Abstract
In 37 Wistar albino rats, we investigated the effects of topical vancomycin on deep sternal wound infection caused by methicillin-resistant Staphylococcus aureus.
Partial median sternotomy was performed under sterile conditions. Group I (n=6) was the sham, and group II (n=7) was the control. Group III (n=8) received topical vancomycin, group IV (n=8) received systemic vancomycin, and group V (n=8) received topical and systemic vancomycin (combined). Rats in groups II through V were inoculated with 0.5 mL × 108 CFU/mL methicillin-resistant S. aureus in the mediastinum and sternum. No medication was given to groups I and II. Twenty-four hours after surgery, 40 mg/kg/day vancomycin was given topically in group III; systemically in group IV; and topically and systemically in group V. After 7 days, smear samples from the mediastinum and tissue cultures from the sternum were obtained.
We found 5.00 ± 0 CFU/mL microorganisms in the mediastinum in group II, 1.90 ± 1.70 in group III, 3.33 ± 0.48 in group IV, and 1.70 ± 1.08 in group V. The quantity of microorganisms per gram of tissue in the sternum was 7.36 ± 0.23 in group II, 6.01 ± 0.33 in group III, 5.81 ± 0.81 in group IV, and 3.99 ± 2.47 in group V. The quantity of microorganisms was less in the 3 treatment groups than in the control group (P <0.05).
We conclude that topical plus systemic vancomycin treatment might be more effective in patients with deep sternal wound infections caused by methicillin-resistant S. aureus.
Key words: Anti-bacterial agents/therapeutic use; drug resistance, bacterial; mediastinitis/prevention & control; postoperative care; rats; staphylococcal infections/drug therapy/prevention & control; sternum/surgery; surgical wound infection/prevention & control; vancomycin/administration & dosage; wound healing
Deep sternal wound infection (DSWI) is a rare but serious complication of cardiac procedures that require sternotomy, with reported incidences ranging from 0.15% to 8%.1–3 Despite medical and surgical therapeutic interventions, DSWI can recur and lead to sepsis, increasing the cost and the length of stay in the hospital. Moreover, the presence of DSWI is also associated with increased mortality, with reported rates from 0.5% up to 47%.1–3 Agents that cause DSWI include staphylococci, streptococci, enterobacteria, and clostridia. However, the morbidity and mortality rates are notably highest in cases of DSWI caused by methicillin-resistant Staphylococcus aureus (MRSA).
Effective surgical treatments of DSWI include wound débridement and washing the omental, pectoral, or rectus abdominis muscle flaps of the mediastinum.4–7 These methods of treatment for DSWI are difficult and lead to an increased risk of complications. Despite these efforts, DSWI still results in high costs and prolonged hospital stays.
Appropriate medical treatment of DSWI usually involves the systemic administration of vancomycin. The systemic use of vancomycin, however, increases the risk of resistance to the drug, as well as the possibility of renal failure as a side effect. Because of these deleterious outcomes, there is a definite need to find alternative treatments for patients with mediastinitis. Vander Salm and colleagues3 have shown that the prophylactic use of topical vancomycin on sternal edges decreased the incidence of DSWI from 3.6% to 0.5% in patients undergoing cardiac operations. This study inspired us to create experimental DSWI models in order to research the effects of topical, systemic, and combined topical–systemic vancomycin on the treatment of DSWI caused by MRSA.
Materials and Methods
The study was approved by our Institutional Ethics Committee. We used 37 Winstar albino male rats (average weight, 300 grams). The rats were divided into 5 groups. Group I (n=6) was the sham group, group II (n=7) was the control group, group III (n=8) was the topical vancomycin group, group IV (n=8) was the systemic vancomycin group, and group V (n=8) was the combined topical–systemic vancomycin group. Each rat was anesthetized with 30 mg/kg ketamine HCl (Ketalar®, Eczacibasi Drug Co.; Istanbul, Turkey) and 2 mg/kg xylazine HCl (Rompun®, Bayer HealthCare; Monheim, Germany). The chest areas of the rats were shaved. The operation site was cleaned with 70% isopropyl alcohol and 2% iodine, and a sterile towel was used to cover the sternum. The skin and presternal layers were incised. A partial upper median sternotomy was performed on all the rats.
The MRSA strains used in this study were isolated from wound samples in our laboratory. The sham group was not given MRSA or medication. The other 4 groups were inoculated in the mediastinal and sternal layers with 0.5 mL × 108 colony-forming units (CFU)/mL MRSA. The sternal and presternal layers were closed with a single 3-0 Prolene® suture. During the treatment period, no medication was given to the rats in groups I and II. Postoperatively, groups III, IV, and V were treated with vancomycin HCl (Vankomisin®, Abbott France S.A.; Rungis Cedex, France) for 7 days, twice daily under ether anesthesia, starting 24 hours after the end of the procedure.8 In group III, the sternotomy was cleaned with 2% iodine, and 40 mg/kg/day vancomycin was administered topically from the fossa jugularis to the substernal area with an insulin syringe. In group IV, 40 mg/kg/day vancomycin was administered intramuscularly into the thigh. A combination of 10 mg/kg/day topical vancomycin and 30 mg/kg/day systemic vancomycin was administered to the rats in group V. After 7 days, tissue samples from the upper ends of the sternum and swab specimens from the upper mediastinum were obtained. Mediastinal swab specimens were mixed with 1 mL of sterile phosphate-buffered saline, and 0.01 mL of the solution was inoculated onto blood agar with a calibrated loop and incubated at 37 °C. The number of bacteria was counted as CFU/mL. Tissue samples were weighed and homogenized under sterile conditions in 1 mL of sterile, phosphate-buffered saline. The serial diluted homogenates were inoculated (0.01 mL) onto blood agar and incubated at 37 °C. After 24 hours, the number of growing of bacteria was determined as CFU/g tissue. The number of bacteria was converted to log10.
Statistical Analysis
Statistical analysis was performed with SPSS 8.0 for Windows (SPSS Inc.; Chicago, Ill). In order to analyze statistical differences between the 5 groups, 1-way ANOVA was used and presented as mean ± standard deviation of log10 CFU. A Bonferroni correction was performed for multiple comparisons. A P value less than 0.05 was considered statistically significant.
Results
The rats were examined for infection. Seven days after inoculation, the mediastina of the rats in group II were full of pus. No pus was observed in groups I, III, IV, and V. Cultures were evaluated microbiologically. The average growth of the microorganisms in the sternum and mediastinum were compared logarithmically (Fig. 1) There was no growth in the sham group. In the mediastinum, bacterial growth was measured as CFU/mL in all groups: 5.00 ± 0 in group II, 1.90 ± 1.70 in group III, 3.33 ± 0.48 in group IV, and 1.70 ± 1.08 in group V. In the sternum, bacterial growth was measured as the number of microorganisms per gram of tissue, and was found to be 7.36 ± 0.23 in group II, 6.01 ± 0.33 in group III, 5.81 ± 0.81 in group IV, and 3.99 ± 2.47 in group V.
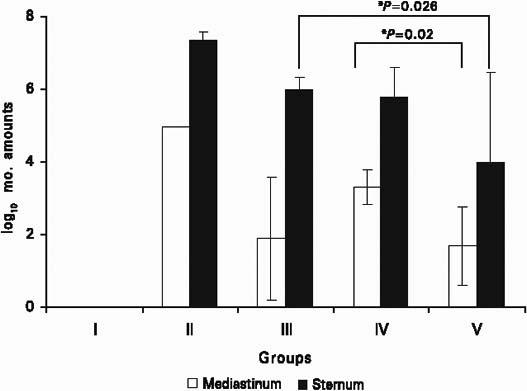
Fig. 1 The average logarithmic growth of microorganisms in the mediastinum (log10 CFU/mL) and in the sternum (log10 CFU/g).
CFU = colony-forming units
The quantities of growing microorganisms in the sternum and mediastinum were significantly smaller in all 3 treatment groups than in the control group (P <0.05). In group V, the quantity of growing microorganisms in the sternum was significantly less than in group III (P=0.026). The quantity of growing microorganisms in the mediastinum was also less in group V than in group IV (P=0.02). There was no statistically significant difference between group III and group IV (P >0.05).
Discussion
Although DSWI is uncommon during the postoperative period, it is associated with high morbidity, prolonged hospitalization, increased cost, and increased mortality after open-heart surgery. When cardiac surgery patients are treated prophylactically with systemic antibiotics, DSWI is observed in 0.3% to 5% of cases.2,4 Risk factors for DSWI are obesity, diabetes mellitus, chronic obstructive pulmonary diseases, suboptimal closure of the sternum, blood loss during operation, and prolonged surgery, mechanical ventilation, or hospitalization.4,9 The usual cause of DSWI after periopera-tive contamination of the mediastinal space is S. aureus. Gram-negative rods have also caused DSWI during the postoperative period.9 The causal factors of DSWI and effective techniques for prevention, early diagnosis, and treatment are still debated. Glycopeptide antibiotics such as vancomycin and teicoplanin are currently being used in the treatment of MRSA.10
According to the U.S. Centers for Disease Control and Prevention definitions of surgical-site infection, a final diagnosis of DSWI involves positive bacterial cultures from the surgical site and obvious clinical signs of infection, such as abscess-like symptoms and drainage with pus.9 Minor criteria are high-grade fever (>38 °C), leukocytosis, rising C-reactive protein level, sternal dehiscence, and localized chest pain. After open-heart surgery, the development of DSWI takes approximately 7 days.11 In our study, the intensity of infection was evaluated by the major infection criteria listed above. After 7 days, the mediastina of the rats in the control group were full of pus, but there was no pus in the mediastina of the rats in the treatment groups. In the sternal tissues of the topical vancomycin-treated group, the higher quantity of microorganisms can be attributed to low penetration of topical vancomycin into bone tissue.
There have been few studies of the pharmacokinetics of topical vancomycin. Desmond and coworkers12 found, after coronary artery bypass surgery on 14 patients, that significant systemic levels of vancomycin were detected 6 hours after application of topical vancomycin. Vancomycin was detected in the urine samples of the patients for 5 days. Desmond's aim was to decrease the high dose of systemic vancomycin by using topical vancomycin, thereby avoiding the side effects of high systemic doses: nephrotoxicity, ototoxicity, reversible neutropenia, and the development of vancomycin-resistant bacteria. Vander Salm and co-authors3 reported that the incidence of postoperative sternal infection was reduced from 3.6% to 0.5% when vancomycin was applied topically to the sternal edges. In our study, the combined treatment group received a decreased systemic dose of vancomycin from 40 mg/kg/day to 30 mg/kg/day and an added 10 mg/kg/day of topical vancomycin. Ikeda and associates13 reported better therapeutic success with 2.5% vancomycin ointment than with systemic vancomycin, in treating cranioplasty sites in MRSA patients. Moreover, topical vancomycin in various doses has successfully treated conjunctivitis (31 mg/mL, ophthalmic solution), chronic suppurative otitis media (25 mg/mL solution), and osteomyelitis (40 mg/kg/day, intravenous) caused by MRSA.14–16 These studies inspired us to conduct this research on topical vancomycin for the treatment of mediastinitis.
Shumacker and Mandelbaum17 introduced a washing system for patients with mediastinitis. Through a previously inserted tube, they irrigated the mediastinum with a solution of 0.1% vancomycin and 5% povidone–iodine, at the rate of 100 mL/h. The mortality rate of irrigated patients, compared with a group treated with systemic vancomycin, decreased from 31% to 1.6%. Merrill and colleagues18 studied 40 patients with mediastinitis and conducted mediastinal irrigation for 7 days with vancomycin, cephalosporins, and selective systemic antibiotics. Their study showed decreased recurrence of mediastinitis, fewer instances of prolonged hospital stay, and decreased surgical cost.
Recently, the use of omental, pectoral, or rectus abdominis muscle flaps has become a preferred surgical treatment for DSWI, with a high success rate.6,7 However, omental transpositions have potential sequelae, such as abdominal wall herniation, hematoma, seroma, and ileus.6 There are also risks of pectoral muscle flaps that prove insufficient to close the sternotomy, hernia in the rectus abdominis flap, bleeding underneath the flap, and dehiscence in the sutures of the sternotomy.7
The therapeutic dose of vancomycin is 30 to 40 mg/kg/day in patients with normal renal function.10 However, in an experimental model of rabbits with meningitis, a high dose of vancomycin (80 mg/day) was administered.19 Similarly, in an experimental model of S. aureus endocarditis, Entenza and coworkers8 used a dose of 23.2 mg/12 h vancomycin. It is believed that resistance developed by S. aureus is caused by the accumulation of vancomycin in components of the cellular wall, which routes vancomycin intravenously from its target to alternative pathways.20 Therefore, vancomycin resistance is a risk associated with prophylactic use in cardiac surgery.21 The reported minimum effective serum concentration of the standard systemic vancomycin dose (500 mg every 6 h) is 5 to 10 mg/L, and the peak serum concentration ranges from 20 to 40 mg/L.12,22 When Desmond's group12 used 1 g topical vancomycin powder for sternotomy, the blood level of vancomycin 3 to 4 hours later was found to be 4.4 mg/L—which is lower than the therapeutic range and could contribute to vancomycin resistance. Our results suggest that combined systemic and topical use of the drug can overcome vancomycin resistance.
Measurement of blood levels of vancomycin would have buttressed our study, but our university's laboratory does not have the technical support to make such determinations.
Conclusion
Although there was no statistically significant difference between the effectiveness of topical and systemic vancomycin, we suggest that systemic plus topical vancomycin will be more effective in treating patients with deep sternal wound infections caused by methicillin-resistant S. aureus. Clinical trials are necessary to clarify this topic. While we recommend combined vancomycin for DSWI, we suggest avoiding the use of topical vancomycin alone, due to the possibility of the patient's developing vancomycin resistance.
Footnotes
Address for reprints: Ali V. Ozcan, MD, Assistant Professor of Surgery, Siteler Mah. Barbaros C. 6248 S., Yenibahcelievler Sit. C-Blok No:3, 20070 Kinikli – Denizli, Turkey. E-mail: vefaozcan@yahoo.com
This study was presented at the 16th Annual Meeting of the Mediterranean Association of Cardiology and Cardiac Surgery (MACCS) in Bodrum, Turkey; 26–29 September 2004.
References
- 1.El Oakley RM, Wright JE. Postoperative mediastinitis: classification and management. Ann Thorac Surg 1996;61:1030–6. [DOI] [PubMed]
- 2.Satta J, Lahtinen J, Raisanen L, Salmela E, Juvonen T. Options for the management of poststernotomy mediastinitis. Scand Cardiovasc J 1998;32:29–32. [DOI] [PubMed]
- 3.Vander Salm TJ, Okike ON, Pasque MK, Pezzella AT, Lew R, Traina V, Mathieu R. Reduction of sternal infection by application of topical vancomycin. J Thorac Cardiovasc Surg 1989;98:618–22. [PubMed]
- 4.Losanoff JE, Richman BW, Jones JW. Disruption and infection of median sternotomy: a comprehensive review. Eur J Cardiothorac Surg 2002;21:831–9. [DOI] [PubMed]
- 5.De Feo M, Gregorio R, Della Corte A, Marra C, Amarelli C, Renzulli A, et al. Deep sternal wound infection: the role of early debridement surgery. Eur J Cardiothorac Surg 2001; 19:811–6. [DOI] [PubMed]
- 6.Weinzweig N, Yetman R. Transposition of the greater omentum for recalcitrant median sternotomy wound infections. Ann Plast Surg 1995;34:471–7. [DOI] [PubMed]
- 7.Nahai F, Rand RP, Hester TR, Bostwick J 3rd, Jurkiewicz MJ. Primary treatment of the infected sternotomy wound with muscle flaps: a review of 211 consecutive cases. Plast Reconstr Surg 1989;84:434–41. [DOI] [PubMed]
- 8.Entenza JM, Vouillamoz J, Glauser MP, Moreillon P. Efficacy of garenoxacin in treatment of experimental endocarditis due to Staphylococcus aureus or viridans group streptococci. Antimicrob Agents Chemother 2004;48:86–92. [DOI] [PMC free article] [PubMed]
- 9.Gardlund B, Bitkover CY, Vaage J. Postoperative mediastinitis in cardiac surgery - microbiology and pathogenesis. Eur J Cardiothorac Surg 2002;21:825–30. [DOI] [PubMed]
- 10.Fekety R. Vancomycin and teicoplanin. In: Mandell GL, Douglas RG, Bennett JE, Dolin R, editors. Mandell, Douglas and Bennett's principles and practice of infectious diseases. 4th ed. New York: Churchill Livingstone; 1995. p. 346–54.
- 11.Aldaz A, Ortega A, Idoate A, Giraldez J, Brugarolas A. Effects of hepatic function on vancomycin pharmacokinetics in patients with cancer. Ther Drug Monit 2000;22:250–7. [DOI] [PubMed]
- 12.Desmond J, Lovering A, Harle C, Djorevic T, Millner R. Topical vancomycin applied on closure of the sternotomy wound does not prevent high levels of systemic vancomycin. Eur J Cardiothorac Surg 2003;23:765–70. [DOI] [PubMed]
- 13.Ikeda H, Kurisu K, Kihira K. Vancomycin ointment for MRSA infection at a cranioplasty site. Ann Pharmacother 2004;38:70–2. [DOI] [PubMed]
- 14.Ross J, Abate MA. Topical vancomycin for the treatment of Staphylococcus epidermidis and methicillin-resistant Staphylococcus aureus conjunctivitis. DICP 1990;24:1050,1053. [DOI] [PubMed]
- 15.Jang CH, Song CH, Wang PC. Topical vancomycin for chronic suppurative otitis media with methicillin-resistant Staphylococcus aureus otorrhoea. J Laryngol Otol 2004; 118:645–7. [DOI] [PubMed]
- 16.Bernard L, Vaudaux P, Vuagnat A, Stern R, Rohner P, Pittet D, et al. Effect of vancomycin therapy for osteomyelitis on colonization by methicillin-resistant Staphylococcus aureus: lack of emergence of glycopeptide resistance. Infect Control Hosp Epidemiol 2003;24:650–4. [DOI] [PubMed]
- 17.Shumacker HB Jr, Mandelbaum I. Continuous antibiotic irrigation in the treatment of infection. Arch Surg 1963;86:384–7. [DOI] [PubMed]
- 18.Merrill WH, Akhter SA, Wolf RK, Schneeberger EW, Flege JB Jr. Simplified treatment of postoperative mediastinitis. Ann Thorac Surg 2004;78:608–12. [DOI] [PubMed]
- 19.Ahmed A, Jafri H, Lutsar I, McCoig CC, Trujillo M, Wubbel L, et al. Pharmacodynamics of vancomycin for the treatment of experimental penicillin- and cephalosporin-resistant pneumococcal meningitis. Antimicrob Agents Chemother 1999;43:876–81. [DOI] [PMC free article] [PubMed]
- 20.Oakley RE, Nimer KA, Bukhari E. Is the use of topical vancomycin to prevent mediastinitis after cardiac surgery justified? J Thorac Cardiovasc Surg 2000;119:190–1. [DOI] [PubMed]
- 21.Sieradzki K, Roberts RB, Haber SW, Tomasz A. The development of vancomycin resistance in a patient with methicillin-resistant Staphylococcus aureus infection. N Engl J Med 1999;340:517–23. [DOI] [PubMed]
- 22.Krivoy N, Yanovsky B, Kophit A, Zaher A, Bar-El Y, Adler Z, et al. Vancomycin sequestration during cardiopulmonary bypass surgery. J Infect 2002;45:90–5. [DOI] [PubMed]


