Since late fall of 2004, we have observed the emergence of new respiratory, digestive, hemolymphatic, vascular, and renal lesions associated with Porcine circovirus-2 (PCV-2) infection in pigs in Ontario. These lesions were seen in late nursery, grower, and finisher pigs, 2–3 wk after placement. Affected pigs had a rasping cough, diarrhea, or both. Pigs were often in poor body condition and had generalized lymphadenopathy.
At necropsy, many pigs with respiratory signs had prominent pulmonary interlobular edema. Histologically, interlobular septa were widely expanded by edema and alveoli were flooded by proteinaceous edema fluid. Lymphohistiocytic bronchointerstitial pneumonia was present, with mild peribronchial and perivascular lymphohistiocytic cuffing, although infiltration of mononuclear inflammatory cells into alveolar septa was generally much less pronounced than in previous PCV-2 cases. Acute lymphoid necrosis was frequently present in spleen and tonsil from multiple pigs. Immunohistochemical (IHC) staining, using rabbit polyclonal anti-PCV-2 antiserum, identified heavier PCV-2 antigen loads in these more recent cases than we had seen prior to the fall of 2004. Although present in the majority of cases, laboratory tests did not consistently identify the presence of other pulmonary pathogens, such as Streptococcus suis, Mycoplasma hyopneumoniae, Swine influenza virus, and Porcine respiratory reproductive syndrome virus (PRRSV).
In addition, enterocolitis with slurry-like to fluid diarrhea was observed in association with PCV-2 infection in grower and finisher pigs. Grossly in affected pigs, there was mural thickening in jejunum, ileum, and colon that was reminiscent of Lawsonia intracellulare-induced porcine proliferative enteritis (PPE). Histologically, these animals had granulomatous enteritis and colitis, with extensive infiltration of histiocytes, lymphocytes, plasma cells, and variable numbers of multinucleated giant cells throughout the lamina propria and submucosa. In multiple lymph nodes and submucosal follicles (GALT), there was widespread, ongoing lymphocytolysis and/or lymphoid depletion with histiocytic infiltration that was more severe and extensive than in cases evaluated prior to late fall, 2004. Large numbers of botryoid basophilic intracytoplasmic inclusions typical of PCV-2 were often present in both histiocytes and multinucleated giant cells. Immunohistochemically, positive staining for PCV-2 antigen was evident in the cytoplasm of variable numbers of histiocytes within foci of granulomatous inflammation.
Concurrently, we saw an increase in the number of animals with PCV-2-associated disease with concurrent vasculitis involving kidney, spleen, and/or lymph node, although actual case numbers remained small. Acute glomerulonephritis was evident in kidney, with multifocal expansion of glomerular tufts by fibrin. Occasional foci of segmental to circumferential fibrinoid vascular necrosis were evident in the target tissues. Single or multifocal splenic infarcts were occasionally observed. Evidence of vasculitis and splenic infarction prompted consideration of classical swine fever as a differential diagnosis in some cases. Cutaneous lesions typical of porcine dermatopathy/nephropathy syndrome (PDNS) were not evident in affected animals. Although variable amounts of PCV-2 antigen were present in other tissues, including lung, antigen was rarely found in association with vascular lesions. However, this does not exclude the possibility of an immune-mediated pathogenesis associated with PCV-2 for these vascular changes. We also saw several cases of pigs with neurological signs, where PCV-2 antigen was demonstrated in endothelial or inflammatory cells in brain, as well as in other tissues including lymphoid organs.
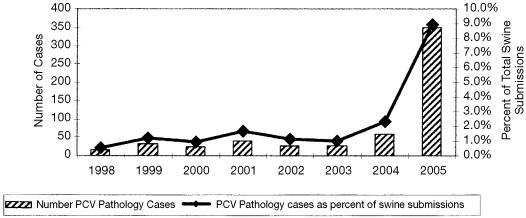
In 2004, PCV-2-associated disease was identified in a total of 60 cases submitted to the Animal Health Laboratory, representing 2.3% of all swine cases submitted. In 2005, our computerized recording system showed a dramatic increase in PCV-2-associated disease, with a total 350 pathology cases associated with PCV-2 and representing 8.9% of all swine cases submitted in 2005 (Figure 1).
Figure 1.
Number of PCV-2 Pathology Cases and Percent of PCV-2 Pathology Cases of Total Swine Submissions Animal Health Laboratory, University of Guelph.
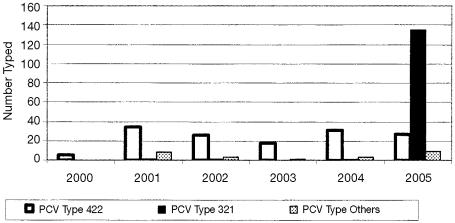
The PCV-2 polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) typing showed a significant change from the RFLP type 422 seen in years prior to 2004 to the RFLP type 321 (Figure 2), with the number of cases with RFLP type 321 increasing from 1 in 2004 to 135 in 2005. This change in RFLP typing patterns reflects a consistent change in the gene sequence recognized by 2 restriction enzymes. These Ontario RFLP type 321 viruses have greater than 99% sequence homology with each other, and 98% sequence homology with those reported for the UK, France, and China. However, these Ontario RFLP type 321 viruses have only 91.6% sequence homology with the previously dominant Ontario RFLP type 422 viruses and are only 92% to 93% similar to those previously reported from the USA.
Figure 2.
PCR-RFLP typing of PCV-2 Animal Health Laboratory, University of Guelph.
References
- 1.van Dreumel T, Josephson G, Lusis P. Porcine circovirus-2 associated conditions in pigs. AHL Newsletter. 2005;9:5. [Google Scholar]
- 2.DeLay J, McEwen B, Carman S, Fairles J, van Dreumel T. Porcine circovirus 2-associated disease is increasing. AHL Newsletter. 2005;9:22. [PMC free article] [PubMed] [Google Scholar]
- 3.Carman S, McEwen B, DeLay J, Cai H, Fairles J, van Dreumel T. Porcine circovirus type 2 — associated disease continues to increase. AHL Newsletter. 2005;9:30. [Google Scholar]
- 4.Carman S, McEwen B, DeLay J, Cai H, Fairles J, van Dreumel T. Porcine circovirus type 2 — associated disease continued over the fall of 2005. AHL Newsletter. 2006;10:6. [Google Scholar]