 160:1078-1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takagi, H., F. Iwamoto, and S. Nakamori. 1997. Isolation of freeze-tolerant laboratory strains of Saccharomyces cerevisiae from proline-analogue-resistant mutants. Appl. Microbiol. Biotechnol. 47:405-411. [DOI] [PubMed] [Google Scholar]
- 18.Takagi, H., K. Sakai, K. Morida, and S. Nakamori. 2000. Proline accumulation by mutation or disruption of the proline oxidase gene improves resistance to freezing and desiccation stresses in Saccharomyces cerevisiae. FEMS Microbiol. Lett. 184:103-108. [DOI] [PubMed] [Google Scholar]
- 19.Whitney, P. A., and B. Magasanik. 1973. The induction of arginase in Saccharomyces cerevisiae. J. Biol. Chem. 248:6197-6202. [PubMed] [Google Scholar]