Abstract
An electric water heater was modified for large-scale cultivation of aerobic acidophilic hyperthermophiles to enable recovery of secreted proteins. Critical changes included thermostat replacement, redesign of the temperature control circuit, and removal of the cathodic anticorrosion system. These alterations provided accurate temperature and pH control. The bioreactor was used to cultivate selected strains of the archaeon Sulfolobus solfataricus and other species within this genus. Reformulation of a basal salts medium facilitated preparation of large culture volumes and eliminated sterilization-induced precipitation of medium components. Substrate induction of synthesis of the S. solfataricus-secreted alpha-amylase during growth in a defined medium supported the utility of the bioreactor for studies of physiologically regulated processes. An improved purification strategy was developed by using strong cation-exchange chromatography for recovery of the alpha-amylase and the processing of large sample volumes of acidic culture supernatant. These findings should simplify efforts to study acidophilic hyperthermophilic microbes and their secreted proteins.
Geothermal environments are often highly acidic. Such extreme environments harbor a wide range of acidophilic hyperthermophilic organisms including members of both the bacterial and archaeal prokaryotic subdivisions. Among the archaeal representatives, members of the Sulfolobus genus have been most intensively characterized. These organisms can grow chemoheterotrophically on reduced carbon compounds (6, 9, 17) and lithoautotrophically on reduced sulfur and carbon dioxide (3, 23). This metabolic versatility is thought to contribute at least in part to the low pH of their growth environment (2), and this together with the high temperature of cultivation creates unique challenges for their physiological manipulation.
Studies of the biomolecules produced by acidophilic hyperthermophiles require sufficient biomass to enable biochemical studies (7, 13, 16, 20, 21). However, large-scale systems suitable for the cultivation of these organisms are not generally available. Conventional microbial fermentors or bioreactors are fabricated out of stainless steel. Stainless steel is not appropriate for cultivation of thermoacidophiles, however, because it rusts. To avoid this problem, it is necessary to minimize steel or stainless steel surface area, particularly for reactor components that cannot be replaced, such as the reactor itself. Fermentors must instead be lined with glass or ceramic materials to circumvent metal oxidation, resulting in significant added reactor cost. One solution to this problem has been the use of plastic or rubber containers placed within secondary containment vessels to allow for reactor insulation. A fabricated lid provides reactor access, and temperature is controlled externally with a hot plate or heating jacket or internally with an immersible heater and thermostat (8, 14, 19). Both Sulfolobus shibatae and Sulfolobus acidocaldarius were cultivated in this manner with complex media and prolonged incubation periods. The resulting biomass was used for the isolation and analysis of intracellular proteins. However, as yet unexamined in such alternative cultivation systems are process control parameters like pH and temperature variation, physiological indicators including growth rate and specific yield, and cultivation criteria such as medium composition. Perhaps because of these issues, the application of these alternative cultivation methods to studies of physiologically regulated processes has remained untested.
Enzymes secreted by hyperthermophiles seldom accumulate in culture supernatants to significant levels (5, 18). These secreted enzymes include various hydrolytic activities including glycosyl hydrolases like the secreted alpha-amylases from Sulfolobus solfataricus (10-12). Synthesis of this enzyme is highly regulated and is dependent upon the maintenance of specific growth temperature and pH values as well as medium composition. The low abundance of such proteins, however, presents a significant technical barrier complicating detailed analysis of their structure, function, and regulation. To overcome this constraint, simplified methods for the preparation and processing of acidophilic hyperthermophilic cultures were developed. These included the development of a low-cost bioreactor, a modified culture medium enabling the use of concentrated stocks, and a high-throughput purification step based on the use of a strong cation-exchange resin.
MATERIALS AND METHODS
Strains and cultivation.
S. solfataricus strain 98/2, S. shibatae strain B12, and S. acidocaldarius strain DG6 were as described previously (17). Small-scale 50-ml batch cultures were prepared in 250-ml screw-cap Erlenmeyer flasks. Inocula for these cultures consisted of thawed frozen cell pellets obtained from laboratory collections stored at −80°C with 0.7% (vol/vol) dimethyl sulfoxide as a cryoprotectant. Cells were cultivated aerobically with agitation by placing flasks in rotary water bath shakers with fitted Lucite lids. Glycerol was used to maintain external temperatures in the rotary baths. Temperatures within the flasks were maintained between 77 and 80°C. Growth in liquid cultures was monitored at a wavelength of 540 nm with a Cary 50 Bio, UV-visible spectrophotometer (Varian). Direct cell counts were determined with a Thoma counting chamber. Strains cultivated in the bioreactor were supplied with oxygen by using compressed ambient air introduced by sparging with aquarium air stones (orange glass beaded air stones; Ginger Inc.). Several other brands tested were found to be acid unstable. Externally supplied air was routed through a Gilmont flow meter (Barnant, model no. 65 MM, GF-5341-1502) at a volumetric airflow rate of 0.6 liter/min. When necessary, air stones were connected in series with Tygon tubing to the flow meter output through single-hole black rubber stoppers embedded in one of the prefabricated bioreactor openings. For induction of alpha-amylase synthesis, cells were grown successively in 50-ml batch cultures in a glucose minimal medium and then in 500-ml batch cultures and then subcultured at an optical density at 540 nm (OD540) of 0.03 into the bioreactor, which contained a minimal medium with glutamate (5 mM) as the sole carbon and energy source (12). A soluble starch (Fluka) stock solution was added at a final concentration of 0.2% (wt/vol) when the cell density had reached an OD540 of 0.1. The bioreactor has an internal glass lining and uses a replaceable steel immersion-type heating element. The bioreactor was not sterilized before use but rather rinsed repeatedly with dilute acid and water. The absorption of uninoculated medium was checked before reactor inoculation to ensure that no significant cell carryover occurred. To preclude contamination, fermentations were conducted by fed batch and the reactor was routinely inoculated to a final cell density of 2 × 107 cells/ml. Provisions to minimize contamination during use included placing sterilized glass wool plugs in the incoming air line and the use of positive air pressure resulting from sparging during venting. Cells were harvested through the reactor drain.
Medium.
The medium of Allen (1) originally developed for the cultivation of moderately thermophilic eukaryotic alga, as modified by Brock et al. (3), was reformulated for the studies reported here. In the reformulated medium, iron citrate (FeC6H5O7) was substituted for iron chloride (FeCl3 · 6H2O) at the same final molarity of iron as originally described (3). This salt provided sufficient iron for growth but negligible levels of carbon. No growth was observed in this medium in the absence of addition of a carbon and energy source. A 10-fold-concentrated stock solution of iron citrate (0.74 mM) with an unadjusted pH of 3.5 was prepared and autoclaved separately. Iron citrate solutions were protected from light by being wrapped in foil after autoclaving to avoid light-induced precipitation. All remaining components of the basal salts medium as described by Brock et al. (3) were combined in the form of a 10-fold-concentrated stock solution and sterilized by autoclaving. This solution was light insensitive. The stock solutions were combined at the time of use with sterilized distilled water by adding first iron citrate, then the other stock salts solution, and finally tryptone as the carbon and energy source to a final concentration of 0.2% (wt/vol). Stock salts solutions are stable for at least 3 months. The pH of the final medium was adjusted to pH 3.0 with sulfuric acid as described previously (3).
Temperature-regulated electrical circuit.
Essential components of the system included the following items. The direct current (DC) power supply was a DC-PACK, alternating current (AC) adapter item LR24173, model DC-950 (Radio Shack), with an input of 120 V (AC) at 60 Hz and 8 W and an output of 9 V (DC) at 500 mA. The thermoregulator was a Quick-Set II adjustable thermoregulator (H-B Instrument; catalog no. 7501) with an immersion depth of 76 mm (3 in.) and an overall length of 390 mm (15[3/8] in.). Contacts were mercury to tungsten with a maximum contact load of 30 mA, noninductive, and scale divisions of 0 to 100°C in 1°C divisions. The thermoregulator had a diameter of 9 to 10 mm in its lower stem and 18 to 19 mm in its upper stem. The upper stem was wrapped in a 2-mm rubber sleeve and inserted through a hole in the top of the bioreactor. A 12-V-DC plug-in relay was used to regulate thermoregulator output. The relay with socket (Radio Shack catalog no. 275-206) was double pole, double throw but used only single pole, single throw (SPST). The relay coil resistance was 60 Ω (±10%) with a pull-in voltage of 9.0 V (DC) and a contact rating of 5 A at 125 V (DC), a nominal current of 75 mA, and absolute maximum ratings at ambient temperature −25 to 60°C with a continuous coil voltage of 3.2 V (DC). The solid-state relay (Electrol, preconditioned 8507) had output poles 1 and 2 at 20 A and 120 V (AC) and input poles 3+ and 4− at 5.5 to 10 V (DC). The bioreactor (water heater) was a point-of-use type (Richmond, model no. 8VP2-1) with a 2.5-gal (9.46-liter)-capacity tank and had waterways rated at 400°F (204.4°C). The heating element is made of steel and will corrode with use. Corrosion can be detected by visual inspection through the surface access ports. The heaters are designed to allow element removal and replacement if necessary. A grounded power supply cord should be used, preferably with a ground fault interrupt circuit.
Protein and enzyme assays.
Protein concentrations were measured with the bicinchoninic acid protein assay reagent kit (Pierce). Unless otherwise indicated, all chemicals were obtained from common chemical suppliers. Alpha-amylase levels were determined by a dextrinization assay (15) as described previously (12) with the following modifications. A reaction mixture containing either culture supernatant clarified by centrifugation at 3,000 × g in an SS-34 rotor and Sorvall RC2B centrifuge at +4°C or purified enzyme and 100 μg of starch in 10 mM sodium acetate (pH 3.5) was incubated at 80°C for 120 min. The reaction was terminated by cooling at 4°C for 5 min, and then the reaction mixture was equilibrated to room temperature. Color was developed by addition of 0.005 ml of an iodine solution (4% [wt/vol] potassium iodide, 1.25% [wt/vol] iodine). The sample absorbance was determined at a wavelength of 600 nm and was corrected for an unincubated but otherwise identical sample. Substrate stability during sample incubation was monitored by evaluating color development in control samples consisting of buffer with and without added substrate. No evidence of substrate instability was apparent. One unit of amylase activity was equivalent to the amount of protein that hydrolyzed 1 μg of starch in 1 min. All samples were assayed in duplicate, and the averages of the sample results are reported.
Alpha-amylase purification and detection.
Alpha-amylase was purified from clarified culture supernatants by passage through a High-S cationic resin (Bio-Rad) at ambient temperature. A 5-ml resin bed was equilibrated with 10 mM sodium acetate buffer (SA; pH 3.5). The culture supernatant was loaded at 1.5 ml/min with a peristaltic pump. The column was then washed with 3 to 5 bed volumes of SA at a reduced flow rate of 0.3 ml/min, and bound protein was eluted with a 100-ml linear gradient of 0 to 1 M sodium chloride in SA at the same flow rate. Fractions were collected in 3-ml volumes and assayed directly. Alpha-amylase activity typically eluted at 0.1 M sodium chloride. Alpha-amylase protein was detected by Coomassie blue staining and Western blot analysis of samples resolved by migration through a 10% (wt/vol) Tris-glycine sodium dodecyl sulfate (SDS)-polyacrylamide gel with a 10% (wt/vol) stacking gel as described previously (12). Prior to loading, fractions from High-S chromatography were dialyzed against water to remove salt and then concentrated by evaporation with a Speed-Vac (Savant).
RESULTS
Bioreactor features.
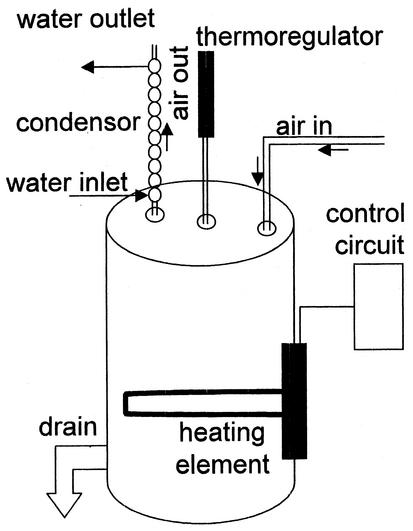
Analysis of low-abundance proteins produced by acidophilic hyperthermophilic archaea necessitates the use of large culture volumes in specialized bioreactors. To accommodate these needs, a bioreactor was developed consisting of a conical 2.5-gal (9.46-liter) 120-V residential electric water heater with dimensions of 14 in. in height (35.6 cm) by 10 in. (25.4 cm) in diameter (Fig. 1). A prefabricated nylon drain was positioned at the bottom side of the housing and was used for sample recovery and tank cleanup. The inner tank was insulated with fiberglass sheeting and was encased by a thin metal enamel-painted housing to maintain a cool surface temperature during operation. Three prefabricated ports were positioned on the top side of the tank and provided ready access to the interior of the heater: two were designed for water supply connections and the third was designed for a pressure relief valve. Water evaporation was avoided by placing a water-cooled glass condensing coil into one of the bioreactor surface ports. Heaters with capacities of 250 gal (946 liters) and above employ identical design features.
FIG. 1.
Bioreactor schematic.
Temperature regulation.
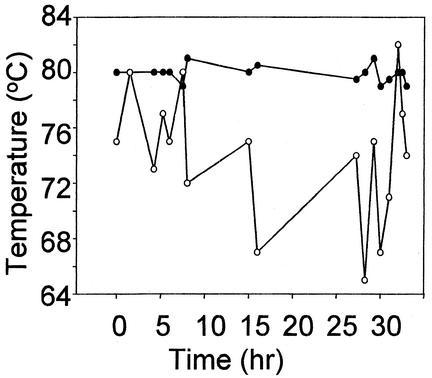
Conventional electric water heaters employ an adjustable surface-mounted thermostat to control water temperatures. This device is preset at 120°F (43°C) to minimize the risk of scalding. Initial attempts to use the supplied thermostat to achieve water temperatures suitable for cultivation of hyperthermophiles were not successful. The surface-mounted thermostat was adjusted to achieve initial water temperatures of 75°C. Water temperatures were then monitored periodically to determine the magnitude of variation which might occur during the time required for a typical growth experiment (Fig. 2, open circles). Temperatures varied over a 20°C range above and below the initial preset temperature value. In addition, it was not possible to achieve adequate temperature control with extra tank insulation consisting of double layers of fiberglass and tinfoil.
FIG. 2.
Bioreactor temperature regulation. Shown are results with temperature control with prefabricated surface-mounted thermostat and circuit (open circles) and temperature control with immersion thermostat and modified heating control circuit (solid circles).
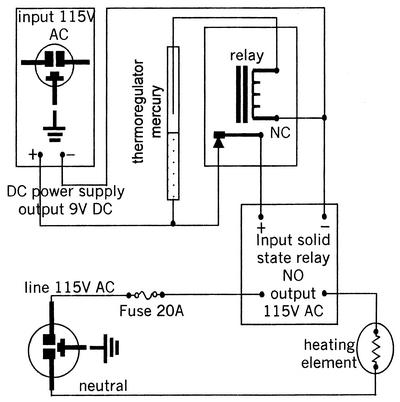
To improve temperature control, the surface-mounted thermostat was replaced by a mercury immersion thermostat inserted through one of the ports in the surface of the tank (Fig. 1). Power (115-V AC) was provided to the electrical water heater's heating element through a 20-A line protected with a 20-A fuse (Fig. 3). A 20-A solid-state relay was used to control line voltage to the heating element. The input side of the solid-state relay (3- to 30-V DC) was powered by a 9-V-DC wall transformer (115-V-AC input). DC voltage from the wall transformer passed through the mercury thermostat that was used to measure the temperature of the culture medium in the bioreactor. As the temperature of the culture medium rose, the mercury also rose in the thermostat, closing the circuit and allowing current to flow. To control the heating element, this logic was reversed, by using an SPST relay. The SPST relay was normally closed with 9-V DC flowing through the normally closed contacts, energizing the input side of the solid-state relay. In this state, the heating element was in the heating mode. As the temperature in the culture medium increased to the set point, the mercury thermostat closed its contact, allowing current to flow through the SPST relay coil. This opened the SPST relay contacts, which turned off the solid-state relay, turning off the heating element. This system greatly improved temperature regulation and allowed temperature to be controlled with a maximum variation of 2°C (Fig. 2, closed circles).
FIG. 3.
Bioreactor heating control circuit. Symbols used represent standard electrical devices.
Regulation of pH.
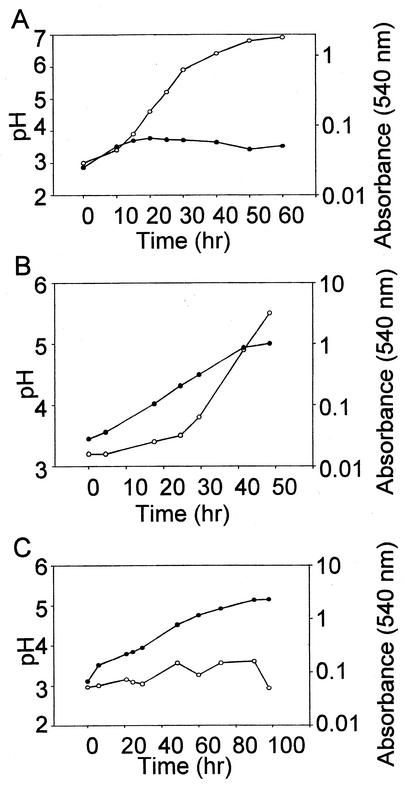
The temperature control modifications were sufficient to cultivate neutrophilic hyperthermophilic microbes that are typically cultivated at or near neutral pH. However, cultivation of acidophilic microbes in acidic media failed because of acid neutralization (Fig. 4A). Anode rods are metal shafts used in all residential water heaters to minimize cathodic corrosion by protruding through the stored water in the tank. They are made of magnesium-aluminum metal alloys and in the present case have the unwanted effect of neutralizing acid. To overcome unwanted changes in medium pH, the anode rod was removed from its site of insertion at the underside of the tank and replaced with a stainless steel bolt.
FIG. 4.
Dependence of S. solfataricus growth on medium pH. (A) Growth curve of S. solfataricus with anode rod in place. (B) Growth curve of S. solfataricus without anode rod. (C) Growth curve of S. solfataricus without anode rod and with acid addition. Symbols: open circles, pH; solid circles, culture absorbance.
In the absence of the anode rod, the acidophilic hyperthermophilic archaeon S. solfataricus was successfully cultured (Fig. 4B). Cells were grown in a reformulated version of Brock's modification (3) of Allen's medium (1) by using compressed air to supply oxygen and mix the reactor. The reformulated medium employs ferric citrate as a replacement for ferric chloride and allows for the use of concentrated stock salts solutions, simplifying preparation of large amounts of growth medium. In addition the reformulation results in a completely soluble medium. When tryptone (0.2%) was used as the sole carbon and energy source, a generation time of 8.5 h and a final absorbance of 1.0 (540 nm) were observed; however, neutralization of the medium occurred, resulting in a final pH of 5.5. This is likely to result from the metabolic consumption of peptides and amino acids and a small reduction in the buffering capacity of the reformulated medium. To realize further improvements in cell yields, the pH of the medium was periodically readjusted by acid titration and increased cell yields a further threefold (Fig. 4C). The original and reformulated media produced identical growth rates and cell yields in smaller-scale shake-flask batch cultures.
Cultivation of Sulfolobus spp.
The bioreactor was suitable for cultivation of other acidophilic hyperthermophiles (Table 1). S. shibatae is closely related to S. solfataricus while S. acidocaldarius is not (4). For both of these organisms, high-cell-density cultures were achieved as indicated by measurements of cell densities, total cell number, and protein assays.
TABLE 1.
Yields of hyperthermophilesa
| Organism | Direct cell count | OD540 | Protein (g) |
|---|---|---|---|
| S. solfataricus | 7.0 × 1011 | 2,260 | 2.23 |
| S. shibatae | 6.6 × 1011 | 1,320 | 1.60 |
| S. acidocaldarius | 2.4 × 1010 | 1,110 | 1.28 |
Values indicated are normalized to liter culture volumes.
Production and recovery of a secreted enzyme.
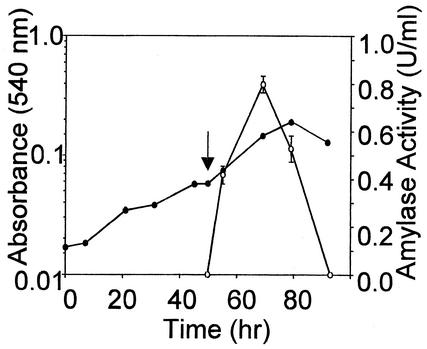
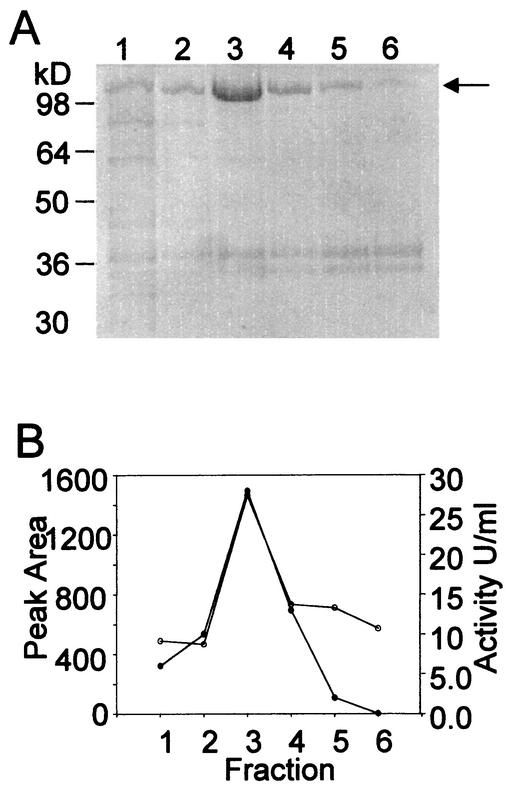
To assess the utility of the bioreactor for analysis of secreted proteins, the S. solfataricus alpha-amylase was chosen for study (12). Previous investigations have shown that synthesis of the alpha-amylase is induced by starch addition and that the degree of induction is further controlled by the presence of other carbon and energy sources (12). Cells were cultivated in the optimized minimal medium containing glutamate as the sole carbon and energy source. At the time indicated, soluble starch was added and levels of the secreted alpha-amylase in the culture medium were monitored by a modified dextrinization assay (Fig. 5). Maximal levels of enzyme activity were 0.8 U/liter in crude culture supernatants. Recovery of this protein from clarified culture supernatants employed chromatography on a High-S strong cation-exchange resin, allowing continued use of low-pH buffers to avoid enzyme inactivation. The High-S resin remains negatively charged even at the low pH value of the bioreactor culture supernatant (pH 3.0), allowing acidic proteins to bind the resin efficiently. Bound protein was eluted with a linear gradient of sodium chloride, and the presence of the alpha-amylase in these fractions was assessed by SDS-polyacrylamide gel electrophoresis (PAGE) and enzyme assay (Fig. 6). Peak activities were repeatedly detected at 0.1 M sodium chloride and coincided with accumulation of the 120-kDa alpha-amylase protein (12). The lack of protein evident in the last active fraction (Fig. 6A, lane 6, and 6B) reflects the use of darker regions of the gel as densitometric background to normalize band intensities. Enzyme yields of pooled active fractions constituted 44% of the amounts of activity present in culture supernatant. The specific activity of this material was significantly increased, allowing more than 100-fold purification in a single chromatographic step.
FIG. 5.
Induction of alpha-amylase secretion. The arrow indicates the time of addition of starch to the culture medium. Symbols: solid circles, culture absorbance; open circles, alpha-amylase activity.
FIG. 6.
SDS-PAGE of fractions eluted from High-S. (A) SDS-PAGE of fractions containing alpha-amylase activity eluted from High-S with an increasing linear sodium chloride gradient. Proteins were visualized by Coomassie blue staining. The arrow indicates the position of the alpha-amylase. (B) Alpha-amylase activity in active fractions. Symbols: open circles, densitometric band intensity of the alpha-amylase; solid circles, alpha-amylase activity.
DISCUSSION
Because of the growing demand for increased hot water quality, residential water heaters have been redesigned so that most commercial systems employ a cast-iron tank whose inner surface is coated with glass. This coating reduces metal-water contact and improves water composition. The reduction in metal surface area in such devices provided the impetus to evaluate and ultimately validate these appliances for cultivation of acidophilic hyperthermophiles. Benefits gained from using this device rather than previously described systems derive from its intended application for producing and maintaining heated water. Multiple top- and bottom-positioned ports enable ready access through both metal layers and reactor insulation. In addition this system has a prefitted immersion heater. Such features ensure control over temperature variation within a 2°C range. Additional improvements in temperature control may be achieved by minimizing power supplied to the immersion heating element.
The bioreactor system should enable more widespread studies of acidophilic hyperthermophiles by reducing equipment expense. Use of a widely available residential appliance for bioreactor construction avoids reliance on highly specialized equipment, thereby minimizing initial costs and maintenance-related expenses. Construction of the system described here cost approximately 10% of that of commercial systems at a similar scale. The bioreactor can be used to assess physiological parameters including variations in temperature, aeration, and pH which might influence protein secretion. The bioreactor design can be applied directly to larger-capacity heating units. These are available in various sizes including the conventional 250-gal (946-liter) model.
Despite removal of the anode rod, the complex medium continued to exhibit a small increase in pH, necessitating periodic acid titration. A more modest increase in pH was observed by using complex reformulated medium in small-scale shake flasks. Cultivation in the bioreactor with a defined medium reduced this pH effect. Neither the original nor the modified medium formulation provides significant buffering capacity, suggesting that the metabolism of complex media such as deamination of amino acids contributed to this effect. The reformulated medium also overcomes sterilization-induced precipitation of medium components noted in the original and modified formulations prepared even at single strength (1, 3). In addition it allows for the use of concentrated stock solutions, thereby simplifying preparation of large culture volumes. Light-induced formation of iron hydroxides leading to precipitation and medium alterations was avoided by blocking light exposure.
The purification strategy described here for the S. solfataricus-secreted alpha-amylase provided increased amounts of the natural form of the enzyme relative to previous methods (10-12). Improved purification of this activity was accomplished by direct chromatographic fractionation of unconcentrated clarified culture supernatants, allowing both concentration and purification in a single step. The previous purification strategy used ultrafiltration to concentrate culture supernatants prior to chromatographic fractionation, which reduced enzyme yields because of nonspecific binding of the protein to filtration membranes (10-12). Current efforts using the bioreactor and new purification system include studies of structural and molecular features of the alpha-amylase and its gene as well as experiments involving complex geothermal microcosms and their secreted factors.
Acknowledgments
The comments of the reviewers are gratefully appreciated.
This research was supported by National Science Foundation grants MCB-9974453 and MCB-0085216.
REFERENCES
- 1.Allen, M. B. 1959. Studies with Cyanidium caldarium, an anomalously pigmented chlorophyte. Arch. Mikrobiol. 32:270-277. [DOI] [PubMed] [Google Scholar]
- 2.Brierley, C. 1978. Bacterial leaching. Crit. Rev. Microbiol. 6:207-262. [DOI] [PubMed] [Google Scholar]
- 3.Brock, T. D., K M. Brock, R. T. Belly, and R. L. Weiss. 1972. Sulfolobus: a new genus of sulfur-oxidizing bacteria living at low pH and high temperature. Arch. Mikrobiol. 84:54-68. [DOI] [PubMed] [Google Scholar]
- 4.Burggraf, S., H. Huber, and K. O. Stetter. 1997. Reclassification of the crenarchaeal orders and families in accordance with 16S rRNA sequence data. Int. J. Syst. Bacteriol. 47:657-660. [DOI] [PubMed] [Google Scholar]
- 5.Canganella, F., M. Andrade, and G. Antranakian. 1994. Characterization of amylolytic and pullulytic enzymes from thermophilic archaea and from a new Fervidobacterium species. Appl. Microbiol. Biotechnol. 42:239-245. [Google Scholar]
- 6.De Rosa, M., A. Gambacorta, and J. D. Bu'lock. 1975. Extremely thermophilic acidophilic bacteria convergent with Sulfolobus acidocaldarius. J. Gen. Microbiol. 86:156-164. [DOI] [PubMed] [Google Scholar]
- 7.Fuchs, T., H. Huber, K. Teiner, S. Burggraf, and K. O. Stetter. 1995. Metallosphaera prunae, sp. nov., a novel metal-mobilizing thermoacidophilic archaeum, isolated from a uranium mine in Germany. Syst. Appl. Microbiol. 18:560-566. [Google Scholar]
- 8.Green, G. R., D. G. Searcy, and R. J. DeLange. 1983. Histone-like protein in the archaebacterium Sulfolobus acidocaldarius. Biochim. Biophys. Acta 741:252-257. [DOI] [PubMed] [Google Scholar]
- 9.Grogan, D. W. 1989. Phenotypic characterization of the archaebacterial genus Sulfolobus: comparison of five wild-type strains. J. Bacteriol. 171:6710-6719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haseltine, C., R. Montalvo-Rodriguez, E. Bini, A. Carl, and P. Blum. 1999. Coordinate transcriptional control in the hyperthermophilic archaeon Sulfolobus solfataricus. J. Bacteriol. 181:3920-3927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haseltine, C., R. Montalvo-Rodriguez, A. Carl, E. Bini, and P. Blum. 1999. Extragenic pleiotropic mutations that repress glycosyl hydrolase expression in the hyperthermophilic archaeon Sulfolobus solfataricus. Genetics 152:1353-1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haseltine, C., M. Rolfsmeier, and P. Blum. 1996. The glucose effect and regulation of α-amylase production in the hyperthermophilic archaeon Sulfolobus solfataricus. J. Bacteriol. 178:945-950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huber, G., C. Spinnler, A. Gambacorta, and K. O. Stetter. 1989. Metallosphaera sedula gen. and sp. nov. represents a new genus of aerobic, metal-mobilizing, thermoacidophilic archaebacteria. Syst. Appl. Microbiol. 12:38-47. [Google Scholar]
- 14.Korkhin, Y., O. Littlefield, P. J. Nelson, S. D. Bell, and P. B. Sigler. 2001. Preparation of components of archaeal transcription preinitiation complex. Methods Enzymol. 334:227-239. [DOI] [PubMed] [Google Scholar]
- 15.Manning, G. G., and L. L. Campbell. 1961. Thermostable alpha-amylase of Bacillus stearothermophilus. J. Biol. Chem. 236:2952-2957. [PubMed] [Google Scholar]
- 16.Rella, R., C. A. Raia, M. Pensa, F. M. Pisani, A. Gambacorta, M. De Rosa, and M. Rossi. 1987. A novel archaebacterial NAD+-dependent alcohol dehydrogenase. Eur. J. Biochem. 167:475-479. [DOI] [PubMed] [Google Scholar]
- 17.Rolfsmeier, M., and P. Blum. 1995. Purification and characterization of a maltase from the extremely thermophilic crenarchaeote Solfataricus solfataricus. J. Bacteriol. 177:482-485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rudiger, A., P. L. Jorgensen, and G. Antranikian. 1995. Isolation and characterization of a heat-stable pullulanase from the hyperthermophilic archaeon Pyrococcus woesei after cloning and expression of its gene in Escherichia coli. Appl. Environ. Microbiol. 61:567-575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Searcy, D. 1995. Culture of Thermoplasma acidophilum, p. 51-61. In F. T. Robb and A. R. Place (ed.), Archaea: a laboratory manual: thermophiles. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 20.Segerer, A. H., A. Trincone, M. Gahrtz, and K. O. Stetter. 1991. Stygiolobus azoricus gen. nov., sp. nov. represents a novel genus of anaerobic, extremely thermoacidophilic archaebacteria of the order Sulfolobales. Int. J. Syst. Bacteriol. 41:495-501. [Google Scholar]
- 21.Stetter, K. O., H. Konig, and E. Stackebrandt. 1983. Pyrodictium gen. nov., a new genus of submarine disc-shaped sulfur reducing archaebacteria growing optimally at 105°C. Syst. Appl. Microbiol. 4:535-551. [DOI] [PubMed] [Google Scholar]
- 22.Sunna, A., M. Moracci, M. Rossi, and G. Antranikian. 1997. Glycosyl hydrolases from hyperthermophiles. Extremophiles 1:2-13. [DOI] [PubMed] [Google Scholar]
- 23.Wood, A. P., D. P. Kelly, and P. R. Norris. 1987. Autotrophic growth of four Sulfolobus strains on tetrathionate and the effect of organic nutrients. Arch. Microbiol. 146:382-389. [Google Scholar]