Abstract
Anomalous origination of a coronary artery can have serious, even fatal, consequences. Intravascular ultrasonography has recently provided new insights into anomalous coronary artery origination from the opposite sinus of Valsalva. On the basis of these insights, we describe 3 typical forms of this anomaly with left coronary artery involvement, including clinical presentations, diagnostic methods (particularly intravascular ultrasonography), and details of surgical treatment. In this case series, the left coronary artery originated from the noncoronary sinus in 1 patient and from the right sinus in another patient. In the 3rd patient, both the left and right coronary arteries originated from the ascending aorta above the sinotubular junction. Baseline areas of stenosis ranged from 48.6% to 70.1%. Intravascular ultrasonography was the only method that enabled us to clarify the mechanisms and the severity of the anomaly. Pharmacologic challenge was useful to predict worsening that might have occurred under physiologic conditions.
We found that, in cases of symptomatic left anomalous coronary artery origination from the opposite sinus of Valsalva, the proximal segment of the left coronary artery consistently has 1) an intramural course inside the aortic wall; 2) hypoplasia, as determined by its circumference; and 3) a cross-sectional ovaloid deformity (lateral compression) with phasic and exercise-induced worsening of the deformity. With regard to surgical treatment, ostioplasty is preferable to coronary bypass.
To establish sound guidelines for managing these anomalies, a larger series should be studied prospectively with quantitative parameters and long-term follow-up.
Key words: Anomalous left coronary artery; coronary angiography; coronary artery bypass; coronary vessel anomalies/classification/diagnosis/pathology/surgery/ultrasonography; death, sudden, cardiac/prevention & control; diagnostic imaging/methods; magnetic resonance imaging; myocardial ischemia/mechanism
Coronary artery anomalies are a heterogeneous group of clinical entities, which are still in the process of nosologic and clinical assessment. Whereas some coronary anomalies are clearly anatomic variants without much clinical relevance, others can cause severe cardiac symptoms and death.1 Anomalous origination of a coronary artery from an opposite sinus of Valsalva (ACAOS)1 comprises a subset of coronary anomalies that may have severe prognostic implications.2–4 In a recent study of 6.3 million U.S. Army recruits, Eckart and associates5 reviewed the medical records and autopsy reports from 277 nontraumatic fatal events and found that 21 of 64 cardiac deaths (34%) were due to coronary anomalies. No coronary anomaly other than anomalous origination of the left coronary artery from the right sinus of Valsalva (left ACAOS) was associated with cardiac death.
A small, specific subgroup of ACAOS cases—those that have an anomalous course of the ectopic coronary artery within the wall of the aortic root, with intussusception—may entail a severe prognosis and require aggressive treatment.1,2 Whereas origination of the right coronary artery from the left sinus of Valsalva is a more common anomaly and its prognosis and treatment better known (although still an open topic in cardiology5–7), left ACAOS is less frequent and its treatment less clearly codified, especially in the adult, sedentary carrier.
To reconsider the clinical presentations, diagnostic methods, and surgical techniques of left ACAOS, we present 3 cases of this anomaly (all involving intussusception of the proximal trunk into the aortic wall). The left coronary artery originated from the noncoronary sinus in one, the right sinus in the second, and the ascending aorta in the third.
Representative Cases
Patient 1
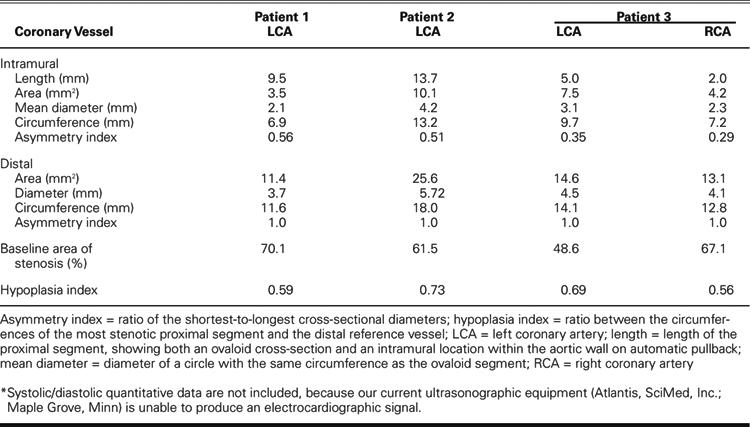
In August 2005, a 67-year-old woman underwent a 2nd cardiologic evaluation related to the recent onset of progressive dyspnea. She had diabetes mellitus and hyperlipidemia. Three years before the current admission, after undergoing elective hip replacement, the patient had presented with syncope, accompanied by ST-segment depression and creatine kinase (CK) elevation (peak, 1,000 U/L; myocardial-band fraction [CK-MB], 12%), which was considered at the time to be consistent with the diagnosis of non-Q-wave myocardial infarction (MI). Nuclear adenosine imaging showed anterior, anterolateral, and apical reversible defects, with a normal ejection fraction (0.65). Heart catheterization showed mild, diffuse coronary plaquing, and revealed that the left main coronary artery originated anomalously from the noncoronary sinus, just to the right of the posterior aortic commissure (Fig. 1). The ectopic ostium could be selectively cannulated only with an Amplatz right coronary curve catheter (4 other catheters failed during a more-than hour-long attempt). Intravascular ultrasonography (IVUS) confirmed the presence of the anomaly and clearly revealed its specific features (Fig. 2; Table I): the left main artery had a slit-like, tangential ostium and an intramural course (with intussusception into the aortic wall) involving a 24-mm-long, retroaortic segment that was hypoplastic (luminal area, 3.5 mm2). The distal, free, left main trunk had an area of 11.4 mm2, indicating a proximal 70% stenosis of the cross-sectional area. Nuclear cardiac magnetic resonance imaging (MRI) confirmed the anomalous origin but could not corroborate any degree of proximal left main trunk stenosis or hypoplasia. The patient was offered surgical treatment to alleviate dyspnea and reversible left ventricular ischemia and to prevent recurrent myocardial infarction and sudden death.
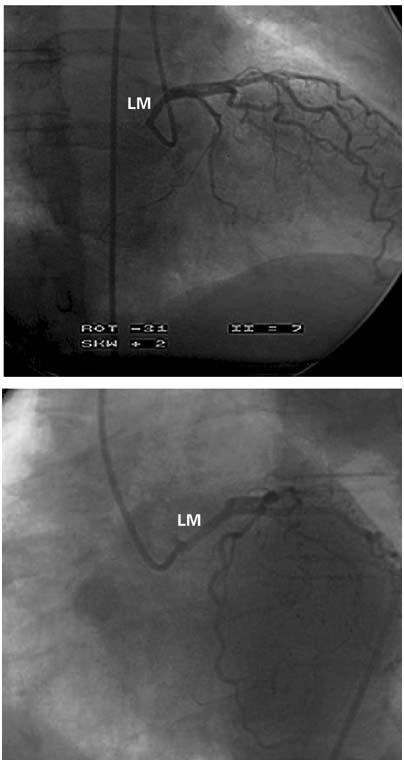
Fig. 1 Case 1. Selective left coronary angiogram in the right anterior oblique (A) and left anterior oblique projections (B). The left main trunk is unusually long and originates quite posteriorly. Subselective injections provided clear evidence that the ostium was located just to the right of the posterior commissure, at the noncoronary sinus (not shown). In (A), it is apparent that the proximal left coronary artery is located at the posterior border of the aortic root, which corresponds to the posterior commissure, in the right anterior oblique projection.
LM = left main
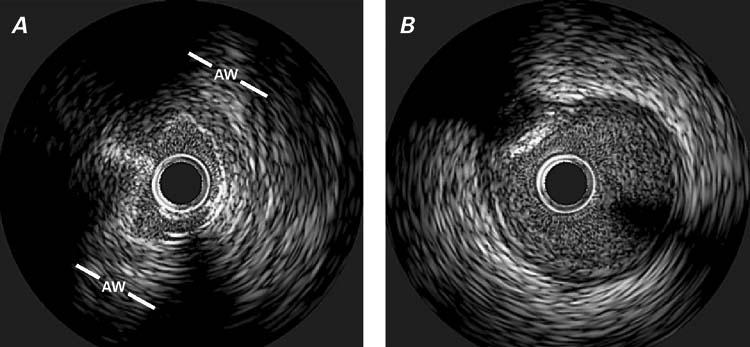
Fig. 2 Case 1. Intravascular ultrasonographic images of the left main trunk at the intramural (aortic) segment (A) and the more distal (epicardial) segment (B). In (A), note the reduced lumen of the proximal segment in the absence of an atherosclerotic intimal build-up, which is present in the distal segment (in [B] at 10 o'clock there is a calcific plaque).
AW = aortic wall
TABLE I. Intravascular Ultrasonography: Quantitative Data*
The patient first chose to try a 4-month enhanced medical regimen that included clonidine (0.1 mg twice a day), amlodipine (5 mg/day), benazepril (10 mg/day), losartan (100 mg/day), hydrochlorothiazide (25 mg/day), and verapamil (200 mg/day); however, this therapy did not relieve her symptoms. Therefore, she underwent surgical repair with the aid of cardiopulmonary bypass. After the aortic root was entered, the intramural, intussuscepted, left main proximal coronary artery was unroofed by transection of the anterior wall of the coronary artery horizontally through the uppermost portion of the posterior aortic-valve commissure, which required resuspension. Postoperative-ly, the patient's condition improved from New York Heart Association (NYHA) functional class III to II. She had residual dyspnea but no chest pain. During the first 18 postoperative months, progressive renal failure developed (serum creatinine level, 1.9 mm/dL) with fluid retention, because of diabetic nephropathy. A new adenosine nuclear stress test revealed no myocardial ischemia and normal left ventricular systolic function. Mild aortic insufficiency was noted on angiography. Selective coronary angiography was readily accomplished with a Judkins left coronary catheter. Repeat IVUS demonstrated excellent relief of the proximal left main stenosis.
Patient 2
In May 2003, a 50-year-old woman was transferred to our institution because of recent-onset unstable angina and dyspnea. The patient had a history of hypercholesterolemia and cigarette smoking and a family history of coronary artery disease. Over the course of several months before the current admission, she had gained 70 pounds and had developed severe hypertension and dyspnea. During the 2 weeks before admission, she had developed angina with transient T-wave changes, which occurred even at rest and lasted for several hours. These episodes had prompted 2 emergency hospital admissions. Coronary angiography showed no apparent coronary obstructive lesions but did reveal a single coronary ostium at the right sinus of Valsalva. The left coronary artery originated from a mixed trunk and coursed anteriorly between the aorta and the pulmonary artery, at the level of the sinotubular junction (Fig. 3). The left ventricular ejection fraction was 0.45. Cardiac MRI could not confirm the intramural aortic course of the left main trunk but confirmed the other angiographic findings related to the ectopic left coronary artery. Only on IVUS did it become evident that the proximal left coronary artery had a smaller cross-sectional area and circumference (Fig. 4; Table I), with an ovaloid cross-section inside the aortic wall. The intussuscepted coronary segment was about 2 cm long and was followed by a larger left main segment, which again veered 90° away from the aorta, becoming round and having a much larger cross-sectional area than that of the intramural segments (Fig. 4; Table I).
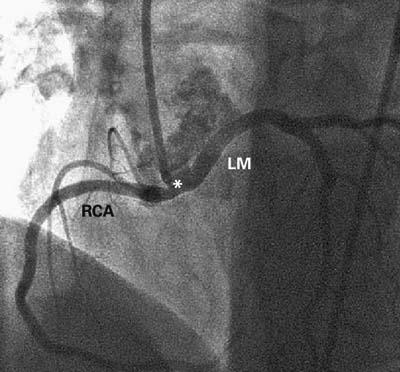
Fig. 3 Case 2. Selective angiogram of the common coronary artery in the left anterior oblique cranial projection. The common trunk (*) is short (about 10 mm) and arises orthogonally from the aortic wall before bifurcating into the right coronary artery (RCA), which has a normal course, and the anomalous left main artery (LM), which wraps around the aortic root.
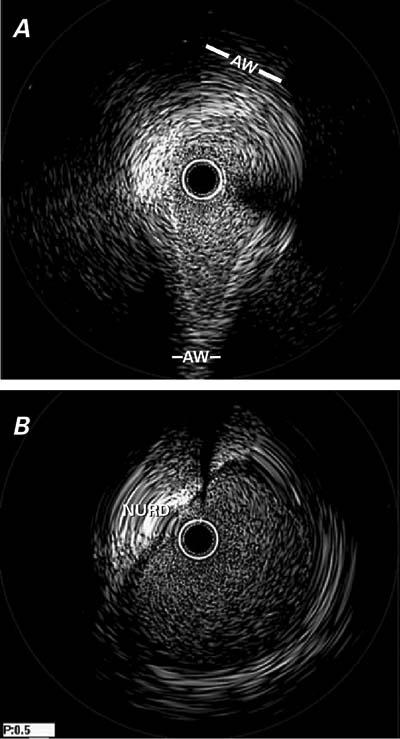
Fig. 4 Case 2. Intravascular ultrasonographic images of the intramural (A) and extramural (B) segments of the left main trunk. In (A), note the severe hypoplasia in the proximal segment (which is not apparent on the angiogram in Fig. 3), the absence of intimal thickening, and the ovaloid cross-section; in contrast, the distal left main artery has a round lumen and an intimal plaque (B). See text and Table I for further details.
AW = aortic wall; NURD = non-uniform rotational distortion
Intravascular ultrasonography included pharmacologic challenge with saline solution (500 mL over 15 minutes) and dobutamine (up to 40 μg/kg/min), in accordance with a prospective research protocol for ACAOS patients approved by our hospital's institutional review board. The maximal challenge elicited typical, sustained chest pain requiring a narcotic agent, accompanied by ventricular tachycardia with syncope that was converted to sinus rhythm by means of electric shock. The patient underwent surgery because of the severe, disabling symptoms and the high risk of sudden death. The operation, performed with the aid of cardiopulmonary bypass, confirmed the described coronary anomaly. Because the proximal mixed trunk and the initial 5 mm of the left main stem were free of the aortic wall, unroofing of these coronary segments would have been problematic (and would also have damaged the anterior aortic commissure). Therefore, a new, large, left main coronary artery ostium was created by opening a window into the inner aortic wall and the posterior wall of the intramural left coronary artery, just proximal to the point of separation from the aortic wall (Fig. 5). The patient recovered uneventfully and remained free of angina and dyspnea. At her most recent follow-up evaluation, 15 months postoperatively, she was asymptomatic. She declined to undergo a scheduled follow-up nuclear stress test.
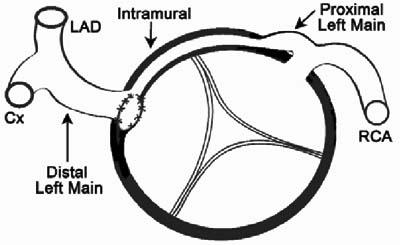
Fig. 5 Case 2. Diagram of the surgical repair shows a cross-sectional view of the aortic root. The new, surgically-created ostium provides unrestricted direct access to the extramural portion of the left main artery, which is distal to the intramural hypoplastic proximal segment.
Cx = circumflex artery; LAD = left anterior descending coronary artery; RCA = right coronary artery
Patient 3
In January 2005, a 53-year-old woman was referred to us because of a long history of chest pain that was typical of angina in location but unrelated to exertion. The patient also had dyspnea and had lately developed mild hypertension. She had recently undergone a nuclear stress test that showed reversible inferolateral ischemia. Two years before the present admission, she had undergone a radical mastectomy, chemotherapy, and radiotherapy for metastatic breast cancer. Previously attempted coronary angiography suggested ectopic origination of both coronary arteries, but selective catheterization could not be achieved at either ostium. After the patient was admitted to our institution, successful selective catheterization and angiography were performed with a 6F ART 4.5 guiding catheter (SciMed, Inc.; Maple Grove, Minn). Both coronary arteries originated ectopically, 1 cm above the left sinus of Valsalva (Fig. 6A). They appeared to be intussuscepted, and the left coronary ostium had a membrane-like stenosis (Fig. 6B). Intravascular ultrasonography clearly showed intussusception of both proximal coronary arteries, as well as significant ostial stenosis due to hypoplasia and lateral compression but not to intimal build-up (Fig. 7; Table I). Rapid saline and dobutamine infusion elicited the patient's typical chest pain and dyspnea, with transient ischemic T-wave changes, for the duration of dopamine infusion. During stimulation, on IVUS, 10% worsening of the stenosis was noted in the left proximal intramural segment. The patient was taken to the operating room, where a coronary ostioplasty was performed with the aid of cardiopulmonary bypass. The presence of slit-like stenotic ostia and ectopic origination were confirmed, and both ostia were enlarged longitudinally (unroofed). Postoperatively, there were no complications. At the 8-month follow-up examination, the patient reported having no angina or dyspnea. Routine follow-up nuclear scintigraphy with an adenosine infusion showed that the reversible ischemia had completely resolved. At the 1-year follow-up, she continued to be asymptomatic.
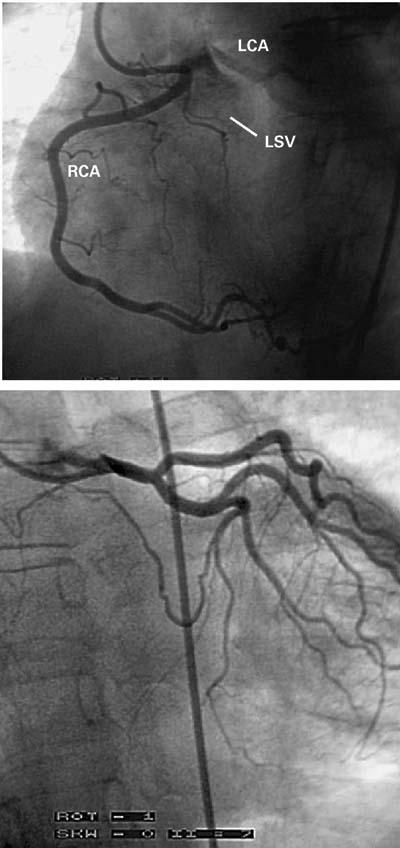
Fig. 6 Case 3. A) Angiogram of the right coronary artery (RCA) in the left anterior oblique projection. Overspilling of the contrast agent identifies the adjacent left coronary ostium (LCA) and the left aortic sinus of Valsalva (LSV). There is no evidence of ostial stenosis. B) Angiogram of the left coronary artery, in the posteroanterior projection, suggests the presence of a membrane-like ostial stenosis.
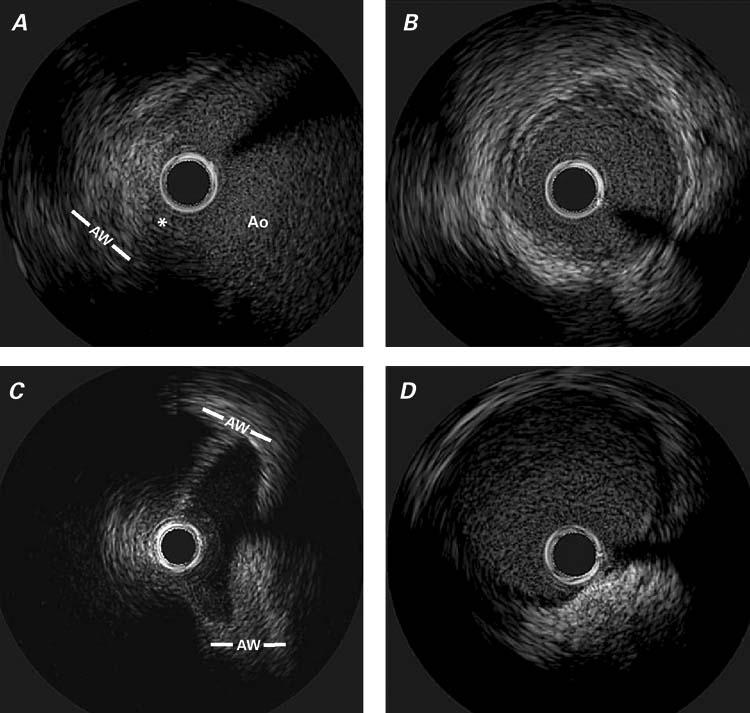
Fig. 7 Case 3. Intravascular ultrasonographic images of the proximal right coronary artery (ostial, A and distal reference, B) segments and the left (C, D) coronary artery. Figure C was obtained during forceful injection of saline (which led to the enhancement of luminal definition). See Table I for related area measurements.
* = ostium; Ao = aortic lumen; AW = aortic wall
Discussion
Clinical Presentation
We recently proposed that there was a clinical benefit in singling out one kind of anomalous origination of a coronary artery from an improper sinus of Valsalva or the ascending aorta, with an intramural course (intussusception into the aortic wall).2 The latter specific feature has been recognized as the likely mechanism of ischemia. We2 proposed the term ACAOS (anomalous origination of a coronary artery from the opposite sinus), which includes cases with the so-called “inter-arterial course” (between the aorta and the pulmonary artery), but also origination from the noncoronary sinus (only the left coronary artery was observed thus far) and the ascending aorta. We further proposed the name “right ACAOS” for cases of anomalous right coronary arteries, and “left ACAOS” for similar cases of anomalous left coronary arteries.
Most of the literature about ACAOS pertains to sudden death in young athletes and soldiers.1–4 The above-mentioned report by Eckart's group4 clarified that, during 8 weeks of intensive military training, 86% of the fatal cardiac accidents (including all of those in left-ACAOS subjects) occurred during or shortly after extreme physical exertion. Atypically, the ages of our 3 female patients at presentation were 50, 53, and 67 years. We theorize that their symptoms may have occurred or worsened because of the onset of hypertension, increased stroke volume, or both. Unfortunately, the exact degree of aortic root dilatation related to aging is not routinely documented. We propose that its quantification be regularly and prospectively pursued in ACAOS patients by means of imaging methods that allow precise systolic and diastolic measurements.2 The postulated pathophysiologic link between progressive aortic dilatation and the onset of symptoms implies increased compression (wall stress) exerted by the dilatation on the intussuscepted coronary segment. To confirm this theory, careful prospective, longitudinal studies will be required.2,8 Our group first attempted to document the severity of a given form of ACAOS by means of IVUS,2 which was performed both at rest and after pharmacologic challenge* aimed at increasing the cardiac output, stroke volume, or both.2,8 Variable aortic dilatation, either at baseline or during exercise or emotional stress, could indeed be the most relevant missing link between a congenital coronary anomaly and the onset of new symptoms in an adult. It is important to note that the clinical expression of ACAOS can occur not only in young athletes (during or after extreme exercise) but also de novo, in sedentary older patients such as ours. In fact, chest pain, dyspnea, and sudden death are known results of similar degrees of left main stenosis due to atherosclerosis.9 The fact that all 3 of the patients presented herein (a continuous though small series of symptomatic left ACAOS patients) were female may suggest a specific liability of this sex to delayed expression of this congenital anomaly, possibly due to aortic root expansion.
Clinical Testing
Recently, cardiologists have begun to believe that, in left-ACAOS patients, the defining characteristic of a poor prognosis is the specific course that the ectopic coronary branch takes in crossing from the abnormal to the normal side of the heart.1,2,7 As in the ectopic right coronary artery, it is not the passage between the aorta and pulmonary artery per se, the slit-like ostium, the ostial ridges, or the tangential origination of an ectopic artery that likely causes clinical manifestations but, rather, the circumstances of the crossing.2,8 Intussusception, which is accompanied by coronary segmental hypoplasia and lateral luminal compression (resulting in an ovaloid cross-section) is the only documented pathophysiologic mechanism of ischemia. In our 3 patients, the IVUS findings were strikingly positive in this respect.
Three features seem to objectively define the severity of an individual case, as evaluated by IVUS:
The amount of hypoplasia of the intramural segment with respect to the distal vessel, as measured by the circumferential length of a cross-section (Table I).
The amount of lateral compression of the intussuscepted segment at baseline, as quantified by the asymmetry index (the ratio of the shortest-to-longest cross-sectional diameters in the ovaloid cross-section) (Table I). Additional, phasic systolic narrowing also occurs at baseline.
The degree of further lateral compression that may occur as the patient ages. In addition, in the catheterization laboratory, a pharmacologic challenge simulating exercise conditions, as with rapid saline, atropine (if needed to reach a heart rate >140 beats/min), and dobutamine infusion (the “SAD” test) can demonstrate further worsening of proximal narrowing during conditions that increase stroke volume.
None of these parameters can be evaluated precisely with traditional contrast angiography, nuclear MRI, or computed tomography.1
Each of our patients (who were all symptomatic) had significant hypoplasia of the intramural segment of the left coronary artery, as shown in Table I. This feature probably resulted from the developmental limitation that affects an intramural artery (which grows within the aortic media) and is aggravated by the phasic lateral compression that the intussuscepted segment undergoes because of increased aortic wall strain during systole, and possibly early diastole. In our patients, the asymmetry index was severely abnormal (average, 0.42; normal, 1.0 in a round lumen) and added substantially to the stenotic effect of hypoplasia by inducing a smaller effective cross-section for the given circumference, as previously discussed.9 During each systolic phase, the shortest diameter narrowed further (by approximately 5%) with respect to resting conditions; it narrowed even more (by 8%–10%) during a simulated exercise protocol using the SAD test. In our patients, the maximum severity of cross-sectional narrowing2 ranged from 48.6% to 70.1% at the intramural left main trunk: these values qualify as significant stenoses of a left main stem according to Coronary Artery Surgery Study (CASS) criteria.10 The most severe degrees of stenosis would likely lead to an early death (even at a neonatal age); the least severe degrees would more probably be compatible with a normal life expectancy and asymptomatic status. Extreme degrees of exertion, as in athletes and military personnel, could increase the ischemic potential of the anomaly, especially in the presence of a compliant aorta.8
Our limited experience with pressure-wire techniques during adenosine challenge2 in right ACAOS did not enable us to identify significant pressure drops, even in patients who were symptomatic and eventually required intervention. This experience is contrary to that described by Lim and associates.11
Although the quest for a clinically significant test with ideal sensitivity and specificity for ACAOS is ongoing, the current investigational algorithm at our8 and other institutions calls for
Clinically identifying patients who have a history of unexplained serious symptoms (recurrent chest pain, dyspnea on exertion, syncope, aborted sudden death, or a combination thereof);
Performing a screening test (cardiac MRI or computed tomographic angiography are the most reliable methods), followed by nuclear treadmill studies and IVUS quantification of stenosis in the selected patients with ACAOS.
Definitive protocols and cutoff values for severe versus mild forms can be established only when sufficient numbers of patients have been tested and empirically followed up for several years in a large, multicenter, prospective study.1
In patients with ACAOS, routine stress testing (even with nuclear myocardial scintigraphy) may be unreliable for the identification of ischemic burden or a prognosis,7,8 either yielding negative results (as often happens when ACAOS is identified by chance during coronary angiography performed for other reasons) or yielding false positive results (as when a positive screening test is the only reason for coronary angiography). In addition, such tests may yield positive results but be incapable of reproducing the presenting symptoms. Our 2nd patient with left ACAOS was so clinically unstable at presentation that a stress test could not be performed before intervention; however, the other 2 patients had positive nuclear stress-test results.
The following facts testify to a cause-and-effect relationship between the coronary anomaly and the symptoms in our patients:
At baseline, significant obstruction of the left main trunk was present.
In patients 2 and 3, the symptoms (angina and dyspnea) were reproducible with the SAD test.
Early after surgical correction in all 3 patients, there was an immediate, substantial improvement in the symptoms.
Treatment
When indicated on the basis of symptoms and IVUS imaging, 2 interventional surgical approaches can be used to treat ACAOS of the left coronary artery: one is local, direct repair of the anomalous origination in the aortic root, and the other is coronary artery bypass surgery.
Bypass surgery is technically feasible with routine techniques but has these disadvantages: vein grafts, because of their limited longevity, are usually a poor choice in these patients, who have a long life expectancy; and arterial conduits tend to atrophy or fail to develop when used to bypass coronary lesions that are not severely obstructive (not causing pressure) at baseline.
Local repair of the anomalous proximal coronary segment has been reported by a few operators,12–16 who became convinced that the critical pathophysiologic mechanism is, indeed, intramural stenosis. Unroofing of the whole intussuscepted segment11–13 or the creation of a new ostium at the distal end of that segment12,13 was the favored option. We chose the unroofing technique for patients 1 and 3; but in patient 2, we created a new ostium. Full unroofing was contraindicated in patient 2, because the intramural segment encroached on the anterior aortic commissure, and the most proximal coronary segment (mixed trunk and left main) was extramural (Fig. 5). In most, if not all, cases of ACAOS that feature a single coronary ostium and a proximal mixed trunk, the proximal trunk is usually extramural in our experience. Creation of a new ostium, at the exact level where the ectopic intussuscepted vessel exits the aortic wall (Fig. 5), is likely the best surgical technique, because it both resolves the stenosis and avoids interfering with aortic valve function, unlike the total unroofing technique.12,13
Less popular surgical approaches consist of relocating a cuff of the aortic wall containing the ectopic orifice into the appropriate side of the aorta (ostial translocation)17 or translocating the pulmonary artery.18
Anomalous origination of a coronary artery from an opposite sinus of Valsalva can occur without intussusception of the ectopic vessel19: when the vessel's course is anterior (prepulmonic), retroaortic, intraseptal (atrial or ventricular), posterior (at the posterior atrioventricular groove), or apical (wrapping around the apex), it is generally extramural and associated with a benign prognosis.
In right ACAOS, stent-angioplasty of the intussuscepted segment has been tried with some early success.2 Technically, left ACAOS could also be treated with stents. However, because of the unknown results (especially late results) of stent use in relatively young individuals with a large myocardial territory at risk, we are postponing this approach until more experience has been gained with right-ACAOS patients and with drug-eluting stents for treating left main stenosis.2
Limitations
This was a hypothesis-generating study comprising just 3 patients; the results cannot be considered definitive until confirmed by large, multicenter studies. Because of technical considerations involving current IVUS software (incapable of identifying systolic times exactly), we did not precisely quantify the degree to which left main obstruction could worsen during SAD testing. We qualitatively observed further phasic narrowing; however, this finding will require precise quantification, because such narrowing could make a borderline stenosis intermittently significant. In this study, we did not consider alternatives to surgery, such as medical treatment or simple follow-up observation, which could be tested in prospective, randomized studies.
Footnotes
*Typically, the saline/atropine/dobutamine protocol (the “SAD” test) increases cardiac output by 250% and stroke volume by about 25% (which could lead to significant aortic-root expansion during systole).
Address for reprints: Paolo Angelini, MD, 6624 Fannin Street, Suite 2780, Houston, TX 77030. E-mail: pangelinimd@houston.rr.com
References
- 1.Angelini P, Velasco JA, Flamm S. Coronary anomalies: incidence, pathophysiology, and clinical relevance. Circulation 2002;105:2449–54. [DOI] [PubMed]
- 2.Hariharan R, Kacere RD, Angelini P. Can stent-angioplasty be a valid alternative to surgery when revascularization is indicated for anomalous origination of a coronary artery from the opposite sinus? Tex Heart Inst J 2002;29:308–13. [PMC free article] [PubMed]
- 3.Cheitlin MD, De Castro CM, McAllister HA. Sudden death as a complication of anomalous left coronary origin from the anterior sinus of Valsalva. A not-so-minor congenital anomaly. Circulation 1974;50:780–7. [DOI] [PubMed]
- 4.Taylor AJ, Byers JP, Cheitlin MD, Virmani R. Anomalous right or left coronary artery from the contralateral coronary sinus: “high-risk” abnormalities in the initial coronary artery course and heterogeneous clinical outcomes. Am Heart J 1997;133:428–35. [DOI] [PubMed]
- 5.Eckart RE, Scoville SL, Campbell CL, Shry EA, Stajduhar KC, Potter RN, et al. Sudden death in young adults: a 25-year review of autopsies in military recruits. Ann Intern Med 2004;141:829–34. [DOI] [PubMed]
- 6.Popma JJ, Bitte J. Coronary angiography and intravascular ultrasonography. In: Braunwald E, Zipes DP, Libby P, editors. Heart disease: textbook of cardiovascular medicine. Philadelphia: WB Saunders; 2001. p. 404–7.
- 7.Basso C, Maron BJ, Corrado D, Thiene G. Clinical profile of congenital coronary artery anomalies with origin from the wrong aortic sinus leading to sudden death in young competitive athletes. J Am Coll Cardiol 2000;35:1493–501. [DOI] [PubMed]
- 8.Angelini P. Coronary artery anomalies—current clinical issues: definitions, classification, incidence, clinical relevance, and treatment guidelines. Tex Heart Inst J 2002;29:271–8. [PMC free article] [PubMed]
- 9.Angelini P, Trivellato M, Donis J, Leachman RD. Myocardial bridges: a review. Prog Cardiovasc Dis 1983;26:75–88. [DOI] [PubMed]
- 10.Coronary artery surgery study (CASS): a randomized trial of coronary artery bypass surgery. Survival data. Circulation 1983;68:939–50. [DOI] [PubMed]
- 11.Lim MJ, Forsberg MJ, Lee R, Kern MJ. Hemodynamic abnormalities across an anomalous left main coronary artery assessment: evidence for a dynamic ostial obstruction. Catheter Cardiovasc Interv 2004;63:294–8. [DOI] [PubMed]
- 12.Mustafa I, Gula G, Radley-Smith R, Durrer S, Yacoub M. Anomalous origin of the left coronary artery from the anterior aortic sinus: a potential cause of sudden death. Anatomic characterization and surgical treatment. J Thorac Cardiovasc Surg 1981;82:297–300. [PubMed]
- 13.Rinaldi RG, Carballido J, Giles R, Del Toro E, Porro R. Right coronary artery with anomalous origin and slit ostium. Ann Thorac Surg 1994;58:829–32. [DOI] [PubMed]
- 14.Nelson-Piercy C, Rickards AF, Yacoub MH. Aberrant origin of the right coronary artery as a potential cause of sudden death: successful anatomical correction. Br Heart J 1990;64:208–10. [DOI] [PMC free article] [PubMed]
- 15.Romp RL, Herlong JR, Landolfo CK, Sanders SP, Miller CE, Ungerleider RM, Jaggers J. Outcome of unroofing procedure for repair of anomalous aortic origin of left or right coronary artery. Ann Thorac Surg 2003;76:589–96. [DOI] [PubMed]
- 16.Karamichalis JM, Vricella LA, Murphy DJ, Reitz BA. Simplified technique for correction of anomalous origin of left coronary artery from the anterior aortic sinus. Ann Thorac Surg 2003;76:266–7. [DOI] [PubMed]
- 17.Di Lello F, Mnuk JF, Flemma RJ, Mullen DC. Successful coronary reimplantation for anomalous origin of the right coronary artery from the left sinus of Valsalva. J Thorac Cardiovasc Surg 1991;102:455–6. [PubMed]
- 18.Rodefeld MD, Culbertson CB, Rosenfeld HM, Hanley FL, Thompson LD. Pulmonary artery translocation: a surgical option for complex anomalous coronary artery anatomy. Ann Thorac Surg 2001;72:2150–2. [DOI] [PubMed]
- 19.Angelini P, Villason S, Chan AV, Diez JG. Normal and anomalous coronary arteries in humans. In: Angelini P, editor. Coronary artery anomalies: a comprehensive approach. Baltimore: Lippincott Williams & Wilkins; 1999. p. 27–150.


