Abstract
In vivo pharmacokinetic/pharmacodynamic characterization for numerous antibacterial compounds has had a significant impact upon optimal dosing regimen design and the development of in vivo susceptibility breakpoints. More recently, similar characterization has been undertaken for antifungal drug classes. Very little is known of these pharmacodynamic relationships for the new echinocandin class of compounds. We utilized a neutropenic murine model of disseminated candidiasis to describe the time course antifungal activity of HMR 3270, a new glucan synthase inhibitor. Single-dose in vivo time kill studies with four 16-fold escalating doses demonstrated concentration-dependent killing when drug levels in serum were more than four times the MIC. Postantifungal effects were dose dependent, ranging from 8 to 80 h duration. Multiple dosing regimen studies utilized six total doses, four dosing intervals, and a treatment duration of 6 days. Shortening the dosing interval from every 144 h (q144h) to q36h resulted in a fourfold rise in the dose necessary to achieve a net fungistatic effect. The peak/MIC ratio most strongly correlated with treatment outcomes (peak/MIC ratio, R2 = 98%; ratio of the area under the concentration-time curve from 0 to 24 h to the MIC, R2 = 79%, percentage of time above the MIC, R2 = 61%). Studies were also conducted with five additional Candida albicans isolates to determine if a similar peak/MIC ratio was associated with efficacy. In vivo concentration-dependent killing was similarly observed in studies with each of the additional isolates. The peak/MIC ratio necessary to produce efficacy was relatively similar among the strains studied (P = 0.42). The peak/MIC ratio (mean ± standard deviation) necessary to achieve a fungistatic effect was 3.72 ± 1.84, and the ratio necessary to achieve maximal killing was near 10.
The incidence of invasive fungal infections continues to rise (11, 14). Despite advances in antifungal therapy, rates of morbidity and mortality remain high. HMR 3270 is one of a new class of echinocandin antifungal compounds. These lipopeptide compounds act by inhibiting the synthesis of (1,3)-β-glucan, leading to cell wall damage (12, 20). This class of drug has been shown to possess a broad antifungal spectrum and potency in vitro and in vivo (1, 12, 15, 16, 17, 18, 19, 20, 24, 29; P. Warn, A. Sharp, G. Morrissey, and D. Denning, Abstr. 41st Intersci. Conf. Antimicrob. Agents Chemother., abstr. J1617, 2001; C. B. Moore and D. W. Denning, Abstr. 41st Intersci. Conf. Antimicrob. Agents Chemother., abstr. F2147, 2001).
The study of antimicrobial pharmacodynamics provides insight into the link between drug exposures, in vitro susceptibility, and treatment efficacy (7, 10). It has become common for these studies with antibacterial drugs to impact dosing regimen design for clinical trials and to impact the development of susceptibility breakpoints (5, 9, 13, 26). Similar pharmacodynamic characterizations have been undertaken with several antifungal compounds (2, 3, 4). The correlation between this information in experimental models and available clinical trial analysis suggests these pharmacodynamic predictions could play a similar role in the development of new antifungal drugs (2, 22, 30; C. J. Clancy, C. A. Kauffman, A. Morris, M. L. Nguyen, D. C. Tanner, D. R. Snydman, V. L. Yu, and M. H. Nguyen, Prog. Abstr. 36th Annu. Meet. Infect. Dis. Soc. Am., abstr. 98, p. 93, 1998). The present studies have been designed to characterize these in vivo pharmacodynamic relationships for a new glucan synthase inhibitor, HMR 3270. We are hopeful this information can be utilized for future clinical development of this and other antifungal compounds.
MATERIALS AND METHODS
Organisms.
Six clinical isolates of Candida albicans (designated K-1, 580, 98-17, 98-234, 2438, and 2183) were utilized. C. albicans K-1 and 580 were clinical isolates from systemic candidiasis. The other isolates were mucosal isolates. Isolates 2438 and 2183 were kindly provided by Lopez-Ribot et al. (23). The organisms were maintained, subcultured, and quantified on Sabouraud dextrose agar (SDA) plates (Difco Laboratories, Detroit, Mich.). Twenty-four hours prior to study, organisms were subcultured at 35°C.
Antifungal agent.
HMR 3270 was obtained as a powder from Aventis Pharmaceuticals (Romainville, France). The powder was stored desiccated at −4°C. Drug solutions were prepared on the day of study by dissolving the powder in 0.85% sterile physiologic NaCl (vol/vol). Subsequent drug dilutions were performed with 0.85% NaCl (vol/vol).
In vitro susceptibility testing.
MICs were determined using a broth microdilution modification of the NCCLS M27-A method (25). The MIC was defined as the lowest concentration that produced an optically clear well. Determinations were performed in duplicate on at least two separate occasions.
Animals.
Six-week-old ICR/Swiss specific-pathogen-free female mice weighing 23 to 27 g were used for all studies (Harlan Sprague-Dawley, Madison, Wis.). Animals were maintained in accordance with the American Association for Accreditation of Laboratory Animal Care criteria (27). All animal studies were approved by the Animal Research Committee of the William S. Middleton Memorial VA Hospital.
Infection model.
Mice were rendered neutropenic (polymorphonuclear leukocytes < 100/mm3) by injecting cyclophosphamide (Mead Johnson Pharmaceuticals, Evansville, Ind.) intraperitoneally 4 days (150 mg/kg of body weight) before infection, 1 day (100 mg/kg) before infection, and 2 days (100 mg/kg) after infection. Absolute white blood cell and neutrophil counts were monitored every 48 h throughout the period of study with a Coulter counter and peripheral blood smears. Neutrophil counts remained at or below 100/mm3 throughout the entire study.
Organisms were subcultured on SDA 24 h prior to infection. Inoculum was prepared by placing three to five colonies into 5 ml of sterile pyrogen-free 0.9% saline warmed to 35°C. The final inoculum was adjusted to 0.6 transmittance at 530 nm. Fungal counts of the inoculum determined by viable counts on SDA were 106 CFU/ml.
Disseminated infection with the Candida organisms was achieved by injection of 0.1 ml of inoculum via the lateral tail vein 2 h prior to start of drug therapy. At the end of the study period animals were sacrificed by CO2 asphyxiation. After sacrifice the kidneys of each mouse were immediately removed and placed in sterile 0.9% saline at 4°C. Homogenate was then serially diluted 1:10, and aliquots were plated on SDA for viable fungal colony counts after incubation for 24 h at 35°C. The lower limit of detection is 100 CFU/ml. Results were expressed as the mean number of CFU/kidneys for two mice (four kidneys).
Pharmacokinetics.
Single-dose pharmacokinetics of HMR 3270 were determined in individual neutropenic infected ICR/Swiss mice following intraperitoneal doses of 4 and 16 mg/kg administered in 0.2-ml volumes. At each dose examined, groups of four mice were sampled by intracardiac puncture, and serum was pooled at each time point. Mice were anesthetized with halothane prior to sampling and euthanized with CO2 immediately afterwards. Drug levels were determined at seven time points over 84 h. The first samples were collected 1 h after drug administration. Subsequent samples were collected at 4- to 24-h intervals. The pooled blood was placed in 1.5-ml tubes (Fisher Scientific) and immediately centrifuged (model MB centrifuge; International Equipment Co.) at 10,000 × g for 5 min. The serum was subsequently removed and stored at −80°C. Serum drug levels were determined by high-performance liquid chromatography assay at Aventis Pharmaceuticals. The interday precision of the assay was between −13 and −1.4%, with a coefficient of variation of 6.4%. The lower level of detection for this assay was 0.04 mg/liter. Pharmacokinetic constants including elimination half-life and C0 were calculated via nonlinear least-squares techniques (MINSQ; Micromath Inc., Salt Lake City, Utah). The area under the concentration-time curve (AUC) was calculated by the trapezoidal rule. For doses for which no kinetics were determined, pharmacokinetic parameters were extrapolated from the values obtained in the actual studies.
In vivo time kill and postantifungal effect.
Infection in neutropenic mice was produced as described above. Two hours after infection with C. albicans K-1, mice were treated with single intraperitoneal doses of HMR 3270 (0.25, 0.5, 1.0, and 4.0 mg/kg). Groups of two treated and control mice were sacrificed at each sampling time, with intervals ranging from 1 to 2 h for the first 6 h for the time kill study and every 6 to 24 h thereafter for remainder of the study period to determine the postantifungal effect (PAFE). Growth with the two lower doses was monitored for 72 and 120 h. The two highest doses were monitored for 144 h to determine if the organisms would recover from the effect of drug treatment. Control growth was determined over 24 h at five sampling times. Kidneys were removed at each time point and processed immediately for CFU determination. The time that serum levels of HMR 3270 remained above the MIC for the organism following the four doses was calculated from the pharmacokinetic data. The postantibiotic effect (PAE) was calculated by determining the time it took for controls to increase 1 log10 CFU/kidneys (C) and subtracting this from the amount of time it took organisms from the treated animals to grow 1 log10 CFU/kidney (T) after drug levels in serum fell below the MIC for the organism: PAE = T − C (8, 31).
Pharmacodynamic parameter determination.
Neutropenic mice were infected with C. albicans K-1 2 h prior to start of therapy. Twenty-four dosing regimens were chosen to minimize the interdependence among the three pharmacodynamic parameters studied and also to observe the complete dose response relationship. Groups of two mice were treated for 144 h with dosing regimens of HMR 3270 using fourfold-increasing total doses administered at dosing intervals of every 36 h (q36h), q48h, q72h, and q144h. Total doses ranged from 0.125 to 4.00 mg/kg/144 h. Drug was administered in 0.2-ml volumes. Mice were sacrificed after 144 h of therapy, and kidneys were removed for CFU determination as described above. Untreated control mice were sacrificed just before treatment and at the end of the experiment. Efficacy was defined as the change in log10 CFU/kidneys over the 144-h treatment period and was calculated by subtracting the mean log10 CFU/kidneys in untreated control mice after 144 h from the mean number of CFU from kidneys of two mice at the end of therapy.
Pharmacodynamic parameter magnitude determination.
Studies similar to those described above were performed with five addition strains of C. albicans (98-17, 98-234, 2438, 580, and 2183). Attempts were made to choose organisms with various susceptibilities to HMR 3270. However, we were unable to identify organisms for which there were various MICs in our stock of organisms. The group of organisms utilized included both fluconazole-susceptible, fluconazole-susceptible dose-dependent, and fluconazole-resistant strains. Dosing studies were designed to vary the magnitude of the pharmacodynamic parameters. The total dose varied from 0.08 to 5.30 mg/kg. Doses were fractionated into three doses (q48h) for the 6-day study period. Groups of two mice were again used for each dosing regimen. At the end of the study, mice were euthanized and kidneys were immediately processed for CFU determination.
Data analysis.
A sigmoid dose-effect model was used to measure the in vivo potency of HMR 3270. The model is derived from the Hill equation: E = (Emax × DN)/(ED50N + DN), where E is the observed effect (change in log10 CFU/kidneys compared with untreated controls at 144 h), D is the cumulative 144-h dose, Emax is the maximum effect, ED50 is the dose required to achieve 50% of Emax, and N is the slope of the dose-effect relationship. The correlation between efficacy and each of the three parameters studied was determined by nonlinear least-squares multivariate regression analysis (Sigma Stat; Jandel Scientific Software, San Rafael, Calif.). The coefficient of determination (R2) was used to estimate the percent of variance in the change of log10 CFU/kidneys over the treatment period for the different dosing regimens that could be attributed to each of the pharmacodynamic parameters.
To allow a more meaningful comparison of potency among the dosing regimens studied we calculated the dose required to produce a fungistatic dose or no net growth over 144 h compared to numbers at the start of therapy. If the doses needed to achieve these benchmarks increased significantly as the dosing interval was lengthened from q36h through q144h regimens, the duration of the time that drug levels in serum remained above the MIC was the parameter predictive of efficacy. On the other hand, if the doses necessary to reach these outcomes decreased with dosing interval lengthening, then the parameter associated with these outcomes would be the peak serum level. If the doses remained similar independent of changes in the dosing interval, then the AUC would be predictive of efficacy.
The static dose was determined for the q48h dosing regimen for each of the six strains. The magnitude of the pharmacodynamic parameter predictive of the efficacy of HMR 3270 was then calculated for each of the six organisms studied to determine if a similar parameter magnitude was associated with efficacy as determined by these indices. The significance of differences among these values was determined by analysis of variance (Sigma Stat; Jandel Scientific Software). A two-tailed P of <0.05 was considered to be statistically significant.
RESULTS
In vitro susceptibility testing.
There was no variation in the MICs for the six C. albicans organisms studied (MICs, 0.50 μg/ml).
Pharmacokinetics.
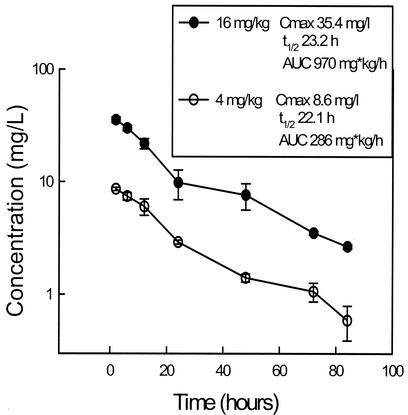
The serum time course of HMR 3270 in infected neutropenic mice following intraperitoneal doses of 4 and 16 mg/kg are shown in Fig. 1. Peak levels in serum and the AUC increased in a linear fashion with dose escalation. Peak levels were achieved within 2 h for each of the doses and ranged from 8.6 ± 0.30 to 35.4 ±2.0 mg/liter (means ± standard deviations). The elimination half-life did not change significantly with the doses studied, ranging from 22.1 to 23.2 h. The AUC, as determined by the trapezoidal rule, ranged from 286 to 970 mg · h/liter with the lowest and highest doses, respectively.
FIG. 1.
Serum HMR 3270 concentrations after administration of intraperitoneal doses of 4 and 16 mg/kg in neutropenic infected mice. Each symbol represents the geometric mean ± standard deviation (error bars) of the levels in the sera of four mice.
In vivo PAE.
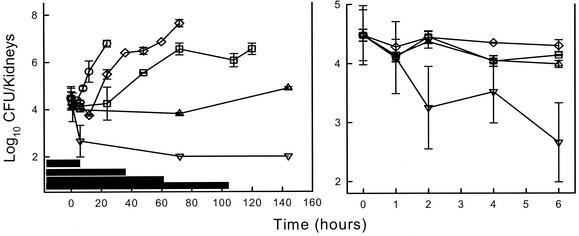
Following tail vein inoculation of 106 CFU/ml, growth of Candida organisms in the kidneys of untreated mice increased 2.31 ± 0.34 log10 CFU/kidneys over 24 h. Control growth of 1 log10 CFU/kidneys in untreated mice was achieved in 11.2 h. No drug carryover was observed in treatment groups. Based upon the above pharmacokinetics, the four doses of HMR 3270 studied (0.25, 0.50, 1.0, and 4.0 mg/kg) would produce serum drug levels above the MIC for the Candida organism (0.50 μg/liter) for 4, 26, 48, and 92 h, respectively. Treatment with the two highest doses produced peak levels 4- and 16-fold greater than the MIC and resulted in a concentration-dependent reduction in colony counts when compared with numbers at the start of therapy of 0.67 and 1.82, respectively. The lower doses produced peak levels at or slightly below the MIC and resulted in little or no net organism killing. Growth curves for both the control group as well as those following the single doses of HMR 3270 are shown in Fig. 2 (left panel). HMR 3270 suppressed regrowth of organisms at each of the four doses studied in a dose-dependent fashion. Although the lower doses did not produce significant killing, they did inhibit organism regrowth with PAEs around 8 h (Fig. 2, right panel). The two higher doses significantly prolonged the time for organism recovery with PAEs exceeding the period of observation. The calculated PAEs with doses of 1 and 4 mg/kg were >52 and >81 h, respectively.
FIG. 2.
(Left panel) In vivo PAE following HMR 3270 doses of 0.25, 0.5, 1, and 4 mg/kg against C. albicans K-1 in neutropenic infected mice. Each symbol represents the mean ± standard deviation (error bars) for two mice. The widths of the horizontal bars represent the time that serum levels exceeded the MIC. (Right panel) In vivo time kill following HMR 3270 doses of 0.25, 0.5, 1, and 4 mg/kg against C. albicans K-1 in neutropenic infected mice. Each symbol represents the mean ± standard deviation (error bars) for two mice.
Pharmacodynamic parameter determination.
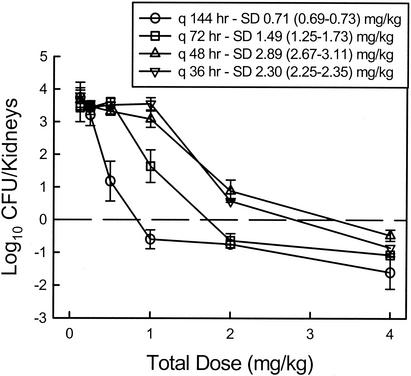
At the start of therapy fungal burdens in kidneys were 4.15 ± 0.15 log10 CFU/kidneys. After 144 h the organisms grew 3.79 ± 0.31 log10 CFU/kidneys in untreated mice, resulting in death in each of the control mice. Drug carryover was not observed in any of the samples. Escalating doses of HMR 3270 produced significant net killing (0.47 to 1.10 log10 CFU/kidneys) compared to the inoculum in control animals at the start of therapy as was observed in the time kill studies. The dose-response curves for each of the four dosing intervals are shown in Fig. 3. As the dosing interval was shortened the dose-response curves shifted to the right indicating less efficacy. The dose necessary to produce a net static effect decreased more than fourfold with widely spaced dosing.
FIG. 3.
Relationship between the total dose and the change in log10 CFU per kidney over the 6-day treatment period for HMR 3270 administered at different dosing intervals in a neutropenic murine model of disseminated candidiasis. Each total dose level was fractionated for each of the dosing intervals. Each symbol represents data for two mice. The dashed horizontal line represents the number of CFU at the start of therapy. SD, the static dose; 95% confidence intervals are given in parentheses.
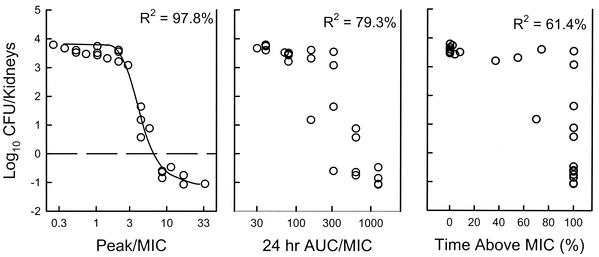
The relationships between microbiologic effect and each of the pharmacodynamic parameters—percent of time above MIC, AUC/MIC ratio, and peak/MIC ratio—are shown in Fig. 4. The data that regressed with the peak level in relation to the MIC had the strongest relationship, with an R2 of 98% (AUC/MIC, R2 = 79%; percentage of time above the MIC, R2 = 61%). The AUC in relation to the MIC appeared slightly more important than the time above MIC.
FIG. 4.
Relationship between the time above the MIC, the AUC/MIC ratio, the peak/MIC ratio, and the change in log10 CFU per kidney after 6 days of treatment. Each symbol represents data for two mice. The dashed horizontal line represents the number of CFU at the start of therapy.
Correlation of the magnitude of the pharmacodynamic parameter with efficacy.
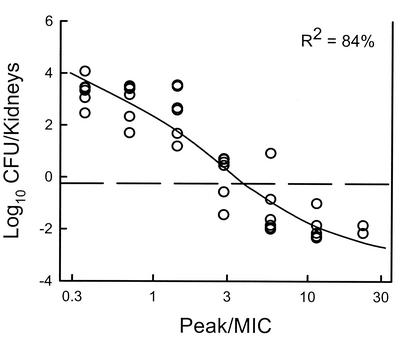
Each of the six C. albicans strains grew well in the animals. At the start of therapy the kidneys had between 3.91 ± 0.06 and 4.26 ± 0.17 log10 CFU/kidneys. The range of organism growth in the control animals was 2.46 ± 0.28 to 4.07 ± 0.26 log10 CFU/kidneys (mean 3.26 ± 0.56 log10 CFU/kidneys). The relationship between the HMR 3270 peak/MIC ratios and efficacy with the six strains is displayed in Fig. 5. The efficacies were very similar for the peak/MIC ratios corresponding to each strain (R2 = 84%). The peak/MIC ratios (Table 1) necessary to achieve a net static effect was around 3 (mean ± SD = 3.72 ± 1.84). Maximal organism killing was observed with ratios near 10.
FIG. 5.
Relationship between the peak/MIC ratio and the change in log10 CFU per kidney after 6 days of treatment for HMR 3270 against six C. albicans organisms. Each symbol represents data for two mice. The dashed horizontal line represents the number of CFU at the start of therapy.
TABLE 1.
HMR 3270 peak/MIC ratios against six Candida strains in a neutropenic disseminated candidiasis model
| C. albicans strain | MIC (mg/liter) | Static dose (mg/kg) | Peak/MIC ratioa |
|---|---|---|---|
| K-1 | 0.5 | 2.26 | 3.24 |
| 2183 | 0.5 | 2.34 | 3.33 |
| 2438 | 0.5 | 1.44 | 2.07 |
| 98-17 | 0.5 | 2.22 | 3.18 |
| 98-234 | 0.5 | 2.19 | 3.14 |
| 5810 | 0.5 | 5.15 | 7.36 |
| Mean ± SD | 2.60 ± 1.29 | 3.72 ± 1.84 |
P = 0.42
DISCUSSION
The antimicrobial effect of increasing drug concentrations and the presence or absence of PAFEs defines the time course activity of antifungal drugs. For drugs such as the polyenes, studies have demonstrated enhanced organism killing with higher drug concentrations and prolonged persistent or postantifungal effects (4, 15). For drugs displaying this pattern of activity the peak/MIC ratio has most often been the pharmacokinetic/pharmacodynamic (PK/PD) parameter predictive of efficacy (4, 6, 7).
The in vivo time course of antifungal activity of the new cyclic lipopeptide antifungal compounds has not described the time course adequately. These drugs inhibit the synthesis of 1,3-β-glucan of the fungal cell wall (12, 20). This class of compounds exhibits a broad antifungal spectrum with particular potency against Candida species (1, 12, 15, 16, 17, 18, 19, 24, 28, 29). For Candida species this activity results in rapid cell lysis and death. Several investigations have characterized in vitro the time course activity of this new antifungal drug class (15, 16, 17). These studies have demonstrated that the antifungal activity of these compounds is enhanced with increasing drug concentrations. Furthermore, these investigations have demonstrated prolonged suppression of organism growth following brief drug exposures (PAEs). An in vitro pattern of activity characterized by concentration-dependent killing and prolonged PAFE would suggest that large infrequent drug dosing may be most efficacious.
Thus far, in vivo studies with several of glucan synthase inhibitors have demonstrated that efficacy against a variety fungal pathogens is enhanced with escalating drug doses (1, 18, 19, 24). However, these in vivo investigations have not characterized the time course activity of these compounds. The experiments presented here have examined these pharmacodynamic relationships in an in vivo model.
Our in vivo time kill results observed significant organism burden reductions when drug doses produced drug levels in serum more than four times the MIC. The magnitude of this drug concentration/MIC ratio resulted in similar killing in in vitro investigations (15, 16, 17). The degree of in vivo killing in our observations was enhanced with higher drug doses. The highest serum concentration in relation to organism MIC achieved in our single dose time kill studies was 16. In postantifungal observations, the duration of regrowth suppression was also concentration dependent. PAFEs ranged from only 8 h to more than 80 h. These time course characteristics would also suggest one of the concentration-dependent PK/PD parameters would best predict drug efficacy.
The multiple dosing regimen observations demonstrated that large infrequent drug administration was most effective. A fourfold-lower total drug dose was necessary to produce a net fungistatic effect with the once-every-6-day dosing compared to the q36h dosing regimen. As one would expect, when these dosing regimen data were regressed with each of the three PK/PD parameters, the strongest relationship was observed when the peak serum level/MIC ratio was utilized. This time course pattern of activity and PK/PD parameter relationship is similar to that we observed with amphotericin B in this model.
We also attempted to discern the magnitude of the peak/MIC ratio that would be necessary to achieve efficacy against organisms with various MICs. Unfortunately, we do not possess C. albicans strains for which HMR 3270 MICs vary. The in vitro susceptibility surveillance literature suggests that this is not unusual (28, 29). In addition, the resistant C. albicans isolates that have been isolated have varied significantly in virulence in animal models which would further complicate treatment comparisons (21). However, among the six isolates with identical MICs studied, outcomes were similar. A net fungistatic effect was observed with peak/MIC ratios near 3 and maximal efficacy was seen when this ratio approached ten. These peak/MIC ratios (3 and 10) should be compared to HMR 3270 population pharmacokinetics to help define an appropriate dosing regimen for early clinical studies in treatment of C. albicans infection. In general free-drug levels should be utilized for PK/PD parameter magnitude comparisons. However, in this case we utilized total drug levels because of the similarity of protein binding determinations across species with the various echinocandins (J. Lowthers, personal communication). For HMR 3270, the degree of protein binding has been >99% for both mice and in humans.
The echinocandin activity against filamentous pathogens such as Aspergillus species is clearly different from that observed with Candida species. For these pathogens, glucan synthase inhibition does slow organism growth by altering hyphal tip structures, but does not result in organism killing. One might thus expect a different in vivo antifungal time course against filamentous species. Certainly one would not see concentration dependent killing, however, one may still observe postantifungal or sub-MIC effects. The PK/PD parameter predictive of activity with these patterns of activity would be either percentage of time above the MIC or AUC/MIC ratio. Even if the PK/PD parameter predictive of treatment outcome for these species remains the peak/MIC ratio, one might anticipate that a different (likely higher) parameter magnitude would be required to produce similar treatment outcomes. These important questions should be the focus of future PK/PD investigations.
REFERENCES
- 1.Abruzzo, G. K., A. M. Flattery, C. J. Gill, L. Kong, J. G. Smith, V. B. Pikounis, J. M. Balkovec, A. F. Bouffard, J. F. Dropinski, H. Rosen, H. Kropp, and K. Bartizal. 1997. Evaluation of the echinocandin antifungal MK-0991 (L-743,872): efficacies in mouse models of disseminated aspergillosis, candidiasis, and cryptococcosis. Antimicrob. Agents Chemother. 41:2333-2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andes, D., and M. I. van Ogtrop. 1999. Characterization and quantitation of the pharmacodynamics of fluconazole in a neutropenic murine model of disseminated candidiasis model. Antimicrob. Agents Chemother. 43:2116-2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andes, D., and M. I. van Ogtrop. 2000. In vivo characterization of the pharmacodynamics of flucytosine in a neutropenic murine candidiasis model. Antimicrob. Agents Chemother. 44:938-942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andes, D. 2001. In vivo pharmacodynamics of amphotericin B against selected Candida species. Antimicrob. Agents Chemother. 45:922-926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andes, D., and W. A. Craig. 1998. In vivo activities of amoxicillin and amoxicillin-clavulanate against Streptococcus pneumoniae: application to breakpoint determinations. Antimicrob. Agents Chemother. 42:2375-2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blaser, J., B. B. Stone, M. C. Groner, and S. H. Zinner. 1987. Comparative study with enoxacin and netilmicin, a pharmacodynamic model to determine importance of ratio of antibiotic peak concentration to MIC for bactericidal activity and emergence of resistance. Antimicrob. Agents Chemother. 31:1054-1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Craig, W. A. 1998. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin. Infect. Dis. 26:1-12. [DOI] [PubMed] [Google Scholar]
- 8.Craig, W. A., and S. Gudmundsson. 1996. Postantibiotic effect, p. 296-329. In V. Lorian (ed.), Antibiotics in laboratory medicine, 4th ed. Williams and Wilkins, Baltimore, Md.
- 9.Craig, W. A., and D. Andes. 1996. Pharmacokinetics and pharmacodynamics of antibiotics in otitis media. Pediatr. Infect. Dis. J. 15:255-259. [DOI] [PubMed] [Google Scholar]
- 10.Craig, W. A., and A. Dalhoff. 1998. Pharmacodynamics of fluoroquinolones in experimental animals, p. 207-232. In J. Kuhlman, A. Dalhoff, and H. J. Zeiler (ed.), Handbook of experimental pharmacology, vol. 127. Quinolone antibacterials. Springer-Verlag, Heidelberg, Germany.
- 11.Dasbach, E. J., G. M. Davies, and S. M. Teutsch. 2000. Aspergillosis-related US hospitalizations. Clin. Infect. Dis. 31:1524-1528. [DOI] [PubMed] [Google Scholar]
- 12.Douglas, C. M., J. A. D'Ippolito, G. J. Shei, M. Meinz, J. Onishi, J. A. Marrinan, W. Li, G. K. Abruzzo, A. Flattery, K. Bartizal, A. Mitchell, and M. B. Kurtz. 1997. Identification of the FKS1 gene of Candida albicans as the essential target of 1,3-β-d-glucan synthase inhibitors. Antimicrob. Agents Chemother. 41:2471-2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dowell, S. F., J. C. Butler, G. S. Giebink, et al. 1999. Acute otitis media: management and surveillance in an era of pneumococcal resistance—a report from the Drug-Resistant Streptococcus pneumoniae Working Group. Pediatr. Infect. Dis. J. 18:1-9. [PubMed] [Google Scholar]
- 14.Edmond, M. B., S. E. Wallace, D. K. McClish, M. A. Pfaller, R. N. Jones, and R. P. Wenzel. 1999. Nosocomial bloodstream infections in United States hospitals: a three-year analysis. Clin. Infect. Dis. 29:239-244. [DOI] [PubMed] [Google Scholar]
- 15.Ernst, E. J., M. E. Klepser, and M. A. Pfaller. 2000. Postantifungal effects of echinocandin, azole, and polyene antifungal agents against Candida albicans and Cryptococcus neoformans. Antimicrob. Agents Chemother. 44:1108-1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ernst, E. J., M. E. Klepser, M. E. Ernst, S. A. Messer, and M. A. Pfaller. 1999. In vitro pharmacodynamic properties of MK-0991 determined by time-kill methods. Diagn. Microbiol. Infect. Dis. 33:75-80. [DOI] [PubMed] [Google Scholar]
- 17.Ernst, M. E., M. E. Klepser, E. J. Wolfe, and M. A. Pfaller. 1996. Antifungal dynamics of LY 303366, an investigational echinocandin B analog, against Candida ssp. Diagn. Microbiol. Infect. Dis. 26:125-131. [DOI] [PubMed] [Google Scholar]
- 18.Groll, A. H., D. Mickiene, R. Petrainiene, V. Pettraitis, C. A. Lyman, C. H. Bacher, S. C. Piscitelli, and T. J. Walsh. 2001. Pharmacokinetic and pharmacodynamic modeling of anidulufungin (LY30336): reappraisal of its efficacy in neutropenic animal models of opportunistic mycoses using optimal plasma sampling. Antimicrob. Agents Chemother. 45:2845-2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ikeda, F., Y. Wakai, S. Matsumoto, K. Maki, E. Watabe, S. Tawara, T. Goto, Y. Wantanabe, F. Matsumoto, and S. Kuwahara. 2000. Efficacy of FK463, a new lipopeptide antifungal agent, in mouse models of disseminated candidiasis and aspergillosis. Antimicrob. Agents Chemother. 44:614-618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kurtz, M. B., and C. M. Douglas. 1997. Lipopeptide inhibitors of fungal glucan synthase. J. Med. Vet. Mycol. 35:79-86. [DOI] [PubMed] [Google Scholar]
- 21.Kurtz, M. B., G. Abruzzo, A. Flattery, K Bertizal., J. A. Marrinan, W. Li, J. Milligan, K. Nollstadt, and C. M. Douglas. 1996. Characterization of echinocandin-resistant mutants of Candida albicans: genetic, biochemical, and virulence studies. Infect. Immun. 64:3244-3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee, S. C., C. P. Fung, J. S. Huang, C. J. Tsai, K. S. Chen, N. L. Chen, L. C. See, and W. B. Shieh. 2000. Clinical correlates of antifungal macrodilution susceptibility test results for non-AIDS patients with severe candida infections treated with fluconazole. Antimicrob. Agents Chemother. 44:2715-2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lopez-Ribot, J. L., R. K. McAtee, L. N. Lee, W. R. Kirkpatrick, T. C. White, D. Sanglard, and T. F. Patterson. 1998. Distinct patterns of gene expression associated with development of fluconazole resistance in serial Candida albicans isolates from human immunodeficiency virus-infected patients with oropharyngeal candidiasis. Antimicrob. Agents Chemother. 42:2932-2937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maesaki, S., M. A. Hossain, Y. Miyazaki, K. Tomono, T. Tashiro, and S. Kohno. 2000. Efficacy of FK463, a (1,3)-β-glucan synthase inhibitor, in disseminated azole-resistant Candida albicans infection in mice. Antimicrob. Agents Chemother. 44:1728-1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.National Committee for Clinical Laboratory Standards. 1997. Reference method for broth dilution antifungal susceptibility testing for yeast. Approved standard M27-A. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 26.National Committee for Clinical Laboratory Standards. 2000. Development of in vitro susceptibility testing criteria and quality control parameters. Approved guidelines, 2nd ed. Document M23-A2. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 27.National Research Council. Committee on the Care and Use of Laboratory Animals of the Institute of Laboratory Animal Resources, Commission on Life Sciences. 1996. Guide for the care and use of laboratory animals. National Academy Press, Washington, D.C.
- 28.Pfaller, M. A., R. N. Jones, G. V. Doern, A. C. Fluit, J. Verhoef, H. S. Sader, S. A. Messer, A. Houston, S. Coffman, and J. R. Hollis. 1999. International surveillance of blood stream infections due to Candida species in the European SENTRY Program: species distribution and antifungal susceptibility including the investigational triazole and echinocandin agents. Diagn. Microbiol. Infect. Dis. 35:19-25. [DOI] [PubMed] [Google Scholar]
- 29.Pfaller, M. A., S. A. Messer, and S. Coffman. 1997. In vitro susceptibilities of clinical yeast isolates to a new echinocandin derivative, LY303366, and other antifungal agents. Antimicrob. Agents Chemother. 41:763-766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rex, J. H., M. A. Pfaller, J. N. Galgiani, M. S. Bartlett, A. Espinel-Ingroff, M. A. Ghannoum, M. Lancaster, F. C. Odds, M. G. Rinadli, T. J. Walsh, and A. L. Barry for the NCCLS Subcommittee on Antifungal Susceptibility Testing. 1997. Development of interpretive breakpoints for antifungal susceptibility testing: conceptual framework and analysis of in vitro and in vivo correlation data for fluconazole, itraconazole, and candida infections. Clin. Infect. Dis. 24:235-247. [DOI] [PubMed] [Google Scholar]
- 31.Turnidge, J. D., S. Gudmundsson, B. Vogelman, and W. A. Craig. 1994. The postantibiotic effect of antifungal agents against common pathogenic yeasts. J. Antimicrob. Chemother. 34:83-92. [DOI] [PubMed] [Google Scholar]