Abstract
A dog was presented with a history of dyspnea, coughing, and ascites. Angiostrongylosis and severe pulmonary arterial hypertension (PAH) were found, as well as a marked discordance between the electrical and mechanical events of the heart. Pulmonary arterial hypertension related to Angiostrongylus vasorum has rarely been reported.
Résumé
Hypertension artérielle pulmonaire importante causée par Angiostrongytosis vasorum chez un chien. Une hypertension artérielle pulmonaire (HTAP) importante, due à une angiostrongylose, a été diagnostiquée chez un chien présentant de la dyspnée, de la toux, une ascite ainsi qu’une discordance marquée entre les évènements électriques et mécaniques cardiaques. L’HTAP due à Angiostrongylus vasorum, a été rarement décrite.
(Traduit par les auteurs)
A 2 year-old, male Yorkshire terrier was presented at the cardiology unit of the national veterinary school of Alfort with a 1-week history of dyspnea, coughing, abdominal distension, and mild exercise intolerance.
Case description
On physical examination, the dog was alert, dyspneic, and tachypneic. Mucous membranes were pink and moist, and capillary refill time was normal. Cardiac auscultation revealed a grade V/VI systolic murmur and a low grade diastolic murmur (I/VI) over the tricuspid area. Tachycardia was present. Auscultation of the lungs revealed increased bilateral respiratory sounds. Routine biochemical analyses were unremarkable.
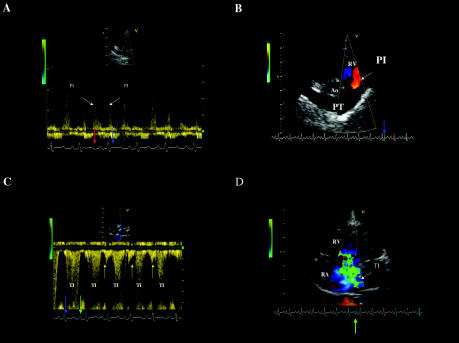
An electrocardiogram demonstrated right axis deviation. Thoracic radiographs showed mild right heart enlargement, with widening of the intrathoracic segment of the caudal vena cava and evidence of enlarged pulmonary arteries. Diffuse bronchial patterns and alveolar infiltrates in the left caudal lung lobe were also found. Abdominal radiographs showed a loss of detail consistent with ascites. Fluid obtained by abdominocentesis was consistent with a modified transudate (serosanguineous fluid, specific gravity = 1.027, proteins = 27 g/L, < 2000 cells/mm3). Echocardiographs were taken according to the recommendations of the American Society of Echocardiography, using a cardiac ultrasound system (Vingmed Vivid 5; General Electric Medical System, Waukesha, Wisconsin, USA) equipped with a 3,5-5 MHz phased-array transducer. The 2-dimensional right parasternal, short-axis transaortic, and transventricular views revealed a mildly enlarged main pulmonary artery associated with severe right atrial and ventricular dilatation. The M-mode echocardiogram confirmed the severe right ventricular dilatation, with an increased myocardial wall thickness, and a reduced left ventricle chamber size associated with paradoxical motion of the interventricular septum. The continuous-wave Doppler plot of the right ventricular outflow tract obtained from the right parasternal short-axis position showed diastolic pulmonary insufficiency ending during the isovolumic contraction period (Figure 1A). Prolongation of pulmonary insufficiency during the isovolumic contraction phase was confirmed by color-flow Doppler (Figure 1B). The end-diastolic velocity of the pulmonary insufficiency was 2.5 m/s. When the modified Bernoulli equation was used, the end-diastolic pressure gradient was 25 mmHg, suggesting that the pulmonary arterial diastolic pressure was elevated. The maximum pulmonary flow velocity was within the normal range (0.85 m/s, normal range = 1 ± 0.3 m/s) (1). The flow profile was, however, abnormal, showing a rapid rise in pulmonary velocity followed by delayed deceleration and midsystolic notching. This pulmonary ejection profile is a common finding in dogs with pulmonary arterial hypertension (PAH) (2). Continuous-wave Doppler examination performed from the left apical 4-chamber view (Figure 1C) identified marked tricuspid insufficiency beginning during the isovolumic contraction phase, continuing during systole and isovolumic relaxation, and ending in the middle of P wave. Prolongation of the tricuspid regurgitation during early electrical diastole was confirmed by using color-flow Doppler (Figure 1D, frame taken between the end of the T wave and the beginning of the P wave). The maximal peak velocity of the tricuspid insufficiency was 5 m/s. When the modified Bernoulli equation was used, the systolic pressure gradient across the tricuspid valve was 100 mmHg. The systolic arterial pulmonary pressure was estimated to be at least 115 mmHg -100 mmHg (for the tricuspid insufficiency) added to the estimate of right atrial pressure (15 mmHg in patients with right heart failure [3]). A late diastolic negative signal occurring after the P wave onset was also observed (Figure 1C). Since the tricuspid diastolic and systolic flows were analyzed by using continuous-wave rather than pulsed-wave Doppler mode, we cannot confirm that the late diastolic negative signal originated at the coaptation point of the tricuspid valves. The signal may have arisen from diastolic tricuspid regurgitation or from reversal of flow (A reversal) in the caudal vena cava. This Doppler study therefore permitted documentation of the presence of severe systolic and diastolic PAH and cor pulmonale.
Figure 1. Doppler echocardiograms from the dog with severe pulmonary arterial hypertension.
A. The continuous-wave Doppler tracing of the right ventricular outflow tract obtained from the right parasternal short axis position showed diastolic pulmonary insufficiency (positive waves, PI) beginning after the T wave (red arrow) and ending during the isovolumic contraction period (blue arrow).
B. The prolongation of pulmonary insufficiency (PI) during isovolumic contraction (blue arrow) was confirmed by color-flow Doppler from the right parasternal short axis position. Blood flow (red color) is directed toward the transducer into the right ventricular outflow tract.
C. Continuous-wave Doppler examination performed from the left apical 4-chamber view identifying a marked tricuspid insufficiency (negative waves, TI) beginning during the isovolumic contraction phase (blue arrow), continuing during systole and isovolumic relaxation, and ending in the middle of P wave (green arrow). A late diastolic negative signal occurring after the P wave onset can also be seen (yellow arrows). This may arise from diastolic tricuspid regurgitation or from reversal of flow (A reversal) in the caudal vena cava.
D. Prolongation of the tricuspid insufficiency (TI) during early electrical diastole was confirmed using color-flow Doppler mode, frame taken between the end of T wave and the beginning of P wave (green arrow). The large turbulent jet of TI extends from the right ventricle to the dorsal wall of the severely enlarged right atrium.
RA — Right atrium; RV — Right ventricle; Ao — Aorta; PT — Pulmonary trunk
Due to these cardiovascular and pulmonary lesions, lungworm infection was suspected. Coproscopic examination (Baermann’s technique and flotation examination) and serologic testing (heartworm infestation) were performed. The dog was free of heartworm, but many Angiostrongylus vasorum larvae were found in the feces (Baermann’s technique).
In this case, the aim of treatment was first to control the right-side heart failure by using standard therapy (diuretics and angiotensin converting enzyme [ACE] inhibitor) and then to treat the cause of the PAH (angiostrongylosis) by using fenbendazole (Panacur; Intervet SA, An Boxmeer, The Netherlands) 30 mg/kg bodyweight (BW), PO, q24h, for 3 wk.
Discussion
This case describes severe PAH induced by angiostrongylosis, with secondary pulmonic valve and tricuspid valve regurgitation. Angiostrongylus vasorum is a metastrongyloid helminth parasite that attacks domestic dogs and related wild carnivores (4,5). The adult worms reside in the pulmonary arterial vasculature, with the adult laying eggs in the terminal pulmonary arterioles (6). Though A. vasorum is known to be endemic in Europe (France, Ireland, Denmark), reports in many other countries, including Canada, are sporadic (4,7).
Reported clinical signs are diverse, but 2 clinical syndromes predominate: 1) respiratory disease as the result of pneumonitis (caused by an inflammatory response to eggs and migrating larvae), pulmonary hemorrhage, or fibrosis, and 2) bleeding diatheses due to coagulapathy, with local or diffuse hemorrhages (neurological, conjunctival, etc.) (4,8,9). Clinical signs associated with PAH and right heart failure have also been reported (exercise intolerance, weight loss, ascites, collapse, sudden death) (8). Though pulmonary lesions are commonplace with A. vasorum, few reports to date have described PAH associated with this lungworm. Little information is available on the frequency or prognosis of PAH under these circumstances.
A low-grade diastolic murmur was heard over the tricuspid area despite the high heart rate (160 bpm). However, no phonocardiography was performed to confirm this murmur, and this is one of the limitations of this case report. Diastolic regurgitation is usually of too low a velocity to be heard. Pulmonary insufficiency should be best heard over the left heart base. We think that the diastolic component of the murmur was due to pulmonary insufficiency, and was heard over the tricuspid area because of the right heart enlargement and the cardiac displacement within the thorax (cor pulmonare).
Angiostrongylosis is diagnosed by detection of A. vasorum larvae in feces by using Baermann’s technique, by smears (bronchoalveolar or endotracheal lavage), and possibly by fine needle aspiration of the lung. A negative fecal sample does not definitively rule out a diagnosis of angiostrongylosis; serial examinations may be necessary to demonstrate the presence of the parasite (4,7,10).
Several additional tests can be performed for a fuller exploration of patients with angiostrongylosis. A complete blood (cell) count (CBC) will usually identify eosinophilia and, less often, neutrophilia, monocytosis, basophilia, and lymphocytosis (4). Thrombocytopenia and anemia have also been reported (4). Biochemical analyses may reveal mild to moderate hyperglobulinemia and, sometimes, mild hypercalcemia (4,6). Echocardiographs may reveal PAH. Thoracic radiographs are usually abnormal, typically showing a patchy, peripheral alveolar-interstitial pattern (4). Unfortunately, because of the owner’s financial limitations, neither a blood gas analysis nor a CBC was done in this case.
Pulmonary arterial hypertension may be primary or due to left ventricular failure, left atrial hypertension, or pulmonary vascular obstruction, or, as in this case, lung disease, pulmonary vascular disease, or both (11). The diagnosis of PAH may be made easily by using color-flow and spectral Doppler, combined with 2-dimensional and M-mode echocardiography. The lesions induced by PAH may include moderate-to-severe right ventricular concentric and eccentric hypertrophy, right atrial dilatation, moderate- to-severe dilatation of the main pulmonary artery and its branches, paradoxical septal motion, systolic septal flattening, reduced left ventricular internal diameter, changes in the pulmonary flow profile and velocity, and pulmonary and tricuspid valve insufficiency (3,12). In this case, PAH was diagnosed on the basis of the Doppler examination findings. The peak velocity of systolic tricuspid insufficiency was measured by using the continuous-wave Doppler mode. The modified Bernouilli equation provides a way for estimating pressure gradient across the tricuspid valve (systolic pressure gradient between right atrium and right ventricle). Without pulmonary stenosis, the right systolic ventricular pressure is nearly equal to the systolic pulmonary pressure. The diastolic right atrial pressure is usually estimated at 5 mmHg and at 10 to 15 mmHg in patients without and with right heart failure, respectively. Systolic pulmonary arterial pressure is estimated by adding the systolic pressure gradient across the tricuspid valve to the right atrial pressure. Therefore, high-velocity tricuspid insufficiency (> 2.6 m/s) usually correlates with high right ventricular systolic pressure (2), and in the absence of right ventricular outflow obstruction, high-velocity tricuspid regurgitation usually indicates systolic PAH (3). Nevertheless, although continuous-wave Doppler gives a good estimation of systolic and diastolic pulmonary arterial pressures when the Bernoulli formula is used, cardiac catheterization is the definitive diagnostic procedure for PAH (3,11). Though catheterization is the gold standard for assessing pulmonary arterial pressure, it would have been too invasive in this case. Since pulmonary pressures were estimated by using the modified Bernouilli equation and not measured invasively, pulmonary arterial pressure may have been underestimated: the gradient method can underestimate pulmonary pressure by anything up to 20 mmHg, even when right atrial pressures are allowed for. Underestimation is greater at very high pulmonary artery pressures (3,12). Nevertheless, it can be assumed that systemic arterial pressure in this dog was at least 115 mmHg.
The unusual feature of this case was the prolongation of some time intervals, which made systolic and diastolic blood flow very discordant with the ECG tracing: systolic tricuspid regurgitation extending into early electrical diastole and diastolic pulmonary insufficiency ending during electrical systole were identified by using continuous and color-flow Doppler modes. Since tricuspid regurgitation occurs as long as right ventricular pressure is higher than atrial pressure, from isovolumic contraction time (IVCT) to isovolumic relaxation time (IVRT), and since PAH is known to be associated with higher IVRT and IVCT, this may explain why tricuspid regurgitation continued in early electrical diastole in this case (13). Furthermore, heart diseases characterized by high afterload and reduced systolic function, such as PAH, are known to be associated with electromechanical delays, regardless of QRS duration (with or without bundle block branch), meaning that “true” systole (mechanical systole with blood ejection) does not strictly match the ECG plot (14–16). Both electromechanical delays and higher IVCT may explain why in this case pulmonary insufficiency finished during electrical systole and not during diastole.
Human PAH patients are frequently treated with digoxin, vasodilators, diuretics, anticoagulants, and supplemental oxygen (17). Use of prostacyclin, prostacyclin analogue, nitric oxide, phosphodiesterase type 5 inhibitor, or dual endothelin-receptor antagonist seems to be beneficial (17,18). In case of angiostrongylosis, several anthelmintic protocols have been described previously (7,19,20): fenbendazole from 20 mg/kg BW, PO, q24h, for 2 or 3 wk, mebendazole (50 to 100 mg/kg BW, PO, q24h, 5 wk), levamisole (5 to 7 mg/kg BW, twice at 10-day intervals), ivermectin (0.2 mg/kg BW, twice at 10-day intervals), milbemycin (0.5 mg/kg BW, twice at 7-day intervals).
As in humans, the prognosis of primary and secondary PAH in canine patients is often poor and treatment often unsuccessful (3,21). However, in this dog, resolution of the clinical signs of severe PAH were expected. Previous authors have already described normalization in PAH after adequate antihypertensive and anthelmintic treatment (22). CVJ
References
- 1.Kienle RD. Echocardiography. In: Kienle RD, Kittelson MD, eds. Small Animal Cardiovascular Medicine. St Louis: Mosby, 1998:95–117.
- 2.Johnson L, Boon J, Orton EC. Clinical characteristics of 53 dogs with Doppler-derived evidence of pulmonary hypertension: 1992–1996. J Vet Intern Med. 1999;13:440–447. doi: 10.1892/0891-6640(1999)013<0440:ccodwd>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 3.Kienle RD, Kittelson MD. Pulmonary arterial and systemic arterial hypertension. In: Kienle RD, Kittelson MD, eds. Small Animal Cardiovascular Medicine. St Louis: Mosby, 1998:433–448.
- 4.Chapman PS, Boag AK, Guitian J, Boswood A. Angiostrongylus vasorum infection in 23 dogs (1999–2002) J Small Anim Pract. 2004;45:435–440. doi: 10.1111/j.1748-5827.2004.tb00261.x. [DOI] [PubMed] [Google Scholar]
- 5.Garosi LS, Platt SR, McConnell JF, Wrayt JD, Smith KC. Intracranial haemorrhage associated with Angiostrongylus vasorum infection in three dogs. J Small Anim Pract. 2005;46:93–99. doi: 10.1111/j.1748-5827.2005.tb00300.x. [DOI] [PubMed] [Google Scholar]
- 6.Boag AK, Lamb CR, Chapman PS, Boswood A. Radiographic findings in 16 dogs infected with Angiostrongylus vasorum. Vet Rec. 2004;154:426–430. doi: 10.1136/vr.154.14.426. [DOI] [PubMed] [Google Scholar]
- 7.Bolt G, Monrad J, Koch J, Jensen AL. Canine angiostrongylosis: a review. Vet Rec. 1994;135:447–452. doi: 10.1136/vr.135.19.447. [DOI] [PubMed] [Google Scholar]
- 8.Bourque A, Conboy G, Miller L, Whitney H, Ralhan S. Angiostrongylus vasorum infection in 2 dogs from Newfoundland. Can Vet J. 2002;43:876–879. [PMC free article] [PubMed] [Google Scholar]
- 9.Cury MC, Lima WS. Rupture of the femoral artery in a dog infected with Angiostrongylus vasorum. Vet Parasitol. 1996;65:313–315. doi: 10.1016/s0304-4017(96)00991-0. [DOI] [PubMed] [Google Scholar]
- 10.Patteson MW, Gibbs C, Wotton PR, Day MJ. Angiostrongylus vasorum infection in seven dogs. Vet Rec. 1993;133:565–570. [PubMed] [Google Scholar]
- 11.Rich S, Braunwald E, Grossman W. Pulmonary hypertension. In: Rich S, Braunwald E, Grossman W, eds. Heart disease – A Textbook of Cardiovascular Medicine. 4th ed. Philadelphia: WB Saunders, 1997:780–806.
- 12.Boon JA. Acquired heart disease, pulmonary hypertension. In: Boon JA, ed. Manual of Veterinary Echocardiography. Baltimore: Williams and Wilkins, 1998:342–352.
- 13.Yeo TC, Dujardin KS, Tei C, Mahoney DW, McGoon MD, Seward JB. Value of a Doppler-derived index combining systolic and diastolic time intervals in predicting outcome in primary pulmonary hypertension. Am J Cardiol. 1998;81:1157–1161. doi: 10.1016/s0002-9149(98)00140-4. [DOI] [PubMed] [Google Scholar]
- 14.Bax JJ, Molhoek SG, van Erven L, et al. Usefulness of myocardial tissue Doppler echocardiography to evaluate left ventricular dyssynchrony before and after biventricular pacing in patients with idiopathic dilated cardiomyopathy. Am J Cardiol. 2003;91:94–97. doi: 10.1016/s0002-9149(02)03009-6. [DOI] [PubMed] [Google Scholar]
- 15.Ghio S, Constantin C, Klersy C, et al. Interventricular and intraventricular dyssynchrony are common in heart failure patients, regardless of QRS duration. Eur Heart J. 2004;25:571–578. doi: 10.1016/j.ehj.2003.09.030. [DOI] [PubMed] [Google Scholar]
- 16.Yu CM, Lin H, Zhang Q, Sanderson JE. High prevalence of left ventricular systolic and diastolic asynchrony in patients with congestive heart failure and normal QRS duration. Heart. 2003;89:54–60. doi: 10.1136/heart.89.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barst RJ, Ivy D, Dingemanse J, et al. Pharmacokinetics, safety, and efficacy of bosentan in pediatric patients with pulmonary hypertension. Clin Pharmacol Ther. 2003;4:372–382. doi: 10.1016/s0009-9236(03)00005-5. [DOI] [PubMed] [Google Scholar]
- 18.Opitz CF, Wensel R, Bettmann M, et al. Assessment of the vasodilator response in primary pulmonary hypertension comparing prostacyclin and iloprost administered by either infusion or inhalation. Eur Heart J. 2003;24:356–365. doi: 10.1016/s0195-668x(02)00302-0. [DOI] [PubMed] [Google Scholar]
- 19.Crawford P, Brooks H, Chantrey J, Gibbons LM. Angiostrongylus vasorum in dogs. Vet Rec. 2001;148:251–252. [PubMed] [Google Scholar]
- 20.Conboy G. Natural infections of Crenosoma vulpis and Angiostrongylus vasorum in dogs in Atlantic Canada and their treatment with milbemycin oxime. Vet Rec. 2004;155:16–18. doi: 10.1136/vr.155.1.16. [DOI] [PubMed] [Google Scholar]
- 21.Bendayan D, Shitrit D, Ygla Y, Huerta M, Fink G, Kramer MR. Hyperuricemia as a prognostic factor in pulmonary arterial hypertension. Respir Med. 2003;97:130–133. doi: 10.1053/rmed.2003.1440. [DOI] [PubMed] [Google Scholar]
- 22.Esteves I, Tessier D, Dandrieux J, et al. Reversible pulmonary hypertension in a dog presenting simultaneously with an atrial septal defect and angiostrongylosis. J Am Vet Med Assoc. 2004;45:206–209. doi: 10.1111/j.1748-5827.2004.tb00226.x. [DOI] [PubMed] [Google Scholar]