I'd like to give you an overall view of what is going on in stem cell therapy for cardiovascular disease. There are 2 major types of cells to consider (Fig. 1). One is skeletal myoblasts, which are already committed skeletal muscle cell precursors or satellite cells that are present in skeletal muscle. These cells can be harvested and, after 2 or 3 weeks of culture, implanted into (for example) an area of scar in the heart, for the purpose of muscle substitution. On the other side of the stem cell world are cells derived from the bone marrow or from other tissues. These include true stem cells in the sense that they may turn into a variety of tissues.
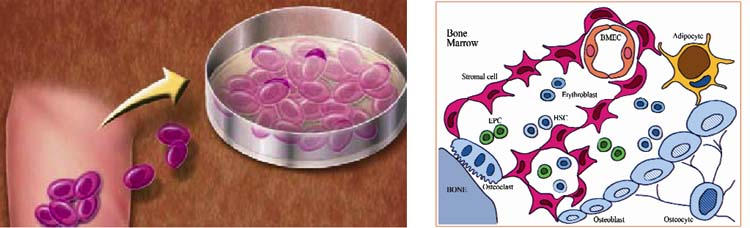
Fig. 1 Cell therapy. Left: Stem cells derived from skeletal myoblasts. Right: Schematic drawing of various stem cell populations found in bone marrow.
There's a lot of effort to understand all the effects of these different cell types, including the potential mechanisms of action.
One of the proposed mechanisms of cellular therapy is angiogenesis; the idea that bone marrow cells may be able to secrete multiple potentially angiogenic substances as well as transdifferentiate into cells that create new blood vessels is fairly well established in pre-clinical studies. Another proposed mechanism is myogenesis, which is a little bit more controversial, especially when we talk about transdifferentiation of bone-marrow-derived cells into heart muscle cells. We know that there may be dedicated precursors of cardiomyocytes in the myocardium. We are still trying to determine if the transplanted cells themselves differentiate, or if paracrine effects from the transplanted cells stimulate stem cells already resident in the heart to differentiate—or if both factors are at work.
Stem cells may be obtained in diverse locations. Aside from the bone marrow, which is traditionally considered a source for stem cells, we may find stem cells in the periphery. For example, we can extract stem cells from fat tissue. There's a lot of ongoing investigation on where to get these cells, how to use them, and exactly which cells work best.
Different approaches can be taken to select cells for therapy. For bone marrow cell transplantation, we can use all the mononuclear cells (which can easily be separated), or we can select endothelial precursor cells (CD34+ cells or AC133 cells, or both), or we can use stromal cells (pluripotent mesenchymal cells that have the ability to turn into any type of tissue). With adult bone marrow, we're generally talking about autologous cells; but you might also think about doing this allogenically, just as with blood transfusions. Of course, the issues of immunogenicity will have to be resolved.
The stem cell program at the Texas Heart Institute is focused primarily on treating heart failure, be it related to a recent myocardial infarction or a chronic disease state. We have done a number of large animal studies with allogenic mesenchymal cells, in both acute and chronic models, in which we explored a variety of delivery modes. We've also just finished some work with bone marrow mononuclear cells (not mesenchymal cells) in a chronic model of heart failure in pigs. This dosing study showed a beneficial effect of certain cell densities and began to answer the key question of what cellular dose is ideal. We're also just starting a cell-trafficking study in which we label the cells so that we can track them in vivo with positron emission tomography and magnetic resonance imaging. We'll be able to see where the cells go and what their fate is.
We have performed an acute study in which we examined 3-dimensional electrical and mechanical mapping of the left ventricle with cell injections, after an infarct. In this model, we created an acute anterior infarct by ligating the left anterior descending coronary artery, which yielded both an electrical and a mechanical defect (Fig. 2, left). Seven days after the infarction, we injected the animal with 100 million allogenic mesenchymal cells at the border zone of the infarct; 2 weeks later, there was significant recovery of both electrical and mechanical function (Fig. 2, right). The same holds true for improvements in wall motion score index and ischemic area, especially when cells are injected transendocardially, rather than by the intracoronary route.
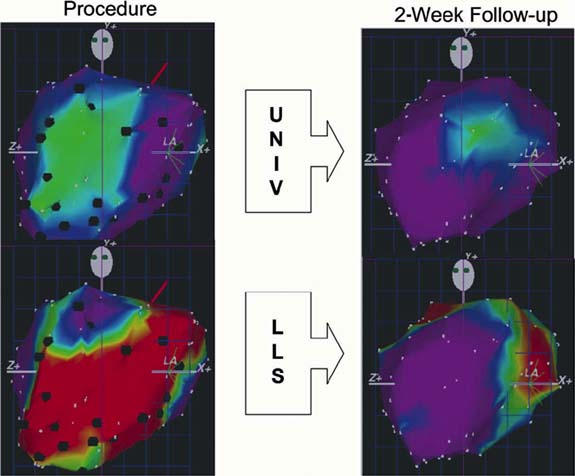
Fig. 2 NOGA™ electrical (UNIV, at top) and mechanical (LLS, at bottom) maps from a canine in the stem cell treatment group. Maps at left are those performed at the time of injection and maps at right are those at 2-week follow-up. The green area in the top left denotes infarcted myocardium with decreased electrical signal. The red area in the bottom left denotes impaired mechanical function corresponding to the infarct. Maps at right show improvement in both electrical and mechanical function.
LLS = linear local shortening; UNIV = unipolar voltage
Looking microscopically, we can identify the different tissues by means of immunohistochemistry: the endothelium is stained for factor VIII, the smooth muscle is stained for actin, and cardiomyocytes are stained for troponin. All the cells that we inject have a red label, which is DI-I; under the microscope, we can see if they co-localize, yielding a yellow color. We actually are pretty confident that we're able to build new arteries using stem cells, because we've shown measurable areas of co-localization in the endothelium and in the smooth muscle walls around blood vessels.
Co-localization is much less prominent in cardiomyocytes, which suggests that transdifferentiation or fusion of the stem cells with resident cardiomyocytes is not the major effect, although it might occur to some degree.
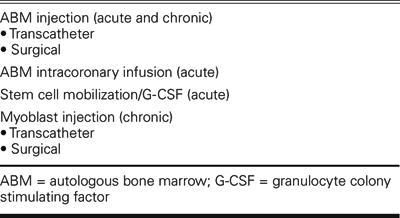
In clinical applications (Table I), autologous bone marrow cells can be injected surgically or delivered with a catheter, in the acute setting after myocardial infarction or in the chronic setting of heart failure or refractory angina. Autologous bone marrow can also be infused down the coronary arteries in the acute setting after myocardial infarction. Finally, substances like G-CSF (granulocyte colony stimulating factor) can be used to stimulate the body to release precursor cells from the bone marrow, which can then be collected for infusion or be allowed to migrate naturally to an area of infarction.
TABLE I. Current Clinical Approaches in Cardiac Stem Cell Therapy
Intracoronary injection of stem cells has been pursued primarily in Europe. The BOOST (BOne marrOw transfer to enhance ST-elevation infarct regeneration) trial—really the only randomized control trial experience out there—involved 60 patients who were treated for about 5 days after myocardial infarction with either autologous bone marrow cells or control therapy. There was a modest but significant increase in left ventricular ejection fraction, with no apparent safety problems in this small experience.
In chronic ischemic heart disease, there are a few studies with small numbers of patients that examine autologous bone marrow cell treatment in the setting of more compromised ventricles. We published a study, in 2003,1 of end-stage patients not eligible for revascularization; 14 patients were treated with direct myocardial injection through a catheter and compared to 7 control patients with 6-month and 1-year follow-up. This example (Fig. 3) shows a patient who had an initial ejection fraction of 0.11 and experienced a very significant improvement in his left ventricular function. No safety issues were noted in this 1st human experience in heart failure. There was no increase in arrhythmias on Holter monitoring, and there were no periprocedural complications. The MVO2 (myocardial oxygen consumption) also showed improvement, particularly when compared with the severely compromised baseline measurement. We're using MVO2 as the primary endpoint of our currently ongoing randomized clinical trial.
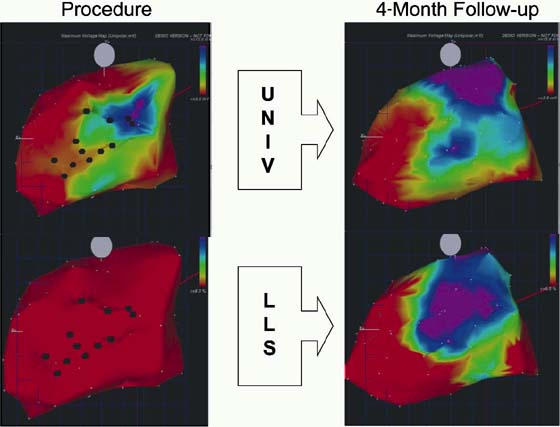
Fig. 3 NOGA™ electrical (UNIV, at top) and mechanical (LLS, at bottom) maps from a human patient in the stem cell treatment group. Maps at left are those performed at the time of injection and maps at right are those at 4-month follow-up. An area of viability, showing normal electrical activity, can be noted on the upper-right map. Maps at the right show improvement in both electrical and mechanical function.
LLS = linear local shortening; UNIV = unipolar voltage
We've also had the unique opportunity to look at the heart of one of the initial Brazilian heart failure patients, who died of unrelated causes after cellular infusion. The treated area had substantially more blood vessels and other unusual histologic features (Fig. 4).
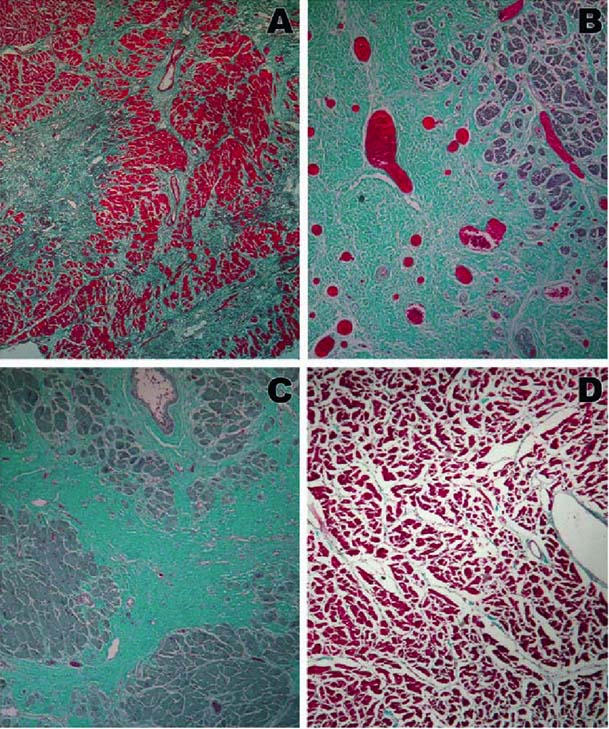
Fig. 4 Gomori trichrome stain of anterolateral (A, B), posterior (C), and septal (D) walls. Increased vascularity is noted in B at the site of prior stem cell injection. Original magnification is ×40 in A, B, and D; ×100 in C.
From: Dohmann HFR, Perin EC, Takiya CM, Silva GV, Silva SA, Sousa ALS, et al. Transendocardial autologous bone marrow mononuclear cell injection in ischemic heart failure: postmortem anatomicopathologic and immunohistochemical findings. Circulation 2005;112:521–6. Reprinted by permission of the American Heart Association.
I'd like to expand a little on our Texas Heart Institute stem cell clinical trial. This is primarily a safety trial, but we're also looking at efficacy endpoints in a single-blinded trial. We are studying severely symptomatic (New York Heart Association functional stage III to IV) patients: 20 treated and 10 control. After the primary 6-month endpoint, if still symptomatic, the control patients can cross over to active therapy. I should note that these patients must have some degree of reversible ischemia, because ischemic tissue is the specific target for cell delivery.
To date, we've randomized 18 patients—12 treated and 6 controls—and 4 of the 6 controls have already crossed over to the active treatment group. We have 2 additional patients scheduled in the next week or two; the trial will probably be finished in the spring of 2006.
In closing, I'd like to report that we have just had another 2 studies approved for initiation by the FDA, one for treatment of postinfarction patients with intracoronary cells and another for treatment with “super cells,” which are selected, very primitive cells with specific markers. Stay tuned; a lot is going to be happening over the next year.
Footnotes
Address for reprints: Emerson C. Perin, MD, PhD, New Interventional Cardiovascular Technology, Southwest Cardiovascular Associates, 6624 Fannin Street, Suite 2220, Houston, TX 77030. E-mail: eperin@crescentb.net
Presented at the Texas Heart Institute's symposium “Evolving Standards in Cardiovascular Care: What Have We Learned? Where Are We Going?” held at the Adam's Mark Hotel; 12 November 2005; Dallas


