Abstract
The population of Bifidobacterium spp. in fecal samples from suckling piglets was investigated, and Beerens, raffinose-bifidobacterium (RB), and modified Wilkins-Chalgren (MW) agar media were evaluated with regard to the enumeration of bifidobacteria in porcine intestinal samples. The results demonstrated that the population of bifidobacteria in the feces of suckling piglets is numerically low, and a phylogenetic analysis of the 16S rRNA gene from bifidobacterial isolates suggested that a possibly new Bifidobacterium species was isolated. Beerens, RB, and MW agar media were not selective for bifidobacteria in the fecal samples. The highest recovery and diversity of bifidobacteria were obtained for MW agar. Nonbifidobacterial isolates from the three agar media were identified and may contribute to the future formulation of improved selective media for the enumeration of bifidobacteria.
The intestinal microbiotas of humans and animals comprise hundreds of different types of microorganisms that play a role in host nutrition and health. In the last decade, there has been major interest in influencing the composition of the gut microbiota to create a more remedial community by providing for the intake of nondigestible dietary supplements (prebiotics). Prebiotics are believed to stimulate the growth and/or activity of health-promoting bacteria, such as bifidobacteria and lactobacilli, and to suppress potentially pathogenic bacteria in the gut (7). In pig production, the inclusion of prebiotic nondigestible oligosaccharides in the diet has the objective of maintaining a beneficial intestinal microbiota dominated by health-promoting bacteria, such as bifidobacteria, during the stressful period of weaning (26). Species of bifidobacteria reported in pigs are Bifidobacterium suis, B. globosum, B. pseudolongum, B. thermophilum, B. boum, and B. choerinum (22, 23, 32, 34). However, little is known of the significance of bifidobacteria in pigs and their potential role in protection against intestinal disorders such as postweaning diarrhea. A prerequisite for studying the effect of prebiotics on the intestinal microbiota is the ability to quantify the different groups of bacteria accurately. Only a few studies have evaluated selective agars for the enumeration and isolation of bifidobacteria in fecal and intestinal samples from pigs (1, 13). In the present study, Beerens agar (4), raffinose-bifidobacterium (RB) agar (12), and modified Wilkin-Chalgreen (MW) agar (29) were assessed for the enumeration and isolation of bifidobacteria in fecal samples from suckling piglets. Beerens agar is reported as a suitable medium for the isolation and enumeration of bifidobacteria from gut microbiota (33) and has often been used to determine bifidobacterial populations in fecal and intestinal samples (8, 11, 27). RB agar is developed as a selective medium for bifidobacteria in intestinal and fecal samples (12), and MW agar has proven very suitable for the isolation and enumeration of bifidobacteria in poultry and rabbit cecal samples (29). The selectivity of MW agar is due to the presence of mupirocin, an antibiotic to which bifidobacteria are resistant and to which many lactobacilli are susceptible (28).
Seven fecal samples were obtained from suckling piglets aged 1 to 4 weeks. For each sample, freshly voided feces from two to three piglets were collected and pooled. Four samples (F1 to F4) represent herds from four different conventional farms, and three samples (F5 to F7) represent the herd of Research Centre Foulum. A total of 10 g of sample was transferred under a flow of CO2 into flasks containing 90 ml of a prereduced salt medium (15). The suspension was homogenized with a stomacher lab blender (Interscience, St. Nom, France) for 2 min in CO2-flushed plastic bags. Tenfold serial dilutions were made in prereduced salt medium according to the technique of Miller and Wolin (25). The total number of culturable anaerobic bacteria was enumerated using anaerobic Wilkins-Chalgren agar (Difco 1805-17-6), which was inoculated and incubated at 38°C for 5 days in an anaerobic cabinet (10% CO2, 10% H2, 80% N2). Bifidobacteria were enumerated and isolated using Beerens agar (4), RB agar (12), and MW agar (29), which were inoculated and incubated at 38°C for 3 days in the anaerobic cabinet. From each selective agar, 30 colonies from the highest dilution of each sample were picked at random and aseptically inoculated into reinforced clostridial broth (Merck 5411) with hemin added at a concentration of 0.005 g/liter. The isolates were grown at 38°C and stored at −80°C in 20% (vol/vol) glycerol. Subcultures of isolates were grown in 10 ml of Trypticase-peptone-yeast extract broth (31) at 38°C. The cells were harvested by centrifugation (5,500 × g for 10 min), and isolates belonging to the genus Bifidobacterium were identified by the detection of fructose-6-phosphate phosphoketolase (F6PPK) in cellular extracts, as described by Scardovi (31). The pH was measured in the supernatants, and the concentrations of various organic acids (short-chain fatty acids, lactic acid, and succinic acid) were determined by gas chromatography as described by Jensen et al. (16). Cells from 1.0-ml samples cultured in Trypticase-peptone-yeast extract broth (31) were harvested by centrifugation (14,500 × g, 5 min), and DNA was extracted as described previously (24). PCRs were performed with a Hybaid PCR Express apparatus. The reaction mixture (50 μl) contained a 0.2 μM concentration of each primer (DNA Technology A/S, Aarhus, Denmark), 0.2 mM each deoxynucleoside triphosphate (BioLabs Inc.), 1.0 U of DyNAzyme II DNA polymerase supplied with the 10× PCR buffer (Finnzymes OY, Espoo, Finland), and approximately 50 ng of template DNA. Amplification was performed under the following conditions: 1 min at 95°C; 30 cycles of 30 s at 95°C, 30 s at 60°C, and 45 s at 72°C; and 10 min at 72°C. Bifidobacterial isolates were identified by PCR as described by Kok et al. (17). We included two universal 16S rRNA gene primers (18) in the PCR assay to generate an approximately 900-bp fragment that served as a positive control for the PCR and to identify nonbifidobacterial isolates. The PCR products were visualized by electrophoresis in a 1% agarose gel stained with ethidium bromide. The 16S rRNA genes from the bifidobacterial isolates and representatives of nonbifidobacterial isolates (see below) were amplified by PCR using the primers TH008 and PH1522 (Table 1). The PCR products were purified using a QIAquick PCR purification kit (QIAGEN GmbH, Hilden, Germany). The 16S rRNA genes of bifidobacterial isolates were digested with the restriction endonuclease HaeIII according to the manufacturer's instructions (New England BioLabs), and restriction digests were resolved by electrophoresis in a 2% agarose gel and visualized by staining with ethidium bromide. The 16S rRNA gene sequences were determined by cycle sequencing using an ABI BigDye Sequencing kit according to the manufacturer's instructions. Based on primers described by Leser et al. (21) and bifidobacterial 16S rRNA gene sequences retrieved from GenBank, primers for bidirectional sequencing were designed for the present study (Table 1). Sequences were read with an automatic sequence analyzer (ABI PRISM 377). The 16S rRNA gene sequences of the bifidobacterial isolates and of reference strains obtained from GenBank were aligned using ALIGN X within Vector NTI Suite 7.1 (Informax Inc.), and phylogenetic analysis was performed using the PHYLIP package (10). The nonbifidobacterial isolates were broadly grouped according to cell morphology, fermentation products, and pH-reducing capacity after growth with glucose as the substrate (results not shown). Partial sequences of 16S rRNA genes of nonbifidobacterial isolates were determined with the primer TH504 (Table 1) and subjected to database comparisons using the basic local-alignment search tool BLAST (3). Representative bifidobacterial isolates and the strains B. boum DSM 20432T, B. pseudolongum subsp. globosum DSM 20092T, B. pseudolongum subsp. pseudolongum DSM 20099T, B. suis DSM 20211T, B. thermophilum DSM 20210T, and B. choerinum DSM 20434T, obtained from Deutsche Sammlung von Mikroorganismen und Zellkulturen (Braunschweig, Germany), were subjected to a phenotypic characterization performed with the PhenePlate system (PhPlate AB, Stockholm, Sweden) according to the manufacturer's instructions.
TABLE 1.
Primers used for 16S rRNA gene sequencing in this study
| Primera | Direction | Nucleotide sequence (5′ → 3′) | Positionb |
|---|---|---|---|
| TH008 | Forward | AGRGTTYGATTMTGGCTCAG | 8-27 |
| LL409 | Forward | GATGGAGGCCTTCGGGTTG | 409-427 |
| LL894 | Forward | CTCAAAGAAATTGACGGGGG | 909-928 |
| TH504 | Reverse | GTATTACCGCGGCTGCTG | 535-504 |
| TH1037 | Reverse | ACGAGCTGACGACGACCATG | 1072-1053 |
| PH1522 | Reverse | AAGGAGGTGATCCAGCCGCA | 1541-1522 |
Modified from primers described in reference 21.
Numbering corresponds to the Escherichia coli 16S rRNA gene sequence.
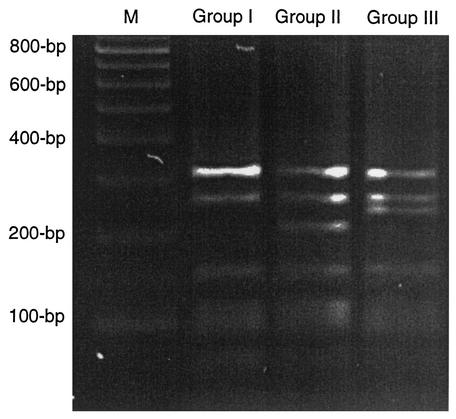
Table 2 shows the bacterial counts obtained in the present study. The counts from Beerens, RB, and MW agar media were lower than the counts of total culturable bacteria. The lowest counts were obtained for MW agar, and in one sample, no colonies appeared on the MW agar above a detection limit of 1.9 × 105 CFU/g (Table 2). Identification of bifidobacterial isolates by demonstration of F6PPK activity was in good agreement with the amplification product from PCRs using genus-specific primers (results not shown). The results demonstrated that the population of bifidobacteria makes up a minor proportion (<1%) of the intestinal microbiota in pigs (results not shown). A low recovery of bifidobacteria from the selective agar media was detected, especially with the Beerens and RB media, showing that the counts were biased towards too-high bifidobacterial numbers. In the literature, bifidobacterial numbers in the range of 107 to 108 bacteria per gram of feces of young and adult pigs are reported (2, 9, 27). However, the selectivity of the agar media used in these studies was not verified or accounted for and therefore these results are to be considered with caution due to potential methodological biases, as demonstrated in the present study. Other studies on bifidobacteria in porcine intestinal samples, in contrast, verified the enumeration of bifidobacteria on the basis of colony form, gram staining, and cell morphology (6, 14). No bifidobacteria at all were found in the feces of young pigs, even with a detection limit of 4 CFU per gram (6); bifidobacteria were detected (2.5 × 108 CFU/g) in only one out of seven cecal samples from pig-flora-associated mice inoculated with fecal microbiota from 20-day-old suckling piglets; and no bifidobacteria were detected with fecal inoculum from 40- and 60-day-old piglets (14). Similarly, in a comprehensive study of intestinal bacterial communities analyzed from a library of 4,270 bacterial 16S rRNA gene sequences cloned from the gut content of 24 pigs, no bifidobacteria were detected (21). These results, which indicate that the population of bifidobacteria is numerically low in the gastrointestinal tract of pigs, are in good agreement with results from the present study. In the present study, distinct results of restriction pattern length polymorphism analysis of the 16S rRNA gene divided the bifidobacterial isolates into three genotypic groups: group I (19 isolates), group II (17 isolates), and group III (25 isolates) (Fig. 1). The 16S rRNA gene sequencing and phylogenetic analysis revealed that the groups represent different bifidobacterial species (results not shown). The group I isolates showed the closest relationship to B. boum JCM 1211, with very high sequence similarities (>99%). B. boum is a species previously isolated from pigs (23). The group II isolates showed low sequence similarities (<95.1%) to any other bifidobacterial species, suggesting that a possible new member of the genus Bifidobacterium was isolated from piglet feces in the present study. The group III isolates showed a close relationship, with high levels of sequence similarity (>99.5%), to B. infantis ATCC 15697T, B. longum ATCC 15707T, and B. suis ATCC 27533T. DNA-DNA hybridization has previously demonstrated that B. infantis, B. longum, and B. suis are genetically closely related (20). However, B. suis is isolated from the gastrointestinal tract of pigs (22) and is apparently host specific (5), while B. infantis and B. longum are primarily of human origin (5). B. suis has been designated as the predominant bifidobacterial species in the gastrointestinal tract of pigs (34). In the present study, the group III isolates (B. suis) were obtained from only one out of seven samples whereas the group I isolates (B. boum) were obtained from four out of seven samples. The group II isolates were obtained from two out of seven samples (results not shown). The biochemical phenotyping demonstrated high similarity among the bifidobacterial isolates from each of the three genotypic groups (results not shown), while the differences between the groups were significant (Table 3). The group II isolates differ from the group I and III isolates, and from the type strains of bifidobacterial species reported to be detected in pigs, by the ability to ferment gentobiose, amygdalin, arbutin, β-methylglucoside, gluconate, and salicine (Table 3). The group I isolates (B. boum) differed from the type strain B. boum DSM 20432T by the ability to ferment lactose and lactulose, and the group III isolates (B. suis) differed from B. suis DSM 20211T by the ability to ferment l-arabinose, d-xylose, lactose, and lactulose (Table 3). The fermentation of lactose by the group I and III isolates may be associated with a high dietary intake of lactose from the maternal milk by the piglets. The low selectivity for bifidobacteria displayed by the Beerens, RB, and MW agar media in the present study showed that these media are not adequate for the enumeration of bifidobacteria in porcine intestinal samples (Table 4). Hartemink and Rombouts (13) reported that only 10% of the colonies on Beerens agar and 30% of distinctive colonies on RB agar could be identified as bifidobacteria when pig ileal content was used as the sample (13). Bifidobacteria appear on RB agar as distinctive yellow colonies with a yellow halo and a surrounding precipitation zone (12), but similar characteristic colonies have been shown by lactobacilli from pigs (13). We found it difficult to recognize these characteristics when performing isolations from RB agar in the present study, and these criteria were not accounted for. This may partly explain the extremely low number of bifidobacteria detected in RB agar (Table 4). MW agar showed the highest number of bifidobacteria and was the only medium that enabled recovery of all three groups of bifidobacteria in the present study (Table 4). The number of bifidobacteria obtained from MW agar differed among samples (Table 2). This may be have been due to other bacterial populations resistant to mupirocin (the selective agent of MW agar) exceeding the number of bifidobacteria in some of the piglet fecal samples. The nonbifidobacterial isolates were identified, and their distribution on Beerens, RB, and MW agar media is also given in Table 4. These results may contribute to a future formulation of improved selective agar for the enumeration of bifidobacteria. It is noteworthy that the group identified as consisting of Actinomyces spp. and growing especially well on MW agar showed the characteristic bifid-shaped cellular morphology (Table 4). Thus, the use of morphology for identifying bifidobacteria on the selective agar media is not sufficient. Instead, the F6PPK assay or the genus-specific PCR should be used for a conclusive evaluation of the presence of bifidobacteria on the selective agar media. With regard to the genus-specific PCR, the present results support the idea that the genus-specific primers have highly specific target regions within the 16S rRNA gene of bifidobacteria, as previously validated by genus-specific in situ hybridization (19), PCR (17), and denaturing gradient gel electrophoresis (30). In conclusion, the present study showed that Beerens, RB, and MW agar media are not adequately selective for bifidobacteria when applied for porcine intestinal samples, although the MW agar exhibits superiority with respect to both selectivity and sensitivity for bifidobacteria. The results also indicated that the population of bifidobacteria in feces of suckling piglets is numerically low and that the predominant species seems to be B. boum. In addition, a possible new Bifidobacterium species was isolated which we will attempt to characterize further in our future work.
TABLE 2.
Bacterial counts of total culturable anaerobic bacteria, bacterial counts obtained for the selective agar media, and number of bifidobacteria isolates recovered from fecal samples
| Samples | Bacterial counts of total culturable bacteriaa (CFU/g) | Beerens agar culture
|
RB agar culture
|
MW agar culture
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Bacterial counts (CFU/g) | No. of isolates investigated | No. of bifidobacteria identified | Bacterial counts (CFU/g) | No. of isolates investigated | No. of bifidobacteria identified | Bacterial counts (CFU/g) | No. of isolates investigated | No. of bifidobacteria identified | ||
| F1 | 9.1 × 109 | 2.2 × 109 | 27 | 0 | 2.7 × 109 | 29 | 0 | 8.3 × 107 | 30 | 5 |
| F2 | 2.0 × 109 | 2.8 × 108 | 24 | 0 | 3.5 × 108 | 29 | 0 | <1.9 × 105 | 0 | |
| F3 | 8.5 × 108 | 8.5 × 107 | 28 | 2 | 1.0 × 108 | 29 | 2 | 3.1 × 106 | 30 | 25 |
| F4 | 9.1 × 108 | 1.0 × 109 | 30 | 0 | 1.3 × 109 | 30 | 0 | 1.9 × 108 | 28 | 0 |
| F5 | 5.2 × 109 | 2.4 × 108 | 28 | 15 | 3.0 × 108 | 25 | 1 | 1.8 × 108 | 12 | 10 |
| F6 | 7.4 × 109 | 4.7 × 108 | 30 | 0 | 1.7 × 109 | 26 | 0 | 8.3 × 108 | 25 | 1 |
| F7 | 1.0 × 109 | 1.5 × 108 | 29 | 0 | 4.7 × 108 | 30 | 0 | 4,6 × 108 | 28 | 0 |
Enumerated on Wilkins-Chalgren agar.
FIG. 1.
Restriction patterns of the 16S rRNA genes from bifidobacteria isolated from piglet fecal samples. The 16S rRNA gene fragments were digested with the enzyme HaeIII and resolved by electrophoresis through a 2% agarose gel. Lane M, molecular size marker (100-bp ladder).
TABLE 3.
Fermentation patterns of bifidobacteria isolated in this study and type strains isolated from pigs
| Substratesb | Bifidobacterial isolatesa
|
B. suis DSM 20211T | B. thermophilum DSM 20210T | B. boum DSM 20432T | B. pseudolongum subsp. pseudolongum DSM 20099T | B. pseudolongum subsp. globosum DSM 20092T | B. choerinum DSM 20434T | ||
|---|---|---|---|---|---|---|---|---|---|
| Group I | Group II | Group III | |||||||
| l-Arabinose | − | + | +/− | − | − | − | − | − | − |
| d-Xylose | − | + | + | − | − | − | − | − | − |
| Galactose | + | + | + | + | + | + | − | + | − |
| Trehalose | − | + | − | − | − | − | − | + | + |
| Palatinose | + | + | + | + | + | + | + | − | + |
| Lactose | + | − | + | − | − | − | − | − | + |
| Melibiose | + | + | + | + | + | + | + | − | + |
| Lactulose | + | − | + | − | − | − | − | − | − |
| Gentobiose | − | + | − | − | − | − | − | − | − |
| Inosine | − | + | − | − | − | − | − | + | + |
| Amygdalin | − | + | − | − | − | − | − | − | − |
| Arbutin | − | + | − | − | − | − | − | − | − |
| β-Methylglucoside | − | + | − | − | − | − | − | − | − |
| Gluconate | − | +/− | − | − | − | − | − | − | − |
| Salicine | − | + | − | − | − | − | − | − | − |
| Urea | − | − | − | − | − | − | − | + | + |
Four representative isolates from each group were analyzed. +, ability to ferment indicated substrate; −, inability to ferment indicated substrate; +/−, variable results within a group.
All strains fermented glucose, maltose, sucrose, and raffinose; none of the strains fermented mannonic acid lactone, cellobiose, melezitose, adonitol, inositol, d-arabitol, glycerol, maltitol, sorbitol, dulcitol, sorbose, deoxyglucose, deoxyribose, rhamnose, d-fucose, l-fucose, tagatose, 5-ketogluconate, melbionate, galacturonic lactone, citrate, furnarate, l-malate, malonate, pyruvate, l-tartrate, or ornithine.
TABLE 4.
Distribution of isolates on Beerens, RB, and MW agars
| Isolate(s) | Recovery (%) of isolates from the indicated selective agarsb
|
Cell morphology | ||
|---|---|---|---|---|
| Beerens | RB | MW | ||
| Bifidobacterial isolates | ||||
| Group I | 1 | 1 | 11 | Rodd |
| Group II | 0 | 0 | 12 | Rodd |
| Group III | 9 | <1 | 7 | Rodd |
| Nonbifidobacterial isolatesa | ||||
| Lactobacillus reuteri (99) | 46 | 49 | 0 | Rod |
| Lactobacillus ruminis (98) | 3 | <1 | 0 | Rod |
| Lactobacillus salivarius (99) | 15 | 1 | 0 | Rod |
| Lactobacillus amylovorus (99) | 4 | 0 | 0 | Rod |
| Lactobacillus mucosae (99) | <1 | 0 | 8 | Rod |
| Streptococcus bovis (99) | 7 | 11 | 0 | Coccus |
| Enterococcus faecalis (99) | 1 | 6 | 0 | Coccus |
| Bacteroides fragilis (98) | 0 | 0 | 11 | Rod |
| Clostridium perfringens (98) | 3 | <1 | 10 | Rodc |
| Escherichia coli (99) | 9 | 18 | 0 | Rodc |
| Actinomyces spp. (94-98) | 5 | 12 | 41 | Rodd |
Gross grouping based on cell morphology, pH-reducing capacity, and production of organic acids from glucose (results not shown). Partial 16S rRNA gene sequence analysis was performed on representatives of each group. Percentages of sequence homology of the isolates to GenBank database entries are given in parentheses.
In percentages of the total number of isolates from each selective agar.
Gas producers.
Typical bifid morphology.
Nucleotide sequence accession numbers.
The nucleotide data reported in this paper have been submitted to the GenBank nucleotide database under the accession numbers AF321295 for isolate group I-3, AF321296 for isolate group II-3, and AF321297 for isolate group III-3.
Acknowledgments
This work was financial supported by The Danish Ministry of Agriculture, Food and Fisheries, The National Committee for Pig Breeding, Health and Production, and The Danish Research Agency.
We thank Sabina van den Braak for help processing the samples and Helle Jensen and Karin Durup for technical assistance.
REFERENCES
- 1.Adami, A., and V. Cavazzoni. 1996. A standard procedure for assessing the faecal microflora of swine. Ann. Microbiol. Enzimol. 46:1-9. [Google Scholar]
- 2.Adami, A., and V. Cavazzoni. 1999. Occurrence of selected bacterial groups in the faeces of piglets fed with Bacillus coagulans as probiotic. J. Basic Microbiol. 39:3-9. [PubMed] [Google Scholar]
- 3.Altschul, S. F., W. Gish, W. Miller, E. W. Myers, and D. J. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403-410. [DOI] [PubMed] [Google Scholar]
- 4.Beerens, H. 1990. An elective and selective isolation medium for Bifidobacterium spp. Lett. Appl. Microbiol. 11:155-157. [Google Scholar]
- 5.Biavati, B., B. Sgorbati, and V. Scardovi. 1992. The genus Bifidobacterium, p. 816-833. In A. Balows, H. G. Truper, M. Dworkin, W. Harder, and K.-H. Schleifer (ed.), The prokaryotes. A handbook on the biology of bacteria: ecophysiology, isolation, identification, applications, 2nd ed., vol. 1. Springer-Verlag, New York, N.Y.
- 6.Brown, I., M. Warhurst, J. Arcot, M. Playne, R. J. Illman, and D. L. Topping. 1997. Fecal numbers of bifidobacteria are higher in pigs fed Bifidobacterium longum with a high amylose cornstarch than with low amylose cornstarch. J. Nutr. 127:1822-1827. [DOI] [PubMed] [Google Scholar]
- 7.Crittenden, R. G. 1999. Prebiotics, p. 141-156. In G. W. Tannock (ed.), Probiotics: a critical review. Horizon Scientific Press, Wymondham, United Kingdom.
- 8.Djouzi, Z., and C. Andrieux. 1997. Compared effects of three oligosaccharides on metabolism of intestinal microflora in rats inoculated with human faecal flora. Br. J. Nutr. 78:313-324. [DOI] [PubMed] [Google Scholar]
- 9.Felix, Y. F., M. J. Hudson, R. W. Owens, B. Ratcliffe, A. J. H. van Es, J. A. van Velthuijsen, and M. J. Hills. 1990. Effect of dietary lactitol on the composition and metabolic activity of the intestinal microflora in the pig and in humans. Microb. Ecol. Health Dis. 3:59-267. [Google Scholar]
- 10.Felsenstein, J. 1989. PHYLIP—phylogeny inference package (version 3.2). Cladistics 5:164-166. [Google Scholar]
- 11.Gavini, F., A.-M. Pourcher, C. Neut, D. Monget, C. Romond, C. Oger, and D. Izard. 1991. Phenotypic differentiation of bifidobacteria of human and animal origin. Int. J. Syst. Bacteriol. 41:548-557. [DOI] [PubMed] [Google Scholar]
- 12.Hartemink, R., B. J. Kok, G. H. Weenk, and F. M. Rombouts. 1996. Raffinose-Bifidobacterium (RB) agar, a new selective medium for bifidobacteria. J. Microbiol. Methods 27:33-43. [Google Scholar]
- 13.Hartemink, R., and F. M. Rombouts. 1999. Comparison of media for the detection of bifidobacteria, lactobacilli and total anaerobes from faecal samples. J. Microbiol. Methods 36:181-192. [DOI] [PubMed] [Google Scholar]
- 14.Hirayama, K., K. Itoh, E. Takahashi, K. Shinozaki, and T. Sawasaki. 1996. Composition of faecal microbiota and metabolism of faecal bacteria of pig-flora-associated (PFA) mice. Microb. Ecol. Health Dis. 9:199-206. [Google Scholar]
- 15.Holdeman, L. V., E. P. Cato, and E. C. Moore. 1977. Anaerobe laboratory manual. Virginia Polytechnic Institute and State University, Blacksburg, Virginia.
- 16.Jensen, M. T., R. P. Cox, and B. B. Jensen. 1995. Microbial production of skatole in the hindgut of pigs fed different diets and its relation to skatole deposition in backfat. Anim. Sci. 61:293-304. [Google Scholar]
- 17.Kok, R. G., A. De Waal, F. Schut, G. W. Welling, G. Weenk, and K. J. Hellingwerf. 1996. Specific detection and analysis of a probiotic Bifidobacterium strain in infant feces. Appl. Environ. Microbiol. 62:3668-3672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kullen, M. J., M. M. Amann, M. J. O'Shaughnessy, D. J. O'Sullivan, F. F. Busta, and L. J. Brady. 1997. Differentiation of ingested and endogenous bifidobacteria by DNA fingerprinting demonstrates the survival of an unmodified strain in the gastrointestinal tract of humans. J. Nutr. 127:89-94. [DOI] [PubMed]
- 19.Langendijk, P. S., F. Schut, G. J. Jansen, G. C. Raangs, G. R. Kamphuis, M. H. F. Wilkinson, and G. W. Welling. 1995. Quantitative fluorescence in situ hybridization of Bifidobacterium spp. with genus-specific 16S rRNA-targeted probes and its application in fecal samples. Appl. Environ. Microbiol. 61:3069-3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lauer, E., and O. Kandler. 1983. DNA-DNA homology, murein types and enzyme patterns in the type strains of the genus Bifidobacterium. Syst. Appl. Microbiol. 4:42-64. [DOI] [PubMed] [Google Scholar]
- 21.Leser, T. D., J. Z. Amenuvor, T. K. Jensen, R. H. Lindecrona, M. Boye, and K. Møller. 2002. Culture-independent analysis of gut bacteria: the pig gastrointestinal tract microbiota revisited. Appl. Environ. Microbiol. 68:673-690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matteuzzi, D., F. Croziana, G. Zani, and L. D. Trovatelli. 1971. Bifidobacterium suis n. sp.: a new species of the genus Bifidobacterium isolated from pig feces. Z. Allg. Mikrobiol. 11:387-395. [DOI] [PubMed] [Google Scholar]
- 23.Maxwell, F., and C. S. Stewart. 1991. Isolation and characteristics of bifidobacteria from pig faeces, p. 412. In Lec bacteries latiques. Adria Normandie, Caen, France.
- 24.Mikkelsen, L. L. 2001. Fructooligosaccharides and galactooligosaccharides as prebiotics for piglets at weaning. Ph.D. thesis. The Royal Veterinary and Agricultural University, Frederiksberg, Denmark.
- 25.Miller, T. L., and M. J. Wolin. 1974. A serum bottle modification of the Hungate technique for cultivating obligate anaerobes. Appl. Microbiol. 27:985-987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mul, A. J., and F. G. Perry. 1994. The role of fructo-oligosaccharides in animal nutrition, p. 57-79. In P. C. Garnsworthy and J. A. Cole (ed.), Recent advances in animal nutrition. Nottingham University Press, Nottingham, United Kingdom.
- 27.Nemcová, R., A. Bomba, S. Gancarciková, R. Heich, and P. Guba. 1999. Study of the effect of Lactobacillus paracasei and fructooligosaccharides on the faecal microflora in weanling piglets. Berl. Münch. Tierärztl. Wochenschr. 112:225-228. [PubMed] [Google Scholar]
- 28.Rada, V. 1997. Detection of Bifidobacterium species by enzymatic methods and antimicrobial susceptibility testing. Biotechnol. Tech. 11:909-912. [Google Scholar]
- 29.Rada, V., K. Sirotek, and J. Petr. 1999. Evaluation of selective media for bifidobacteria in poultry and rabbit caecal samples. J. Vet. Med. Ser. B 46:369-373. [DOI] [PubMed] [Google Scholar]
- 30.Satokari, R. M., E. E. Vaughan, A. D. Akkermans, M. Saarela, and W. M. De Vos. 2001. Polymerase chain reaction and denaturing gradient gel electrophoresis monitoring of fecal Bifidobacterium populations in a prebiotic and probiotic feeding trial. Syst. Appl. Microbiol. 24:227-231. [DOI] [PubMed] [Google Scholar]
- 31.Scardovi, V. 1986. Genus Bifidobacterium, p. 1418-1434. In P. H. A. Sneath, N. S. Mair, M. E. Sharpe, and J. G. Holt (ed.), Bergey's manual of systematic bacteriology, vol. 2. Williams & Wilkins, Baltimore, Md.
- 32.Sgorbati, B., B. Biavati, and D. Palenzona. 1995. The genus Bifidobacterium, p. 279-306. In B. J. B. Wood and W. H. Holzapfel (ed.), The lactic acid bacteria, vol. 2. Blackie Academic, London, United Kingdom.
- 33.Silvi, S., C. J. Rumney, and I. R. Rowland. 1996. An assessment of three selective media for bifidobacteria in faeces. J. Appl. Bacteriol. 81:561-564. [DOI] [PubMed] [Google Scholar]
- 34.Zani, G., B. Biavati, F. Crosiani, and D. Matteuzzi. 1974. Bifidobacteria from the faeces of piglets. J. Appl. Bacteriol. 37:537-547. [Google Scholar]