Abstract
A new, coculture-inducible two-peptide bacteriocin named plantaricin NC8 (PLNC8) was isolated from Lactobacillus plantarum NC8 cultures which had been induced with Lactococcus lactis MG1363 or Pediococcus pentosaceus FBB63. This bacteriocin consists of two distinct peptides, named α and β, which were separated by C2-C18 reverse-phase chromatography and whose complementary action is necessary for full plantaricin NC8 activity. N-terminal sequencing of both purified peptides showed 28 and 34 amino acids residues for PLNC8α and PLNC8β, respectively, which showed no sequence similarity to other known bacteriocins. Mass spectrometry analysis showed molecular masses of 3,587 Da (α) and 4,000 Da (β). The corresponding genes, designated plNC8A and plNC8B, were sequenced, and their nucleotide sequences revealed that both peptides are produced as bacteriocin precursors of 47 and 55 amino acids, respectively, which include N-terminal leader sequences of the double-glycine type. The mature α and β peptides contain 29 and 34 amino acids, respectively. An open reading frame, orfC, which encodes a putative immunity protein was found downstream of plNC8B and overlapping plNC8A. Upstream of the putative −35 region of plNC8B, two direct repeats of 9 bp were identified, which agrees with the consensus sequence and structure of promoters of class II bacteriocin operons whose expression is dependent on an autoinduction mechanism.
Many lactic acid bacteria (LAB) produce antimicrobial peptides known as bacteriocins, which are directed mainly to inhibit the growth of related species or species with the same nutritive requirements (14, 31, 35, 52). Hence, bacteriocin production seems to be aimed to compete against other bacteria which are present in the same ecological niche (4, 19, 20, 43). It has been shown that bacteriocin production provides the producer strain with a selective advantage over other nonproducing, isogenic bacteria (46). On the other hand, it might be expected that biosynthesis of bacteriocins is a high-energy-consuming process, providing an advantage to the producer strain only if the cost/benefit ratio is favorable (19, 43). It is well known that biosynthesis of proteinaceous substances is a high-energy-consuming process and for this reason is well controlled by molecular regulatory systems such as induction and catabolic repression, for instance. This suggests that bacteriocin production by LAB should also be controlled and regulated by these molecular mechanisms.
Bacteria must be able to adapt their metabolism to the changing environmental conditions. This adaptation requires that bacteria sense the multitude of extracellular signals and respond by controlling the expression of an adequate repertoire of genes. Thus, bacteriocin production has been shown to be influenced by pH (1, 5, 6, 32, 34), temperature (17, 37, 54), medium composition (3, 13, 26, 27, 55), DNA-damaging agents (24, 25, 42), and growth conditions (8, 15, 28, 32). However, how the presence of competing microorganisms affects production of bacteriocins is still quite unknown. To date, only two reports have addressed effects of other microorganisms on bacteriocin producer strains. Thus, production of lactacin B and divercin, produced by Lactobacillus acidophilus N2 and Carnobacterium divergens AS7, respectively, was enhanced in the presence of the sensitive strains Lactobacillus delbrueckii ATCC 4797 (5, 6) and Carnobacterium piscicola NCDO 2765 (50), respectively. However, none of these bacteriocins have been further characterized either biochemically or genetically.
We have recently shown that Lactobacillus plantarum NC8 was unable to produce bacteriocin when it was grown alone in broth cultures. However, cocultivation of L. plantarum NC8 with certain gram-positive bacteria in liquid cultures resulted in production of bacteriocin by this strain (A. Maldonado, J. L. Ruiz-Barba, and R. Jiménez-Díaz, submitted for publication). In this study, we show the purification by chromatographic methods of such an inducible bacteriocin, which we have named plantaricin NC8 (PLNC8), as well as the genes responsible for it.
MATERIALS AND METHODS
Bacterial strains and media.
L. plantarum NC8 was originally isolated from grass silage (2) and was provided by Lars Axelsson (MATFORSK, Norwegian Food Research Institute, Osloveien, Norway). Lactococcus lactis MG1363 was obtained from the Cranfield Institute of Technology (Cranfield, Bedford, United Kingdom), Pediococcus pentosaceus FBB63 was obtained from TNO (Nutrition and Food Research, Zeist, The Netherlands), and Lactobacillus plantarum 128/2 belongs to our own collection. They were maintained as frozen stocks at −80°C in MRS (Oxoid, Basingstoke, Hampshire, England) plus 20% (vol/vol) glycerol and propagated in MRS broth at 30°C before use.
Escherichia coli DH5α was grown at 37°C in Luria-Bertani broth (48) with vigorous agitation. DH5α transformant cells harboring recombinant plasmid pUC18 or pBluescript II KS(+) (Stratagene, La Jolla, Calif.) were selected on Luria-Bertani agar plates supplemented with 150 μg of ampicillin (Fluka Chemie GmbH, Buchs, Switzerland) per ml (final concentration), 16 μl of X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) (50 mg/ml; Promega Co., Madison, Wis.) per plate, and 4 μl of IPTG (isopropyl β-d-thiogalactoside) (200 mg/ml; Gibco BRL, Basel, Switzerland) per plate.
Bacteriocin assays.
The agar drop diffusion test (32) was used for detection of bacteriocin activity in cell-free supernatants from L. plantarum NC8 cultures which had been previously induced with gram-positive bacteria.
During purification, PLNC8 activity was quantified with a microtiter plate assay system (27). Detection and quantification of complementary activity among peptides PLNC8α and PLNC8β of PLNC8 were also carried out by the microtiter plate assay system. In all cases, L. plantarum 128/2 was used as the indicator strain, as we have done previously (22, 32, 33, 46). The inhibitory activity was expressed as bacteriocin units (BU) per milliliter, as described previously (33).
Bacteriocin purification.
All of the purification steps were carried out at room temperature, and all of the chromatographic equipment and media were purchased from Amersham Biosciences Europe GmbH, Freiburg, Germany. PLNC8 was purified from 5-liter mixed cultures of L. plantarum NC8 plus either autoclaved L. lactis MG1363 cells (PLNC8 resistant, PLNC8 inducer) or a living culture of P. pentosaceus FBB63 (PLNC8 sensitive, PLNC8 inducer). For the L. plantarum NC8-L. lactis MG1363 culture, 100 ml of an overnight L. plantarum NC8 culture was mixed with 100 ml of an autoclaved (121°C, 15 min) overnight L. lactis MG1363 culture and added to 5 liters of MRS. For the L. plantarum NC8-P. pentosaceus FBB63 mixed culture, 100 ml of an overnight culture of each strain was inoculated into 5 liters of MRS. In both cases, after 8 h of incubation at 30°C without shaking, the cells were removed by centrifugation at 10,000 × g for 10 min at 4°C and then the bacteriocin was purified by the same method described for the bacteriocins plantaricin S and enterocin I (22, 33). Briefly, the cell-free supernatant was precipitated with ammonium sulfate, desalted, and consecutively applied to cation-exchange and hydrophobic-interaction columns. Finally, samples were subjected to C2-C18 reverse-phase chromatography. Fractions showing inhibitory activity after the C2-C18 reverse-phase chromatography were pooled and subjected to several runs until the PLNC8α and PLNC8β peptides were separately purified.
SDS-PAGE.
C2-C18 reverse-phase column-purified PLNC8α and PLNC8β peptides were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (49) with an 18.5% acrylamide resolving gel. After electrophoresis at 100 mV for 2 h, the gel was divided in two parts; one part was silver stained (48), and the other was used to detect inhibitory activity with L. plantarum 128/2 as the indicator strain (7).
N-terminal amino acid sequence and mass spectrometry.
The N-terminal amino acid sequences of purified PLNC8α and PLNC8β were determined by automated Edman degradation with a Beckman LF3000 sequencer-phenylthiohydantoin amino acid analyzer (System Gold; Beckman, Fullerton, Calif.). The molecular mass of each purified bacteriocin component was determined by matrix-assisted laser desorption ionization-time-of-flight (MALDI-TOF) mass spectrometry. Both techniques were carried out by F. Canals, Institut de Biologia Fonamental Vicent Villar Palasí, University of Barcelona, Barcelona, Spain.
DNA isolation.
To prepare total genomic DNA from L. plantarum NC8, the method described by Cathcart (10) was used. Plasmid DNA from E. coli was extracted as described previously (48).
DNA amplification (PCR) techniques.
All primers used in PCRs (Table 1) were synthesized by TIB Molbiol (Berlin, Germany). The degenerate primers NC8-4 and NC8-6 were used to amplify a 74-bp fragment corresponding to part of the plNC8B gene. DNA was amplified in 100-μl reaction mixtures containing 2.5 mM Mg Cl2, 1× reaction buffer, 100 μM concentrations of each of the deoxynucleoside triphosphates, 100 pmol of each of the primers, 250 ng of genomic L. plantarum NC8 DNA as the template, and 5 U of Taq DNA polymerase (Promega) with a GeneAmp PCR System 2400 thermal cycler (Perkin-Elmer Corporation, Norwalk, Conn.). Amplification included denaturation at 94°C for 4 min, followed by 30 cycles of denaturation at 94°C for 30 s, annealing at 42°C for 1 min, and polymerization at 72°C for 1 min. An extra final polymerization step at 72°C for 10 min was performed to ensure that all PCR fragments were complete and A-tailed.
TABLE 1.
Oligonucleotide primers used in this study
| Primer | Sequencea |
|---|---|
| NC8-4 | 5′-TGRTARAAICCYTTRTTRAA-3′ |
| NC8-6 | 5′-GGIATHAARATHYTITGG-3′ |
| NC8-7b | 5′-GGTCTGCGTATAAGCATCGC-3′ |
| M13(−20) | 5′-GTAAAACGACGGCCAGT-3′ |
| M13R(−48) | 5′-AGCGGATAACAATTTCACACAGGA-3′ |
I, deoxyinosine; R, A or G; Y, C or T; N, A, C, G or T; H, A, C, T.
NC8-7 was also used as a probe for Southern hybridization after tailing at the 3′ end with fluorescein-11-dUTP.
Primary screening of E. coli DH5α transformant cells harboring pUC18 or pBluescript II KS(+) plasmids plus the fragment of interest was performed by PCR with the M13(−20) and M13R(−48) universal primers. Reaction mixtures and conditions for the PCR were as described above with the following modifications: DNA was amplified in 24-μl reaction mixtures containing 10 pmol of each of the primers, the annealing temperature was raised to 60°C, and transformant E. coli colonies were used as the source of DNA. Plasmid DNA from positive clones detected after colony blotting (see below) was used as the template for new PCR with the plNC8B-specific primer NC8-7 in combination with the vector-specific primers.
For DNA sequencing, PCR with vector-specific primers [M13(−20) and M13R(−48)] was performed with plasmid DNA from the selected positive clones by using the Expand High-Fidelity PCR system (Roche, Mannheim, Germany) under the conditions recommended by the manufacturer.
Molecular cloning, Southern hybridization, colony hybridization, and DNA sequencing.
Restriction enzymes, T4 DNA ligase, T4 DNA polymerase, and other DNA-modifying enzymes were used as recommended by the manufacturer (Boehringer Mannheim, Indianapolis, Ind.).
DNA fragments amplified by PCR with the degenerate primers NC8-4 and NC8-6 were cloned into pUC18 by use of standard molecular techniques (2a, 18, 48) and sequenced. DNA sequencing was performed by the Servicio de Secuenciación Automática de DNA, CIB-CSIC, Madrid, Spain, with an ABI PRISM 377 DNA sequencer (Applied Biosystems, Perkin-Elmer). From the known DNA sequence of plNC8B, the specific oligonucleotide NC8-7 (Table 1) was designed to be used as a hybridization probe in Southern blotting after 3′ end labeling with fluorescein-11-dUTP by using the ECL 3′-oligolabeling system (Amersham Pharmacia Biotech Europe GmbH, Freiburg, Germany). Chromosomal DNA from L. plantarum NC8 was digested with several restriction enzymes, and the DNA fragments were separated by size on 0.7% agarose gels and then blotted onto Genebind 45 nylon membranes (Pharmacia Biotech, Uppsala, Sweden). This was challenged with the labeled NC8-7 probe.
Those DNA fragments that hybridized with the probe were purified, ligated to pUC18 and pBluescript II KS(+) cloning vectors, and transformed into E. coli DH5α. The colony blot technique was used to screen out the minilibraries obtained (48). DNA fragments from selected clones containing the region encoding PLNC8β were amplified by PCR and subsequently sequenced by the primer-walking strategy (2a, 48).
Computer analysis of DNA and protein sequences.
The Wisconsin Package version 10.2 (http://www.gcg.com; Genetics Computer Group, Madison, Wis.) was used for analysis of both DNA and protein sequences, and the FASTA3 program (http://www.ebi.ac.uk) was used for protein database homology searching. For alignment of the bacteriocin leader peptides, the Multialign program was used (11) (available from http://prodes.toulouse.inra.fr/multalin/multalin.html). For physicochemical analysis of peptides (isoelectric point, molecular weight, hydropathy plot, and helical wheel), the WinPep program was used (30) (available from http://www.ipw.agrl.ethz.ch/∼lhennig/winpep.html).
Nucleotide sequence accession number.
The DNA sequence presented in this article has been deposited in the GenBank database with accession no. AF522077.
RESULTS
Purification of PLNC8.
The bacteriocin was isolated from 5-liter broth cultures of L. plantarum NC8 that had been induced with autoclaved cells of L. lactis MG1363 or with living cells of P. pentosaceus FBB63. We have observed that in the case of L. lactis MG1363, even the addition of autoclaved cells to pure cultures of L. plantarum NC8 was enough to induce bacteriocin production. This alternative method improved the isolation of the bacteriocin from the supernatant of an induced NC8 culture. Like that of most of the known bacteriocins, the behavior of PLNC8 throughout the purification process was that of an ammonium sulfate-precipitable, cationic, and highly hydrophobic substance. The results were the same whichever strain was used to induce NC8.
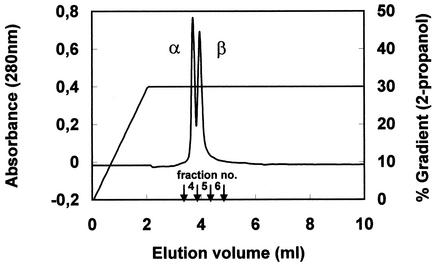
Evidence that two peptides were involved in PLNC8 activity was obtained after the second run in the C2-C18 reverse-phase column (Fig. 1). The bacteriocin assay of the eluted fractions revealed that most of the PLNC8 activity was present in fraction 5 (5,120 BU/ml), where two absorbance peaks overlapped. Minor inhibitory activity was showed by fraction 6 (1,280 BU/ml), whereas neither fraction 4 nor other collected fractions from the same run showed any inhibitory activity. The peptides contained in the two major absorbance peaks were termed α and β, in the order in which they were eluted from the column.
FIG. 1.
C2-C18 reverse-phase chromatography of PLNC8. The numbers above the arrows indicate the fractions (0.5 ml each) involved in PLNC8 activity. Maximum bacteriocin activity was detected in fraction 5. The α and β peaks, corresponding to PLNC8α and PLNC8β, respectively, are indicated.
Isolation of α and β peptides whose complementary action is necessary for full PLNC8 activity.
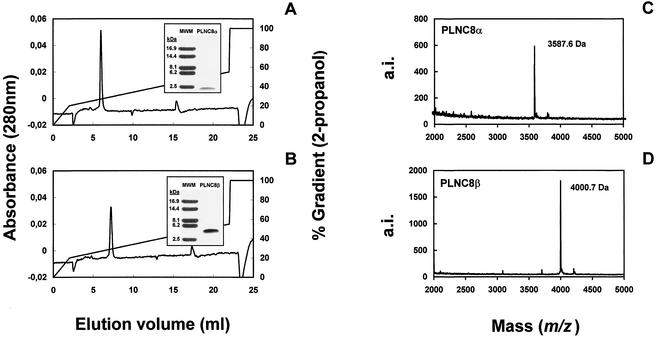
Fractions corresponding to the α and β peaks were pooled separately and then rechromatographed and eluted in the reverse-phase column. Two additional runs were necessary to obtain fractions containing the isolated β peak, and one was necessary for the α peak (Fig. 2). When the bacteriocin activity of these fractions was titrated, the fraction containing pure PLNC8β showed 80 BU/ml, while that containing pure PLNC8α did not show any bacteriocin activity at all. SDS-PAGE analysis showed a single peptide band with an apparent molecular mass of 2.5 kDa in the case of the α peak (Fig. 2A) and of 4 kDa in the case of the β peak (Fig. 2B). In SDS-PAGE activity gels, inhibition of the indicator strain was observed in samples containing the β peptide, whereas the α peptide did not show any inhibitory activity (data not shown).
FIG. 2.
(A and B) C2-C18 reverse-phase chromatography and SDS-PAGE corresponding to PLNC8α and PLNC8β, respectively. MWM, molecular mass markers. (C and D) MALDI-TOF mass spectra corresponding to PLNC8α and PLNC8β, respectively. a.i., arbitrary intensity.
Maximum inhibitory activity was obtained when the molar ratio of PLNC8α to PLNC8β was 1:16. Thus, a titer of 640 BU/ml was obtained when 0.01 μM PLNC8α was combined with 0.16 μM PLNC8β, approximately 8 times more than for PLNC8β assayed alone.
Amino acid sequences and mass spectrometry of PLNC8α and PLNC8β.
The N-terminal amino acid sequencing of both PLNC8 peptides showed 28 and 34 amino acid residues for PLNC8α and PLNC8β, respectively. The molecular masses deduced from their respective amino acid sequences were 3,440 and 4,000 Da, whereas MALDI-TOF mass spectrometry analysis showed molecular masses of 3,587 (Fig. 2C) and 4,000 Da (Fig. 2D), respectively. These results indicated that the amino acid sequence of the β peptide was complete, while in the α peptide a C-terminal end residue (a phenylalanine residue) escaped sequencing. The same results were obtained whether NC8 was induced by autoclaved cells from L. lactis MG1363 or by living P. pentosaceus FBB63 cells.
Genetic analysis and DNA sequencing of plNC8.
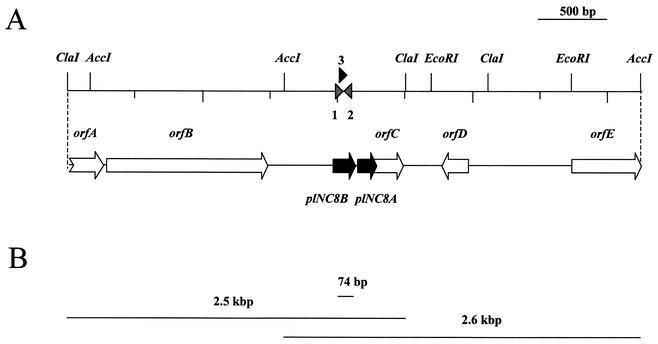
As expected, a 74-bp product corresponding to part of the plNC8B gene was obtained when total genomic DNA from L. plantarum NC8 was used as the template in PCRs with the degenerate primers NC8-4 and NC8-6. The PCR product was successfully cloned and sequenced, and the DNA sequence obtained corresponded to that predicted from the amino acid sequence of PLNC8β. When total genomic L. plantarum NC8 DNA was digested with EcoRI, HindIII, ClaI, and AccI restriction enzymes, fragments of 20, 15.5, 2.5, and 2.6 kbp, respectively, which hybridized with the NC8-7 probe were detected.
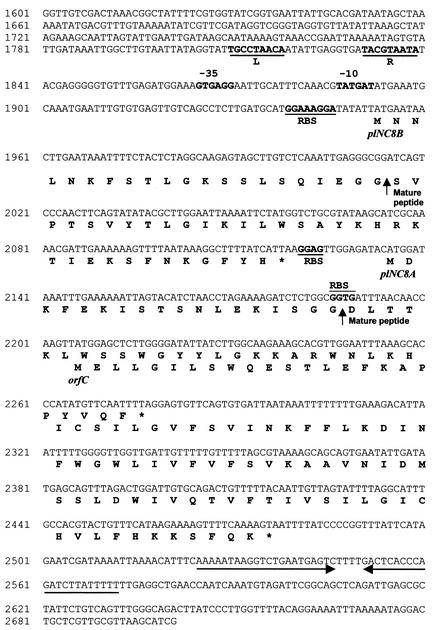
The 2.5-kbp ClaI fragment was successfully cloned and sequenced. Analysis of this sequence revealed the presence of six complete open reading frames (ORFs) and one partial ORF (Fig. 3 and 4). The ORFs designated plNC8A and plNC8B, encoding two peptides of 47 and 55 amino acids, respectively, were located close together, with plNC8B 14 bp upstream of plNC8A. Both peptides contain leader sequences of the double-glycine type that, once processed, give rise to mature peptides of 29 and 34 amino acids, respectively, which coincided with the purified bacteriocins PLNC8α and PLNC8β (Fig. 4). The theoretical isoelectric point (pI) for the mature PLNC8α peptide is 10.7, and its molecular mass is 3,587 Da; for the mature PLNC8β peptide, the pI is 10.8 and its molecular mass is 4,000 Da. Both the amino acid sequence and the MW deduced from the DNA sequencing corresponded to those obtained for the C2-C18 reverse-phase column-purified β peptide and, with the exception of a C-terminal phenylalanine residue that escaped sequencing, also to those of the purified α peptide. Furthermore, when the sequences of PLNC8α and PLNC8β were plotted on an Edmunson helical wheel, both peptides showed amphiphilic regions if they adapt an alpha-helical structure.
FIG. 3.
Genetic map of the plNC8 locus. (A) Restriction map showing plNC8 genes and putative ORFs. Numbers show the locations of the degenerate PCR primers used for cloning part of the plNC8 gene (1, primer NC8-6; 2, primer NC8-4) and of the probe used for hybridization (3, primer NC8-7) (Table 1). (B) DNA fragments that have been cloned and sequenced independently, showing their relative positions into the plNC8 locus.
FIG. 4.
Nucleotide sequence of part of the plNC8 locus and deduced proteins. Putative promoter −35 and −10 sites and RBSs are indicated by boldface. Vertical arrows indicate the cleavage sites in the PLNC8α and PLNC8β native peptides and the beginnings of the respective mature peptides. Direct repeats (L and R) upstream of plNC8B are indicated by lines under boldface letters. A potential Rho-independent transcription terminator is indicated by arrows downstream of orfC. Stop codons are indicated by asterisks at the ends of the protein sequences.
The mature α and β peptides did not show significant homology with any of the bacteriocins previously described. In contrast, the leader sequences of both the α and β peptides are very similar to the double-glycine type of those reported for other class IIb LAB bacteriocins (12, 16, 21, 23, 38, 51).
At 11 and 7 bp upstream of plNC8A and plNC8B, respectively, putative ribosome binding sites (RBSs) were detected (Fig. 4). A putative regulated promoter was found upstream of plNC8B, containing the typical −10 and −35 regions (Fig. 4). At 26 nucleotides upstream of the −35 region, two direct repeats (L and R) were identified. Each repeat consists of 9 bp separated by an AT-rich region of 12 nucleotides. The nucleotide sequences of the L (TGCCTAACA) and R (TACGTAATA) repeats agree with the consensus sequence obtained from direct repeats observed in promoters of bacteriocin genes whose expression is dependent on an autoinduction mechanism (16, 36, 38, 41).
Downstream of plNC8B, and overlapping the end of plNC8A, a third ORF named orfC was found (Fig. 3 and 4). orfC encodes a putative protein of 90 amino acid residues with a theoretical pI of 9.13 and a molecular mass of 10,328 Da, which is a good candidate for the immunity protein. The hydropathy profile analysis of the theoretically encoded protein showed the presence of three putative transmembrane segments, which are thought to be necessary for insertion of immunity proteins into the membrane of the bacteriocin producer strain (25, 38). Just downstream of orfC (Fig. 4), an inverted repeat which may function as a Rho-independent transcription terminator was found, thus suggesting that the genes plNC8B and plNC8A plus orfC would be produced on the same transcript. A putative RBS identified upstream of orfC, together with the putative RBS upstream of plNC8B and plNC8A, could suggest that the products of these three ORFs would be translated independently.
Upstream of plNC8B, a partial ORF (orfA) and a complete one (orfB) were found (Fig. 3). The incomplete orfA encodes a putative protein fragment of 60 amino acid residues which showed 50% homology with the C-terminal part of a branched-chain amino acid transporter (BRNQ) from Lactobacillus gasseri (47), and the putative protein of 396 amino acid residues encoded by orfB showed a high degree of homology (50%) to Na+/H+ antiporter proteins from several other bacteria (9).
Finally, the 2.6-kbp AccI fragment which overlapped with the ClaI fragment described above was cloned and sequenced to further extend the sequence downstream of the PLNC8 operon (Fig. 3). Although several new putative ORFs were found, none of them showed homology with any known protein. However, it was noticeable that orfE encoded a putative protein of 178 amino acid residues which showed 98% identity in 109 residues to the C-terminal part of orfB (upstream from plNC8B and plNC8A). Also, the putative protein encoded by orfD and those from several DNA fragments placed between orfD and orfE showed high homology with the transposase IS1380-SPN1 from Streptococcus pneumoniae (53).
DISCUSSION
In this study we describe the purification and genetic characterization of a novel coculture-inducible two-peptide bacteriocin, named plantaricin NC8, produced by L. plantarum NC8. Our results clearly indicate that this bacteriocin consists of two different peptides, PLNC8α and PLNC8β, whose complementary action is necessary for full PLNC8 activity. To our knowledge, this is the first-well characterized bacteriocin which is produced only after coculture with the inducing bacteria. Previous work on enhancement of bacteriocin production in LAB by induction with other bacteria have been published (6, 50), but none of those bacteriocins have been shown to be produced only after the induction event has taken place.
Purification of the PLNC8α and PLNC8β peptides from PLNC8 was achieved by the same protocol described for plantaricin S (33) and enterocin I (22), consistent with the well-conserved biochemical characteristics of many LAB bacteriocins (35, 38). However, amino acid sequence comparisons indicated that PLNC8 is new, thus being a novel class IIb bacteriocin from L. plantarum (38). Remarkably, the same bacteriocin was produced independently of the strain and method used to induce NC8, indicating that the same induction mechanism is most probably started by different inducer strains, which, in turn, must share the production of certain inducing compounds.
Although no significant homology among the amino acid sequences of the mature peptides PLNC8α and PLNC8β and other known bacteriocins was found, the leader peptide was very similar to those from other class IIb bacteriocins (12, 16, 23, 33, 38, 40, 51). These N-terminal leader sequences show the consensus sequence (-LS—EL-I-GG), thus indicating that both peptides are processed and secreted out of the cell via a dedicated ABC transporter (29), which is a typical feature of most class I and class II bacteriocins. Typically, genes coding for ABC transporters are located close to the bacteriocin-encoding genes (38). Although it has not been found in the cloned and sequenced 4,243-bp ClaI-AccI fragment, an ABC transporter system almost certainly must be present in NC8. In order to locate the ORF whose product could show homology to any of the ABC transport proteins described, cloning and sequencing upstream and downstream from the DNA sequence presented here is currently being carried out.
In most of bacteriocin operons described to date, a gene encoding the immunity protein, which protects the producing bacteria against self-toxicity, usually follows the bacteriocin structural genes (38). The bacteriocin- and the immunity protein-encoding genes are generally cotranscribed to ensure that the producer strain is not killed by its own bacteriocin (38). In our case, orfC is a good candidate to encode the PLNC8 immunity protein, although no homology between the putative peptide encoded and other immunity proteins in the data banks has been found, as is the case with the rest of the bacteriocin immunity proteins described to date (38). There are some indications that could reinforce the hypothesis that orfC could encode the PLNC8 immunity protein. It is located close to the PLNC8 structural genes, just downstream of plNC8B and overlapping plNC8A, and a putative Rho-independent transcription terminator was found just downstream of orfC. That could indicate that the structural bacteriocin genes, plNC8A and plNC8B, would be cotranscribed together with orfC. Also, as in other immunity proteins, the putative peptide showed the presence of three putative transmembrane segments, which is thought to be necessary for insertion into the membrane of the bacteriocin producer strain (25, 38).
Very significant are the two direct repeats found upstream of the PLNC8 operon, which could indicate that the expression of the bacteriocin is regulated via an autoinduction mechanism as has been found to be the case in other bacteriocin operons (16, 38, 39, 44, 45). In these systems, the DNA direct repeats are binding sites for the response regulator involved in regulation of bacteriocin synthesis (44, 45). Current investigation in our laboratory is focused on finding evidence for the existence of such autoinduction mechanism in L. plantarum NC8, as well as elucidating the nature of the PLNC8 induction mechanism.
Acknowledgments
We thank L. Axelsson (MATFORSK, Norwegian Food Research Institute, Osloveien, Norway) for providing the L. plantarum NC8 strain.
This work was supported by the Spanish Government through MCYT project AGL2000-1611-CO3-01. A.M. was the recipient of a grant from MCYT, Spain.
REFERENCES
- 1.Ahn, C., and M. E. Stiles. 1990. Plasmid-associated bacteriocin production by a strain of Carnobacterium piscicola from meat. Appl. Environ. Microbiol. 56:2503-2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aukrust, T., and H. Blom. 1992. Transformation of Lactobacillus strains used in meat and vegetable fermentations. Food Res. Int. 25:253-261. [Google Scholar]
- 2a.Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl. 1992. Current protocols in molecular biology. Greene Publishing and Wiley-Interscience, New York, N.Y.
- 3.Barefoot, S. F., and T. R. Klaenhammer. 1983. Detection and activity of lactacin B, a bacteriocin produced by Lactobacillus acidophilus. Antimicrob. Agents Chemother. 45:1808-1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barefoot, S. F., and G. A. Grinstead. 1993. Bacteriocins from dairy propionibacteria and inducible bacteriocins of lactic acid bacteria, p. 219-231. In D. G. Hoover and L. R. Steenson (ed.), Bacteriocins of lactic acid bacteria. Academic Press, New York, N.Y.
- 5.Barefoot, S. F., C. G. Nettles, and Y. R. Chen. 1994. Lactacin B, a bacteriocin produced by Lactobacillus acidophilus, p. 353-376. In L. de Vuyst and E. J. Vandamme (ed.), Bacteriocins of lactic acid bacteria: microbiology, genetics and applications. Blackie Academic and Professional, London, United Kingdom.
- 6.Barefoot, S. F., Y. R. Chen, T. A. Hughes, A. B. Bodine, M. Y. Shearer, and M. D. Hughes. 1994. Identification and purification of a protein that induces production of the Lactobacillus acidophilus bacteriocin lactacin B. Appl. Environ. Microbiol. 60:3522-3528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhunia, A. K., M. C. Johnson, and B. Ray. 1987. Direct detection of an antimicrobial peptide of Pediococcus acidilactici in sodium dodecyl sulfate-polyacrylamide gel electrophoresis. J. Ind. Microbiol. 2:319-322. [Google Scholar]
- 8.Biswas, S. R., P. Ray, M. C. Johnson, and B. Ray. 1991. Influence of growth conditions on the production of a bacteriocin, pediocin AcH, by Pediococcus acidilactici AcH. Appl. Environ. Microbiol. 57:1265-1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Booth, I. R., M. A. Jones, D. McLaggan, Y. Nikolaev, L. S. Ness, C. M. Wood, S. Miller, S. Tötemeyer, and G. P. Ferguson. 1996. Bacterial ion channels, p. 693-729. In W. N. Konings, H. R. Kaback, and J. S. Lolkema (ed.), Transport processes in eukaryotic and prokaryotic organisms. Elsevier Press, New York, N.Y.
- 10.Cathcart, D. P. 1995. Purification, characterization and molecular analysis of plantaricin S, a two-peptide bacteriocin from olive fermenting Lactobacillus plantarum strains. Ph.D. thesis. Cranfield University, Bedford, United Kingdom.
- 11.Corpet, F. 1988. Multiple sequence alignment with hierarchical clustering. Nucleic Acids Res. 16:10881-10890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cuozzo, S. A., F. Sesma, J. M. Palacios, A. P de Ruiz Holgado, and R. R. Raya. 2000. Identification and nucleotide sequence of genes involved in the synthesis of lactocin 705, a two-peptide bacteriocin from Lactobacillus casei CRL705. FEMS Microbiol. Lett. 185:157-161. [DOI] [PubMed] [Google Scholar]
- 13.de Klerk. 1967. Bacteriocinogeny in Lactobacillus fermenti. Nature 214:609. [Google Scholar]
- 14.de Vuyst, L. 1994. Bacteriocins and bacteriocin-like substances from Lactobacillus, p. 319-329. In L. de Vuyst and E. J. Vandamme (ed.), Bacteriocins of lactic acid bacteria: microbiology, genetics and applications. Blackie Academic & Professional, London, United Kingdom.
- 15.de Vuyst, L., R. Callewaert, and K. Crabbe. 1996. Primary metabolite kinetics of bacteriocin biosynthesis by Lactobacillus amylovorus and evidence for stimulation of bacteriocin production under unfavourable growth conditions. Microbiology 142:817-827. [DOI] [PubMed] [Google Scholar]
- 16.Diep, D. B., L. S. Håvarstein, and I. F. Nes. 1996. Characterization of the locus responsible for the bacteriocin production in Lactobacillus plantarum C11. J. Bacteriol. 178:4472-4483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Diep, D. B., L. Axelsson, C. Grefsli, and I. F. Nes. 2000. The synthesis of the bacteriocin sakacin A is a temperature-sensitive process regulated by a pheromone peptide through a three-component regulatory system. Microbiology 146:2155-2160. [DOI] [PubMed] [Google Scholar]
- 18.Dower, W. J., F. J. Miller, and W. C. Ragsdale. 1998. High efficiency transformation of E. coli by high voltage electroporation. Nucleic Acids Res. 16:6127-6145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dykes, G. A. 1995. Bacteriocins: ecological and evolutionary significance. Tree 10:186-189. [DOI] [PubMed] [Google Scholar]
- 20.Dykes, G. A., and J. W. Hastings. 1997. Selection and fitness in bacteriocin-producing bacteria. Proc. R. Soc. London B 264:683-687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ehrmann, M. A., A. Remiger, V. G. H. Eijsink, and R. F. Vogel. 2000. A gene cluster encoding plantaricin 1.25β and other bacteriocin-like peptides in Lactobacillus plantarum TMW1.25. Biochim. Biophys. Acta 1490:355-361. [DOI] [PubMed] [Google Scholar]
- 22.Floriano, B., J. L. Ruiz-Barba, and R. Jiménez-Díaz. 1998. Purification and genetic characterization of enterocin I from Enterococcus faecium 6T1a, a novel antilisterial plasmid-encoded bacteriocin which does not belong to the pediocin family of bacteriocins. Appl. Environ. Microbiol. 64:4883-4890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Flynn, S., D. van Sinderen, G. M. Thornton, H. Holo, I. F. Nes, and J. K. Collins. 2002. Characterization of the genetic locus responsible for the production of ABP-118, a novel bacteriocin produced by the probiotic bacterium Lactobacillus salivarius subsp. salivarius UCC118. Microbiology 148:973-984. [DOI] [PubMed] [Google Scholar]
- 24.Fremaux, C., C. Ahn, and T. R. Klaenhammer. 1993. An inducible promoter controls the expression of helveticin J, a large heat-labile bacteriocin produced by Lactobacillus helveticus. FEMS Microbiol. Rev. 12:121. [Google Scholar]
- 25.Fremaux, C., C. Ahn, and T. R. Klaenhammer. 1993. Molecular analysis of the lactacin F operon. Appl. Environ. Microbiol. 59:3906-3915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fricourt, B. V., S. F. Barefoot, R. F. Testin, and S. S. Hayasaka. 1994. Detection and activity of plantaricin F, an antibacterial substance from Lactobacillus plantarum BF001 isolated from processed channel catfish. J. Food Prot. 57:698-702. [DOI] [PubMed] [Google Scholar]
- 27.Geis, A., J. Singh, and M. Teuber. 1983. Potential of lactic streptococci to produce bacteriocin. Appl. Environ. Microbiol. 45:205-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gutowski-Ecked, Z., C. Klein, K. Siegers, K. Bohm, M. Hammelmann, and K.-D. Entian. 1994. Growth phase-dependent regulation and membrane localization of SpaB, a protein involved in biosynthesis of the lantibiotic subtilin. Appl. Environ. Microbiol. 60:1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Håvarstein, L. S., D. B. Diep, and I. F. Nes. 1995. A family of bacteriocin ABC transporters carry out proteolytic processing of their substrates concomitant with export. Mol. Microbiol. 16:229-240. [DOI] [PubMed] [Google Scholar]
- 30.Hennig, L. 1999. WinGene/WinPep: user-friendly software for the analysis of aminoacid sequences. BioTechniques 26:1170-1172. [DOI] [PubMed] [Google Scholar]
- 31.Jack, R. W., J. R Tagg, and B. Ray. 1995. Bacteriocins of gram-positive bacteria. Microbiol. Rev. 59:171-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jiménez-Díaz, R., R. M. Rios-Sánchez, M. Desmazeaud, J. L. Ruiz-Barba, and J. C. Piard. 1993. Plantaricin S and T, two new bacteriocins produced by Lactobacillus plantarum LPCO10 isolated from a green olive fermentation. Appl. Environ. Microbiol. 59:1416-1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jiménez-Díaz, R., J. L. Ruiz-Barba, D. P. Cathcart, H. Holo, I. F. Nes, K. H. Sletten, and P. J. Warner. 1995. Purification and partial amino acid sequence of plantaricin S, a bacteriocin produced by Lactobacillus plantarum LPCO10, the activity of which depends on the complementary action of two peptides. Appl. Environ. Microbiol. 61:4459-4463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kaiser, A. L., and T. J. Montville. 1993. The influence of pH and growth rate on production of the bacteriocin, bavaricin MN, in batch and continuous fermentations. J. Appl. Bacteriol. 75:536-540. [Google Scholar]
- 35.Klaenhammer, T. R. 1993. Genetics of bacteriocins produced by lactic acid bacteria. FEMS Microbiol. Rev. 12:39-86. [DOI] [PubMed] [Google Scholar]
- 36.Kleerebezem, M., O. P. Kuipers, W. M. de Vos, M. E. Stiles, and L. E. N. Quadri. 2001. A two-component signal transduction cascade in Carnobacterium piscicola LV17B: two signaling peptides and one sensor-transmitter. Peptides 22:1597-1601. [DOI] [PubMed] [Google Scholar]
- 37.Mørtvedt-Abildgaard, C. I., J. Nissen-Meyer, B. Jelle, B. Grenov, M. Skaugen, and I. F. Nes. 1995. Production and pH-dependent bactericidal activity of lactocin S, a lantibiotic from Lactobacillus sake L45. Appl. Environ. Microbiol. 61:175-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nes, I. F., D. B. Diep, L. S. Håvarstein, M. B. Brurberg, V. Eijsink, and H. Holo. 1996. Biosynthesis of bacteriocins in lactic acid bacteria. Antonie Leeuwenhoek 70:113-128. [DOI] [PubMed] [Google Scholar]
- 39.Nes, I. F., and V. G. H. Eijsink. 1999. Regulation of group II peptide bacteriocin synthesis by quorum sensing mechanisms, p. 175-192. In G. M. Dunny and S. C. Winans (ed.), Cell-cell signaling in bacteria. ASM Press, Washington, D.C.
- 40.Nissen-Meyer, J., H. Holo, S. L. Håvarstein, K. Sletten, and I. F. Nes. 1992. A novel lactococcal bacteriocin whose activity depends on the complementary action of two peptides. J. Bacteriol. 174:5686-5692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Quadri, L. E. N., M. Kleerebezem, O. P. Kuipers, W. M. De Vos, K. L. Roy, J. C. Vederas, and M. E. Stiles. 1997. Characterization of a locus from Carnobacterium piscicola LV17B involved in bacteriocin production and immunity: evidence for global inducer-mediated transcriptional regulation. J. Bacteriol. 179:6163-6171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rammelsberg, M., E. Muller, and F. Radler. 1990. Caseicin 80: purification and characterization of a new bacteriocin from Lactobacillus casei. Arch. Microbiol. 154:1901-1906. [Google Scholar]
- 43.Riley, M. A. 1998. Molecular mechanisms of bacteriocin evolution. Annu. Rev. Genet. 32:255-278. [DOI] [PubMed] [Google Scholar]
- 44.Risøen, P. A., L. S. Håvarstein, D. B. Diep, and I. F. Nes. 1998. Identification of the DNA-binding sites for two response regulators involved in control of bacteriocin synthesis in Lactobacillus plantarum C11. Mol. Gen. Genet. 259:224-232. [DOI] [PubMed] [Google Scholar]
- 45.Risøen, P. A., M. B. Brurberg, V. G. H. Eijsink, and I. F. Nes. 2000. Functional analysis of promoters involved in quorum sensing-based regulation of bacteriocin production in Lactobacillus. Mol. Microbiol. 37:619-628. [DOI] [PubMed] [Google Scholar]
- 46.Ruiz-Barba, J. L., D. P. Cathcart, P. J. Warner, and R. Jiménez-Díaz. 1994. Use of Lactobacillus plantarum LPCO10, a bacteriocin producer, as a starter culture in Spanish-style green olive fermentations. Appl. Environ. Microbiol. 60:2059-2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Russell, W. M., and T. R. Klaenhammer. 2001. Identification and cloning of gusA, encoding a new beta-glucuronidase from Lactobacillus gasseri ADH. Appl. Environ. Microbiol. 67:1253-1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 49.Schägger, H., and G. Von Jagow. 1987. Tricine-sodium dodecyl sulphate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal. Biochem. 166:368-379. [DOI] [PubMed] [Google Scholar]
- 50.Sip, A., W. Grajek, and P. Boyaval. 1998. Enhancement of bacteriocin production by Carnobacterium divergens AS7 in the presence of a bacteriocin-sensitive strain Carnobacterium piscicola. Int. J. Food Microbiol. 42:63-69. [DOI] [PubMed] [Google Scholar]
- 51.Stephens, S., B. Floriano, D. P. Cathcart, S. A. Bayley, V. F. Witt, R. Jiménez-Díaz, P. J. Warner, and J. L. Ruiz-Barba. 1998. Molecular analysis of the locus responsible for production of plantaricin S, a two-peptide bacteriocin produced by Lactobacillus plantarum LPCO10. Appl. Environ. Microbiol. 64:1871-1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tagg, J. R., A. S. Dajani, and L. W. Wannamaker. 1976. Bacteriocins of gram-positive bacteria. Bacteriol. Rev. 40:722-756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tettelin, H., K. E. Nelson, I. T. Paulsen, J. A. Eisen, T. D. Read, S. Peterson, J. Heidelberg, R. T. Deboy, D. H. Haft, R. J. Dodson, A. S. Durkin, M. Gwinn, J. F. Kolonay, W. C. Nelson, J. D. Peterson, L. A. Umayan, O. White, S. L. Salzberg, M. R. Lewis, D. Radune, E. Holtzapple, H. Khouri, A. M. Wolf, T. R. Utterback, C. L. Hansen, L. A. McDonald, T. V. Feldblyum, S. Angivoli, T. Dickinson, E. K. Hickey, I. E. Holt, B. J. Loftus, F. Yang, H. O. Smith, J. C. Venter, B. A. Dougherty, D. A. Morrison, S. K. Hollingshead, and C. M. Fraser. 2001. Complete genome sequence of a virulent isolate of Streptococcus pneumoniae. Science 293:498-506. [DOI] [PubMed] [Google Scholar]
- 54.Tichaczeck, P. S., J. Nissen-Meyer, I. F. Nes, R. F. Vogel, and W. P. Hammes. 1992. Characterization of the bacteriocins curvacin A from Lactobacillus curvatus LTH1174 and sakacin P from L. sake LTH673. Syst. Appl. Microbiol. 15:460-468. [Google Scholar]
- 55.West, C. A., and P. J. Warner. 1988. Plantaricin B, a bacteriocin produced by Lactobacillus plantarum NCDO1193. FEMS Microbiol. Lett. 49:163-165. [Google Scholar]