A 10-year-old, spayed female, beagle-cross dog was presented to the Small Animal Clinic (SAC) of the Western College of Veterinary Medicine (WCVM) because of the recent onset of polyuria and polydipsia. The owners reported that the dog was otherwise behaving normally. Physical examination of the dog was difficult and incomplete, because she was fractious and uncooperative. Blood and urine were collected for a complete blood (cell) count (CBC), serum chemical analysis, and urinalysis. Abnormalities were present as follows: mild erythrocytosis (8.68 × 1012/L; reference interval, 5.5 to 8.5 × 1012/L); elevation in the hematocrit (0.592 L/L; reference interval, 0.370 to 0.550 L/L), and reticulocytosis (4.1%; normal < 1%); marked hypercalcemia (3.97 mmol/L; reference interval, 1.91 to 3.03 mmol/L); mild hypercholesterolemia (6.10 mmol/L; reference interval, 2.70 to 5.94 mmol/L); increased alkaline phosphatase (ALP) activity (236 U/L; reference interval, 18 to 128 U/L); increased alanine aminotransferase (ALT) activity (114 U/L; reference interval, 19 to 59 U/L), and mild hyperproteinemia (74 g/L; reference interval, 55 to 71 g/L). The serum albumin concentration was 37 g/L, within the reference interval of 28 to 38 g/L. The urine specific gravity was 1.002.
Hypercalcemia is always a concern, because concentrations greater than about 4 mmol/L can result in renal failure, mineralization of the kidneys and other soft tissues, cardiac dysrhythmia and dysfunction, and other medical problems and emergencies (1–4). Hypercalcemia is also worrisome, because the most common cause in dogs, as in humans, is cancer (hypercalcemia of malignancy) (2–4). Some other causes of hypercalcemia in dogs include acute and chronic renal failure, primary hyperparathyroidism, hypoadrenocorticism, hypervitaminosis D, bone diseases associated with osteolysis, and granulomatous inflammation (1,3–6).
On review of the WCVM SAC medical records for the 10-year period between July 1, 1994, and June 30, 2004, inclusive, 59 dogs that had had persistent hypercalcemia were found. In 16 (27.1%) of these cases, an underlying cause for the hypercalcemia was not determined. In 43 dogs, the underlying cause was associated with malignancy in 20 (46.5%), idiopathic hyperplasia or adenoma of one or more parathyroid glands in 13 (30.2%), renal failure in 6 (14.0%), granulomatous disease in the form of blastomycosis in 2 (4.7%), and hypoadrenocorticism in 2 (4.7%). Of the 20 dogs with a malignant neoplasm, 14 (70.0%) had lymphosarcoma.
When hypercalcemia is being evaluated, it is helpful to recall that serum calcium is distributed into 3 major fractions. There is some ambiguity, if not confusion, about the terminology used to characterize these fractions.
Determining the cause of hypercalcemia can be difficult. The first step in investigating a laboratory finding of elevated serum calcium concentration is to ensure that the elevation is not artifactual — for example, related to lipemia or hemolysis (1,3–5) — or due to the use of an inappropriate reference interval. Dogs less than 1 y of age, especially large breed dogs, have been reported to have serum calcium concentrations that may be 0.1–0.5 mmol/L higher than those of the reference intervals for adult dogs (4,6). Once an elevated serum calcium concentration has been determined to reflect true hypercalcemia, it is helpful to determine if it is persistent and related to an underlying derangement in calcium homeostasis or due to transient hemoconcentration, as is the case in dehydration. To illustrate this point, all calcium analyses done on canine serum samples submitted to Prairie Diagnostic Services (PDS) at the University of Saskatchewan (U of S) in 2003 were reviewed and it was found that 174 of 3677 samples (4.7%) had serum calcium values above the reference interval. The cause of an elevation in serum calcium often cannot be determined from routine serum chemical analysis; however, in the case of the above 174 samples, 43 (24.7%) were from dogs in which dehydration was thought to have been the most likely cause of the elevated serum calcium concentration and at least another 36 (20.1%) where dehydration was thought to have contributed to the elevation of the serum calcium concentration. Sixteen (9.2%) of the 174 samples were from dogs < 6 mo old. Eight (4.6%) of the 174 samples contained evidence of marked hemolysis or lipemia; however, the serum chemistry analyzer used by PDS in 2003 was capable of providing reliable serum calcium concentrations in samples with high concentrations of hemoglobin or lipid.
Although there was evidence of mild hemoconcentration in the sample from this dog, the magnitude of the hypercalcemia exceeded that which would be expected.
When hypercalcemia is being evaluated, it is helpful to recall that serum calcium is distributed into 3 major fractions. There is some ambiguity, if not confusion, about the terminology used to characterize these fractions. Some authors refer to calcium as being either ionized or bound (4,5), while others state that all calcium in body fluids is ionized, and that the ionized calcium is either free or bound (6). If this terminology is combined, about 50% of serum calcium exists as free ionized calcium, about 40% to 45% is ionically bound to anionic proteins, principally albumin, and 5% to 10% is bound to nonprotein anions such as citrate, phosphate, and lactate. Free ionized calcium is the portion of serum calcium that is biologically active, hormonally regulated, and contributes to pathologic states (4–6). Typically, free ionized calcium is elevated in dogs with hypercalcemia associated with malignancy and primary hyperparathyroidism, but not in dogs with renal failure, hypoadrenocorticism, or dehydration.
Although there was evidence of mild hemoconcentration in the sample from this dog, the magnitude of the hypercalcemia exceeded that which would be expected. Therefore, to determine the cause of the hypercalcemia, the dog was sedated and reexamined, and a sample of her serum was submitted to the medical laboratory of Royal University Hospital, U of S, for determination of the free ionized calcium concentration. Several different types of cancer have been associated with hypercalcemia of malignancy; however, lymphosarcoma is the most common (3,5). Since most dogs with lymphosarcoma and hypercalcemia will have enlargement of peripheral lymph nodes and internal organs, or a mediastinal mass (3,5), the peripheral lymph nodes were aspirated for cytologic evaluation and the thorax and abdomen were radiographed. During the physical examination, several small subcutaneous masses, found over the neck, were also aspirated for cytologic evaluation. The physical examination also included a thorough rectal examination, as adenocarcinoma of the apocrine glands of the anal sac is the second most common neoplasm associated with hypercalcemia of malignancy in dogs, and approximately 50% of dogs with this cancer are hypercalcemic at the time of diagnosis (3,5).
Serum free ionized calcium in this dog was elevated at 1.92 mmol/L (reference interval, 1.25 to 1.45 mmol/L). Cytologic evaluations did not reveal evidence of lymphosarcoma, radiographs of the thorax and abdomen were unremarkable, and there were no abnormalities detected during the rectal examination. The0 small subcutaneous masses over the neck were composed of adipocytes and free lipid, consistent with lipomas.
To determine the cause of the elevation in total and free ionized calcium concentrations, a serum sample was submitted to the Animal Health Diagnostic Laboratory at Michigan State University for measurement of free ionized calcium, intact parathyroid hormone (PTH), and parathyroid hormone-related protein (PTHrP) concentrations. A portion of PTHrP has an amino acid sequence similar to that of PTH. Parathyroid hormonerelated protein also shares many actions of PTH, causes hypercalcemia, and is secreted by some neoplastic cells (1,3). Free ionized calcium was elevated at 2.02 mmol/L (reference interval, 1.25 to 1.45 mmol/L), intact PTH was 10.1 pmol/L (reference interval, 2 to 13 pmol/L), and PTHrP was 0.4 pmol/L (normal < 1.0 pmol/L). While the PTH concentration was within the reference interval, it was considered an abnormal finding given the concurrent hypercalcemia, as its production should have been suppressed. For this reason and because there was no evidence of malignancy, including a PTHrP concentration within the reference interval, a tentative diagnosis of primary hyperparathyroidism was made and surgical examination of the parathyroid glands was recommended to the owner.
Three weeks later, the dog was readmitted for surgery. At the time of admission, the dog’s total calcium had increased to 4.28 mmol/L. She was treated with IV fluids (0.9% NaCl at 150 mL/h) and furosemide (Lasix; Hoechst Marion Roussel, Laval, Quebec), 50 mg, IV, 3 times over the course of the night, in an attempt to reduce the serum calcium concentration. The following morning her serum calcium had decreased to 3.46 mmol/L. During surgery, a firm round nodule, approximately 1 cm in diameter, was detected in the cranial pole of the left lobe of the thyroid gland, excised, placed in 10% buffered formalin, and submitted for histologic evaluation. Following the surgery, the dog was monitored carefully for signs of hypocalcemia, such as twitches, tremors, or seizures. The dog’s serum calcium concentration was low (1.81 mmol/L) immediately after surgery; therefore, she was administered vitamin D (Hytakeral; Sanofi Canada, Markham, Ontario), 250 mg, PO, and calcium, as calcium carbonate (Tums; GlaxoSmithKline, Oakville, Ontario), 700 mg, PO. Several hours later, her serum calcium concentration was within the reference interval at 2.63 mmol/L. The following morning her serum calcium was slightly elevated at 3.06 mmol/L. Four days after surgery, the dog continued to be polyuric and polydipsic; however, total serum calcium concentration at that time was within the reference interval at 2.90 mmol/L.
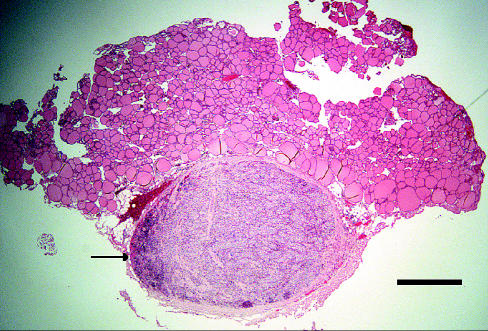
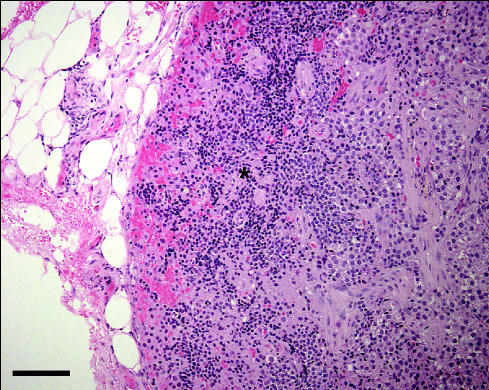
The portion of thyroid gland submitted for histologic examination included a firm, brown nodule, approximately 3 mm in diameter by 2 mm thick. Microscopic evaluation of this nodule revealed a uniform population of polygonal cells arranged in cords and nests of varying sizes, surrounded by a relatively thick fibrous tissue capsule (Figure 1). Each polygonal cell featured a moderate-sized, centrally located, round nucleus; a moderate amount of pale, foamy appearing cytoplasm; and an indistinct cell border. There appeared to be a focus of normal parathyroid gland, which was displaced and compressed at one edge of the nodule (Figure 2), and both tissues were surrounded by normal appearing thyroid gland (Figure 1). Based on these observations, a diagnosis of primary hyperparathyroidism due to a parathyroid gland adenoma was made.
Figure 1.
The histologic appearance of the nodule of tissue removed from the cranial pole of the left lobe of the thyroid gland of a 10-year-old, spayed female, beagle-cross dog with persistent hypercalcemia. The central, well demarcated, pale stained mass was diagnosed as a parathyroid gland adenoma. Note the increased cellularity at one edge of the mass (arrow). The mass was surrounded by normal thyroid gland follicles. Hematoxylin and eosin stain; bar = 1 mm.
Figure 2.
A higher magni.cation image of the mass as in Figure 1. The small cells with the small, dark staining nuclei at the edge of the mass (*) were interpreted to be normal parathyroid gland cells that had been displaced and compressed by the expanding adenoma. Hematoxylin and eosin stain; bar = 10 μm.
Primary hyperparathyroidism is defined by the autonomous and excessive secretion of PTH from 1 or more of the parathyroid glands resulting in persistent hypercalcemia. The disease is usually caused by a solitary PTH-secreting adenoma, but focal and multifocal primary parathyroid gland hyperplasia and parathyroid gland carcinoma have also been described (2,3).
The dog’s serum calcium concentration remained within the reference interval at 4, 11, and 18 d after surgery and the dog’s owners reported that the polyuria and polydipsia waned. The dog was treated with vitamin D (Hytakeral; Sanofi Canada), 250 mg, PO, q24h, and calcium, as calcium carbonate (Tums; GlaxoSmithKline), 300 mg, PO, q24h; these medications were tapered over 3 to 4 wk. Serum calcium concentration 10 mo after surgery was within the reference interval at 2.42 mmol/L. This was an important finding, because a small proportion (< 10%) of dogs with primary hyperparathyroidism and successful removal of a single adenoma will have recurrence of hypercalcemia due to a second parathyroid mass (3). A CBC done 2.5 y following surgery again showed mild erythrocytosis and mildly elevated hematocrit. Multiple serum chemical analyses have been performed since the dog had surgery and mild elevations in cholesterol concentration, ALP activity, and ALT activity have persisted, but a cause has not been determined. Serum calcium concentration has remained within the reference interval. The owner has reported that while the dog’s polyuria waned, it never resolved.
This case serves as a reminder of the complexities sometimes associated with determining the cause of hypercalcemia in the dog and emphasizes the valuable role that diagnostic laboratories can play in the process.
Footnotes
Dr. Sakals’ current address is Small Animal Clinic, Veterinary Teaching Hospital, Western College of Veterinary Medicine.
Dr. Sakals was an undergraduate veterinary student supported by a Western College of Veterinary Medicine Interprovincial Undergraduate Student Summer Research Award when this work was conducted.
Dr. Fernandez’s addresses are Calgary Animal Referral and Emergency Centre, 7140–12 Street, SE, Calgary, Alberta T2H 2Y4 and Faculty of Veterinary Medicine, University of Calgary, Health Sciences G380, 3330 Hospital Drive, NW, Calgary, Alberta T2N 4N1.
References
- 1.Bergman PJ. Paraneoplastic syndromes. In: Withrow SJ, MacEwen EG, eds. Small Animal Clinical Oncology. 3rd ed. Philadelphia: Saunders, 2001:35–53.
- 2.Capen CC. Tumors of the endocrine glands. In: Meuten DJ, ed. Tumors in Domestic Animals. 4th ed. Ames, Iowa: Iowa State Univ Pr, 2002:607–696.
- 3.Feldman EC, Nelson RW. Canine and Feline Endocrinology and Reproduction, 3rd ed. Philadelphia: Saunders, 2004:660–715.
- 4.Nelson RW, Turnwald GH, Willard MD. Endocrine, metabolic, and lipid disorders. In: Willard MD, Tvedten H, eds. Small Animal Clinical Diagnosis by Laboratory Methods. 4th ed. St. Louis: Saunders, 2004: 165–207.
- 5.Ferguson DC, Hoenig M. Endocrine system. In: Latimer KS, Mahaffey EA, Prasse KW, eds. Duncan and Prasse’s Veterinary Laboratory Medicine Clinical Pathology. 4th ed. Ames, Iowa: Iowa State Univ Pr, 2003: 270–303.
- 6.Stockham SL, Scott MA. Fundamentals of Veterinary Clinical Pathology. Ames, Iowa: Iowa State Press, 2002:401–432.