Abstract
The culturability of 10 strains of Campylobacter jejuni and Campylobacter coli was studied after the bacteria were exposed to acid conditions for various periods of time. Campylobacter cells could not survive 2 h under acid conditions (formic acid at pH 4). The 10 Campylobacter strains could not be recovered, even when enrichment media were used. Viable cells, however, could be detected by a double-staining (5-cyano-2,3-ditolyl tetrazolium chloride [CTC]-4′,6′-diamidino-2-phenylindole [DAPI]) technique, demonstrating that the treated bacteria changed into a viable but nonculturable (VBNC) form; the number of VBNC forms decreased over time. Moreover, some VBNC forms of Campylobacter could be successfully resuscitated in specific-free-pathogen fertilized eggs via two routes, amniotic and yolk sac injecting.
In recent years, the frequency of human enteritis caused by Campylobacter jejuni and Campylobacter coli has increased in many developed countries. The potential source of the infection has been identified as the consumption of undercooked poultry products or water contaminated with Campylobacter species (2, 28). Hence, poultry contaminated with Campylobacter is a source of human Campylobacter infection. At broiler farms, drinking water is the prime suspect as the vehicle that spreads Campylobacter throughout the flock (23). Therefore, decontamination of the water is needed. The use of acidified water (using organic acids to control pathogenic agents) is a convenient and safe decontamination method.
Several studies have shown that strong acids, such as formic, acetic, ascorbic, and lactic acids, rapidly inhibit the growth of Campylobacter species (9, 11, 12, 15, 34). Organic acids have also been used (5, 11, 13, 33) to reduce the amount of infection with Campylobacter and Salmonella on carcasses or to prevent broilers from becoming infected. Acidified water is intensively used in food industries and animal husbandry. However, it is still not known whether Campylobacter bacteria were just below the detection limit and had changed into dormant cells or whether the bacteria had died after exposure to the acid conditions. The survival of Campylobacter depends on many factors, such as aerobiosis, presence of nutrients, temperature, and pH levels (17, 21, 24). In unfriendly environments, Campylobacter may be able to enter a nonculturable stage. The viability of these cells cannot be detected by a routine culture method. This phenomenon, the viable but nonculturable (VBNC) stage, was first described by Rollins and Colwell (26). The VBNC stage has been considered to play a role in human health. In recent years, several studies have explored the recovery of VBNC forms of Campylobacter cells. However, the recovery of VBNC forms of Campylobacter cells is still controversial (3, 6, 8, 19, 20, 22, 27, 29, 30, 33).
The aim of this study was to better understand the survival of Campylobacter in acidified Mueller-Hinton broth over time. Hence, experiments were conducted to determine the recovery of 10 Campylobacter strains after exposure to acidity; four experiments checking survival, resuscitation in enrichment media, formation of VBNC forms, and recovery of VBNC forms were performed. The potential resuscitation of the treated bacteria was checked by injecting VBNC Campylobacter in 9-day-old specific-free-pathogen (SPF) embryonated chicken eggs.
Campylobacter strains.
Ten C. jejuni and C. coli strains isolated from chickens were used (9). They were kept at −70°C in glycerol (20% [vol/vol]). A 100-μl sample of each strain was thawed and then inoculated in 10 ml of brucella broth (BBL; Becton Dickinson, Sparks, Md.). Subsequently, the inoculated broth tubes were incubated at 37°C for 48 h under microaerophilic conditions (5% O2, 10% CO2, and 85% N2) generated by using a Gas Pak Plus envelope (BBL; Becton Dickinson) in anaerobic jars. Thereafter, all strains were subcultured onto Columbia agar with 5% (vol/vol) defibrinated horse blood (Bio Trading Benelux BV, Mijdrecht, The Netherlands) and were kept under microaerophilic conditions at 37°C for 48 h. Then, one typical Campylobacter colony was transferred to Mueller-Hinton (MH) broth (Oxoid, Hampshire, United Kingdom) and incubated under microaerophilic conditions at 37°C for 48 h. These 10 strains were the active Campylobacter stock solutions used in this study.
Campylobacter under acid conditions.
For each of the 10 Campylobacter strains, 1 ml of the active stock solution was transferred to 4 ml of MH broth, with an adjusted pH of 4 by using formic acid (analytical grade) (Merck KgaA, Darmstadt, Germany). The acid condition was used throughout the experiments. The cultures were kept at 37°C under anaerobic conditions by using a Gas Pak Plus envelope with palladium catalyst (BBL; Becton Dickinson) to prevent exposure of the Campylobacter to the excess oxygen atmosphere in the jars. As a general method, 1 ml of each treated culture was taken and diluted 10-fold, by putting the 1-ml culture sample in 9 ml of buffer peptone water (BPW) (10 g of peptone, 5 g of NaCl, 4.5 g of Na2HPO4 · 2H2O, and 1.5 g of KH2PO4 [all amounts given per liter]), after which 0.1 ml of each diluted suspension was placed onto Colombia agar plate supplemented with horse blood for 0, 0.5, 1, 2, and 4 h, respectively. All plates were incubated at 37°C for 72 h under microaerophilic conditions. Typical colonies of Campylobacter were counted and expressed as log10 CFU milliliter−1. This experiment was performed in triplicate.
Resuscitation in liquid medium.
The resuscitation experiment was performed to check the potential recovery of injured or culturable Campylobacter cells after exposure to acid conditions. For each Campylobacter strain, 1 ml of active stock solution was transferred to 4 ml of acidified MH broth. All treated cultures were kept under microaerobic conditions at 37°C for 2 h. The cultures were then centrifuged in a Centrifuge 5415C (Merck, Darmstadt, Germany) at 10,000 rpm for 10 min. The pellets were retained and resuspended with 1 ml of phosphate-buffered saline; 0.5 ml of each resuspended culture was then transferred to 9 ml of a modified selective enrichment medium, charcoal-cefoperazone-deoxycholate broth (CCDB), which contains the same ingredients as the CCDA plates (4) but without the addition of antibiotics. All resuspended cultures in CCDB were microaerophilically incubated at 37 or 43°C for 2 days. After incubation, 1 ml of the resuspended culture in CCDB was taken and put into 9 ml of BPW, thereby diluting it 10-fold. Samples (0.1 ml) of the diluted and undiluted suspension were plated on CCD blood agar plates. The plates were microaerophilically incubated at 37 or 43°C for another 2 days. Typical colonies of the bacteria were examined and counted. This experiment was done in duplicate.
Direct culturable, total, and viable cell counts.
Campylobacter strains C350, C4602, and C144 were randomly selected to study and compare the numbers of culturable, total, and viable cells after exposure to acid conditions. One milliliter of each active culture strain was transferred to 4 ml of the acidified MH broth. Campylobacter samples were taken at 0, 1, 2, and 4 h, and the samples were processed as described below for culturable, active or viable, and total cell counts. For culturable cell counts, 1 ml of each Campylobacter strain in the acid solution was put into 9 ml of BPW, thereby diluting it 10-fold, and 0.1 ml of the diluted solution was placed on Columbia blood agar plates. The plates were microaerophilically incubated at 37°C for 3 days. Colonies were counted and compared to the original concentration; counts are in log CFU milliliter−1. The remaining solution (4 ml) was centrifuged at 10,000 rpm for 10 min. The pellets were collected to determine total and viable cell counts by using a double-staining (5-cyano-2,3-ditolyl tetrazolium chloride [CTC]-4′,6′-diamidino-2-phenylindole [DAPI]) technique described by Cappelier et al. (7). Results were expressed as the number of corresponding bacteria per milliliter of the original sample. This experiment was performed in triplicate.
Resuscitation via SPF embryonated chicken eggs.
For each of the 10 Campylobacter strains, 1 ml of active stock solution was transferred to 4 ml of the acidified MH broth. Three 9-day-old SPF embryonated eggs were aseptically injected with 0.5 ml of each treated Campylobacter strain. All inoculated eggs were kept at 37°C on shaking trays for 3 days. Thereafter, the eggshell was gently peeled off. Yolk sac and amniotic fluid were carefully separated immediately. Fifty microliters of the yolk sac fluid was then taken and streaked onto Columbia blood agar plates with a sterilized swab, while 50 μl of amniotic fluid was spread onto Columbia blood agar plates. The plates were incubated at 37°C under microaerophilic atmosphere for 5 days. The positive-control group was inoculated with Campylobacter solution without acid treatment, and the negative-control group was inoculated with 0.5 ml of formic acid without Campylobacter. Typical colonies of each Campylobacter strain were counted per plate. The identification of Campylobacter was done with a phase-contrast microscope when necessary.
Survival of Campylobacter in acidified MH broth.
The culturability of each of the 10 Campylobacter strains rapidly decreased during 1 h of incubation with formic acid at pH 4. After a 2-h incubation period, the bacteria could not be cultured. These results were similar to those of our previous study, in which formic acid was in a mixture of organic acids and feed (9). It has been confirmed that formic acid at pH 4 inhibits Campylobacter growth. The use of acidified water (organic acids) as a decontamination method is widespread in food industries and animal husbandry in an effort to control pathogenic agents. However, the failure of acidified water to prevent growth of pathogenic agents has also been reported (1, 5, 25). The lack of effectiveness of acidified water may be influenced by the short exposure time, trace amounts of the remaining acid, or cell survival traits.
Recovery of Campylobacter by CCD broth.
Injured cells may recover under appropriate conditions, such as enrichment media and a microaerophilic atmosphere. All undiluted (original) and diluted suspensions of each treated strain in CCDB were spread onto the CCD agar plates supplemented with blood, and no growth was observed on these plates for all 10 Campylobacter strains at different temperatures.
Total, culturable, and viable cell count results.
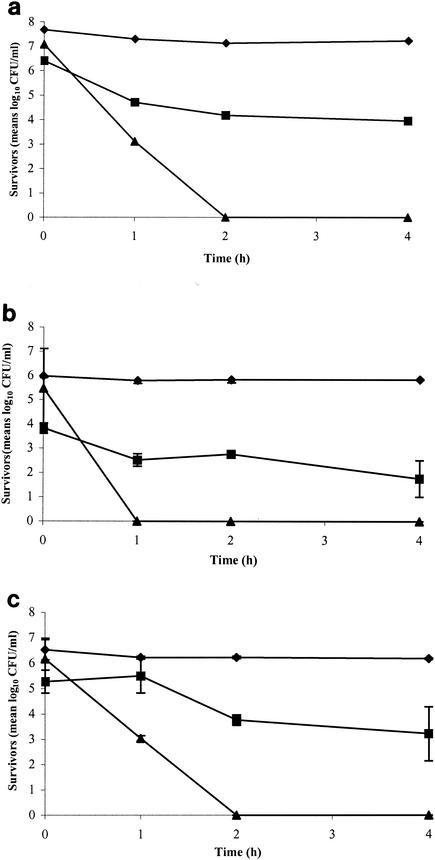
Fig. 1a, b, and c show the total, culturable, and viable cell counts for strains C4602, C144, and C350, respectively. The total cell counts of these three Campylobacter strains were constant throughout the 4-h incubation time. At the beginning, the culturable cells of C4602, C144, and C350 were at 7.08 ± 0.05, 5.47 ± 1.65, and 6.18 ± 0.81 log10 CFU ml−1, respectively. The culturability of C144 cells could not be observed on the spread blood agar plate after 1 h under acid conditions, while the culturability of C4602 and C350 dramatically decreased by 3.97 and 3.15 log10 CFU ml−1, respectively. After incubation for 2 h, the culturability of these strains could not be observed. It has been demonstrated that microcosm water can induce Campylobacter to change to the VBNC form (7, 14, 21, 30, 31). To demonstrate the formation of the VBNC form of Campylobacter under acid conditions, the double staining (CTC-DAPI) technique was used (7). The results demonstrated that Campylobacter cells could enter the VBNC stage under acid conditions, while no colony growth was observed. The numbers of viable cells of the three strains decreased slightly over time (Fig. 1). After 2 h of incubation, the numbers of viable cells of strains C4602 and C350 were 4.18 ± 0.24 and 3.76 ± 0.20 log10 CFU ml−1, respectively, whereas the number of viable C144 cells was 2.76 ± 0.07 log10 CFU ml−1. At the end of the experiment, the numbers of viable cells of C4602, C144, and C350 remained at 3.96 ± 0.00, 1.76 ± 0.00, and 3.22 ± 0.01 log10 CFU ml−1, respectively. Each Campylobacter strain responded to the acid differently. Nevertheless, the number of Campylobacter (viable cells) after exposure to acid in our experiment was less than those in other studies (e.g., number after starvation) (6-8, 14, 21, 26). Probably, in our experiment, the undissociated acid form of formic acid could diffuse into the cells and destroy DNA synthesis (10) and/or could cause loss of outer cell membrane (9), which would yield a reduced number of VBNC.
FIG. 1.
Numbers of total (⧫), viable (▪), and culturable (▴) cells of C. coli C4602 (a), C. jejuni C144 (b), and C. jejuni C350 (c) over time. The bacteria were incubated with acidified MH broth (formic acid at pH 4), and the double-staining (CTC-DAPI) and culture technique was used.
Recovery of Campylobacter via SPF embryonated eggs.
The recovery experiment demonstrated that after exposure to acidity for 2 h, 4 of the 10 Campylobacter strains were resuscitated via embryonated eggs successfully. In the positive-control group, 8 of the 10 Campylobacter strains could be found in the yolk sac in higher numbers than in the amniotic fluid (Table 1). It is extremely difficult to be 100% sure that no culturable cells were inoculated into the eggs, but the recovery experiment of treated Campylobacter cells, after 2 h of incubation, in the enrichment liquid media demonstrated that no culturable cells remained in the acid solution. Our experiment is the first to demonstrate that Campylobacter under acid conditions, where the cells were probably in the VBNC stage, can resuscitate in embryonated eggs. Comparing the results from the three strains, a higher number of VBNC cells may give more probability of recovery. This explains the unsuccessful resuscitation when the number of VBNC cells was low (8). The recovery may depend on the animal model and the strain used. Studies have shown that some researchers have been successful in recovering VBNC form (6, 19, 27, 29) while some have had no success (3, 20, 22, 32). Our results revealed the successful resuscitation of VBNC cells via both the allantoic and yolk sac routes of embryonated eggs. The yolk sac, containing sufficient nutrients and growth factors, yielded more colonies of Campylobacter than the allantoic sac, where biological defensive lysozymes and a high pH are present (16, 18). These results are in agreement with the experimental results of Cappelier et al. (8), in which passage in embryonated eggs is the preferable model for recovery of the VBNC stage of Campylobacter. In conclusion, our findings indicate that Campylobacter can enter into a nonculturable (VBNC) stage under acid conditions. Moreover, the VBNC stage can be resuscitated after passage through embryonated eggs. These results could help to explain the failure of the use of acidified water (using organic acids) to control Campylobacter at the farm level or in the slaughter process. From the epidemiological point of view, these findings demonstrate that the spread of Campylobacter through acidified drinking water can still be the source of recontamination in commercial chicken-rearing houses.
TABLE 1.
Colonization of 10 strains of Campylobacter cells treated with formic acid at pH 4 via two injection routes of SPF embryonated chicken eggs
| Campylobacter straina | No. of fertilized eggs colonizedb/ no. of inoculated eggs
|
||
|---|---|---|---|
| Amniotic fluid | Yolk sac | Total | |
| Treated group | |||
| C4596 | 0/3 | 0/3 | 0/6 |
| C2150 | 0/3 | 0/3 | 0/6 |
| C4601 | 0/3 | 0/3 | 0/6 |
| C2146 | 3**/3 | 3***/3 | 6/6 |
| C186 | 3**/3 | 3**/3 | 6/6 |
| C350 | 3**/3 | 3**/3 | 6/6 |
| C591 | 0/3 | 0/3 | 0/6 |
| C690 | 0/3 | 0/3 | 0/6 |
| C4602 | 2**/3 | 2**/3 | 4/6 |
| C144 | 0/3 | 0/3 | 0/6 |
| Formic solution (negative control) | 0/3 | 0/3 | 0/6 |
| Positive-control group | |||
| C4596 | 2**/2 | 2**/2 | 4/4 |
| C2150 | 2**/2 | 2***/2 | 4/4 |
| C4601 | 2**/2 | 2***/2 | 4/4 |
| C2146 | 2**/2 | 2***/2 | 4/4 |
| C186 | 2**/2 | 2***/2 | 4/4 |
| C350 | 2*/2 | 2**/2 | 4/4 |
| C591 | 2*/2 | 2**/2 | 4/4 |
| C690 | 2**/2 | 2***/2 | 4/4 |
| C4602 | 2*/2 | 2**/2 | 4/4 |
| C144 | 2*/2 | 2*/2 | 4/4 |
C. coli strains C4596, C4601, and C4602 and C. jejuni strains C2150, C2146, C186, C350, C591, C690, and C144 were studied. Campylobacter strains are available from and were provided by the ID-DLO Institute of Animal Science and Health, Lelystad, The Netherlands.
The number of colonies formed is indicated as follows: *, less than 5 CFU per plate; **, between 6 and 50 CFU per plate; ***, more than 51 CFU per plate.
Acknowledgments
We greatly appreciate the excellent technical assistance of Thaweesak Songserm and Dirk van Roozelaar.
This study was funded in part by financial support from the Royal Thai government (to P.C.).
REFERENCES
- 1.Al-Chalaby, Z. A., M. H. Hinton, and A. H. Linton. 1985. Failure of drinking water sanitisation to reduce the incidence of natural salmonella in broiler chickens. Vet. Rec. 116:364-365. [DOI] [PubMed] [Google Scholar]
- 2.Altekruse, S. F., N. J. Stern, P. I. Fields, and D. L. Swerdlow. 1999. Campylobacter jejuni-an emerging foodborne pathogen. Emerg. Infect. Dis. 5:28-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beumer, R. R., J. de Vries, and F. M. Rombouts. 1992. Campylobacter jejuni non-culturable coccoid cells. Int. J. Food Microbiol. 15:153-163. [DOI] [PubMed] [Google Scholar]
- 4.Bolton, F. J., D. N. Hutchinson, and D. Coates. 1984. Blood-free selective medium for isolation of Campylobacter jejuni from feces. J. Clin. Microbiol. 19:169-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Byrd, J. A., B. M. Hargis, D. J. Caldwell, R. H. Bailey, K. L. Herron, J. L. McReynolds, R. L. Brewer, R. C. Anderson, K. M. Bischoff, T. R. Callaway, and L. F. Kubena. 2001. Effect of lactic acid administration in the drinking water during preslaughter feed withdrawal on Salmonella and Campylobacter contamination of broilers. Poult. Sci. 80:278-283. [DOI] [PubMed] [Google Scholar]
- 6.Cappelier, J. M., C. Magras, J. L. Jouve, and M. Federighi. 1999. Recovery of viable but non-culturable Campylobacter jejuni cells in two animal models. Food Microbiol. 16:357-383. [Google Scholar]
- 7.Cappelier, J. M., B. Lazaro, A. Rossero, A. Fernandez-Astorga, and M. Federighi. 1997. Double staining (CTC-DAPI) for detection and enumeration of viable but non-culturable Campylobacter jejuni cells. Vet. Res. 28:547-555. [PubMed] [Google Scholar]
- 8.Cappelier, J. M., J. Minet, C. Magras, R. R. Colwell, and M. Federighi. 1999. Recovery in embryonated eggs of viable but nonculturable Campylobacter jejuni cells and maintenance of ability to adhere to HeLa cells after resuscitation. Appl. Environ. Microbiol. 65:5154-5157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chaveerach, P., D. A. Keuzenkamp, H. A. Urlings, L. J. Lipman, and F. van Knapen. 2002. In vitro study on the effect of organic acids on Campylobacter jejuni/coli populations in mixtures of water and feed. Poult. Sci. 81:621-628. [DOI] [PubMed] [Google Scholar]
- 10.Cherrington, C. A., M. Hinton, G. R. Pearson, and I. Chopra. 1991. Short-chain organic acids at pH 5.0 kill Escherichia coli and Salmonella spp. without causing membrane perturbation. J. Appl. Bacteriol. 70:161-165. [DOI] [PubMed] [Google Scholar]
- 11.Cudjoe, K. S., and G. Kapperud. 1991. The effect of lactic acid sprays on Campylobacter jejuni inoculated onto poultry carcasses. Acta Vet. Scand. 32:491-498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cuk, Z., A. Annan-Prah, M. Janc, and J. Zajc-Satler. 1987. Yoghurt: an unlikely source of Campylobacter jejuni/coli. J. Appl. Bacteriol. 63:201-205. [DOI] [PubMed] [Google Scholar]
- 13.Dickens, J. A., and A. D. Whittemore. 1997. Effects of acetic acid and hydrogen peroxide application during defeathering on the microbiological quality of broiler carcasses prior to evisceration. Poult. Sci. 76:657-660. [DOI] [PubMed] [Google Scholar]
- 14.Ferderighi, M., J. L. Tholozan, J. M. Cappelier, J. P. Tissier, and J. L. Jouve. 1998. Evidence of non-coccoid viable but non-culturable Campylobacter jejuni cells in microcosm water by direct viable count, CTC-DAPI double staining, and scanning electron microscopy. Food Microbiol. 15:539-550. [Google Scholar]
- 15.Fletcher, R. D., A. C. Albers, A. K. Chen, and J. N. Albertson, Jr. 1983. Ascorbic acid inhibition of Campylobacter jejuni growth. Appl. Environ. Microbiol. 45:792-795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Freeman, B. M. 1984. Physiology and biochemistry of the domestic fowl, vol. 5. Academic Press, London, United Kingdom.
- 17.Hazeleger, W. C., J. D. Janse, P. M. Koenraad, R. R. Beumer, F. M. Rombouts, and T. Abee. 1995. Temperature-dependent membrane fatty acid and cell physiology changes in coccoid forms of Campylobacter jejuni. Appl. Environ. Microbiol. 61:2713-2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hughey, V. L., and E. A. Johnson. 1987. Antimicrobial activity of lysozyme against bacteria involved in food spoilage and food-borne disease. Appl. Environ. Microbiol. 53:2165-2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jones, D. M., E. M. Sutcliffe, and A. Curry. 1991. Recovery of viable but non-culturable Campylobacter jejuni. J. Gen. Microbiol. 137:2477-2482. [DOI] [PubMed] [Google Scholar]
- 20.Korsak, D., and J. Popowski. 1997. Campylobacter jejuni in coccoid form does not reverse into spiral form in chicken guts. Acta Microbiol. Pol. 46:409-412. [PubMed] [Google Scholar]
- 21.Lazaro, B., J. Carcamo, A. Audicana, I. Perales, and A. Fernandez-Astorga. 1999. Viability and DNA maintenance in nonculturable spiral Campylobacter jejuni cells after long-term exposure to low temperatures. Appl. Environ. Microbiol. 65:4677-4681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Medema, G. J., F. M. Schets, A. W. van de Giessen, and A. H. Havelaar. 1992. Lack of colonization of 1 day old chicks by viable, non-culturable Campylobacter jejuni. J. Appl. Bacteriol. 72:512-516. [DOI] [PubMed] [Google Scholar]
- 23.Pearson, A. D., M. Greenwood, T. D. Healing, D. Rollins, M. Shahamat, J. Donaldson, and R. R. Colwell. 1993. Colonization of broiler chickens by waterborne Campylobacter jejuni. Appl. Environ. Microbiol. 59:987-996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reezal, A., B. McNeil, and J. G. Anderson. 1998. Effect of low-osmolality nutrient media on growth and culturability of Campylobacter species. Appl. Environ. Microbiol. 64:4643-4649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Refregier-Petton, J., N. Rose, M. Denis, and G. Salvat. 2001. Risk factors for Campylobacter spp. contamination in French broiler-chicken flocks at the end of the rearing period. Prev. Vet. Med. 50:89-100. [DOI] [PubMed] [Google Scholar]
- 26.Rollins, D. M., and R. R. Colwell. 1986. Viable but nonculturable stage of Campylobacter jejuni and its role in survival in the natural aquatic environment. Appl. Environ. Microbiol. 52:531-538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saha, S. K., S. Saha, and S. C. Sanyal. 1991. Recovery of injured Campylobacter jejuni cells after animal passage. Appl. Environ. Microbiol. 57:3388-3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Skirrow, M. B. 1991. Epidemiology of Campylobacter enteritis. Int. J. Food Microbiol. 12:9-16. [DOI] [PubMed] [Google Scholar]
- 29.Stern, N. J., D. M. Jones, I. V. Wesley, and D. M. Rollins. 1994. Colonization of chicks by non-culturable Campylobacter spp. Lett. Appl. Microbiol. 18:333-336. [Google Scholar]
- 30.Talibart, R., M. Denis, A. Castillo, J. M. Cappelier, and G. Ermel. 2000. Survival and recovery of viable but noncultivable forms of Campylobacter in aqueous microcosm. Int. J. Food Microbiol. 55:263-267. [DOI] [PubMed] [Google Scholar]
- 31.Tholozan, J. L., J. M. Cappelier, J. P. Tissier, G. Delattre, and M. Federighi. 1999. Physiological characterization of viable-but-nonculturable Campylobacter jejuni cells. Appl. Environ. Microbiol. 65:1110-1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van de Giessen, A. W., C. J. Heuvelman, T. Abee, and W. C. Hazeleger. 1996. Experimental studies on the infectivity of non-culturable forms of Campylobacter spp. in chicks and mice. Epidemiol. Infect. 117:463-470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van Netten, P., J. Huis in't Veld, and D. A. Mossel. 1994. An in-vitro meat model for the immediate bactericidal effect of lactic acid decontamination on meat surfaces. J. Appl. Bacteriol. 76:49-54. [DOI] [PubMed] [Google Scholar]
- 34.Waterman, S. R., and P. L. Small. 1998. Acid-sensitive enteric pathogens are protected from killing under extremely acidic conditions of pH 2.5 when they are inoculated onto certain solid food sources. Appl. Environ. Microbiol. 64:3882-3886. [DOI] [PMC free article] [PubMed] [Google Scholar]