Abstract
In vivo studies have characterized the pharmacodynamic characteristics of the triazole fluconazole. These investigations demonstrated that the ratio of the area under the concentration-time curve from 0 to 24 h to the MIC (24-h AUC/MIC ratio) is the critical pharmacokinetic/pharmacodynamic (PK/PD) parameter associated with treatment efficacy. Further analysis demonstrated that a fluconazole 24-h AUC/MIC ratio of 20 to 25 was predictive of treatment success in both experimental models and clinical trials. We used a neutropenic murine model of disseminated Candida albicans infection to similarly characterize the time course activity of the new triazole ravuconazole. The PK/PD parameters (percent time above the MIC, AUC/MIC ratio, and peak level in serum/MIC ratio) were correlated with in vivo efficacy, as measured by organism number in kidney cultures after 24 and 72 h of therapy. Ravuconazole kinetics and protein binding were performed in neutropenic infected mice. Peak/dose and AUC/dose values ranged from 0.03 to 0.04 and 0.30 to 0.34, respectively. Serum elimination half-life ranged from 3.9 to 4.8 h. Protein binding was 95.8%. Single-dose postantifungal effect studies demonstrated prolonged suppression of organism regrowth after serum ravuconazole levels had fallen below the MIC. Treatment efficacies with the five dosing intervals studied were similar, supporting the argument for the AUC/MIC ratio as the PK/PD parameter predictive of efficacy. Nonlinear regression analysis also suggested that the AUC/MIC ratio was strongly predictive of treatment outcomes (AUC/MIC ratio, R2 = 91%; peak/MIC ratio, R2 = 85%; percent time above the MIC, R2 = 47 to 65%). Similar studies were conducted with seven additional C. albicans isolates with various ravuconazole susceptibilities (MIC, 0.016 to 0.12 μg/ml) to determine if a similar 24-h AUC/MIC ratio was associated with efficacy. The ravuconazole free-drug AUC/MIC ratios were similar for all of the organisms studied (10 to 36; mean ± SD = 20.3 ± 8.2; P = 0.43). These free-drug AUC/MIC ratios are similar to those observed for fluconazole in this model.
Antimicrobial pharmacodynamic characterizations have provided insight into the link between drug exposures and treatment efficacy. Therapeutic outcome predictions based upon these pharmacodynamic relationships have correlated well in treatment against both susceptible and resistant pathogens (2). This has proven useful for the design of optimal dosing regimens and the development of susceptibility breakpoint guidelines (7, 8, 18).
Numerous studies with a variety of antibacterial drugs have demonstrated that the pharmacokinetic/pharmacodynamic (PK/PD) parameter predictive of in vivo efficacy is similar for drugs within the same class (4, 28). Furthermore, studies have suggested that the magnitude of the PK/PD parameter necessary for efficacy is relatively similar for drugs within the same class. These investigations have also shown that similar parameter magnitudes are needed to produce efficacy in different animal species including humans.
Prior in vivo studies have demonstrated that the PK/PD parameter predictive of fluconazole efficacy against Candida albicans is the ratio of the area under the concentration-time curve from 0 to 24 h to the MIC (24-h AUC/MIC ratio) (1, 16). Studies have also suggested that a 24-h AUC/MIC ratio in the range of 20 to 25 is associated with treatment efficacy in experimental in vivo models when defined as the microbiologic endpoint 50% effective dose (ED50) or 80% survival in animals (1, 16, 22, 23, 27; K. Sorenson, S. Corcoran, S. Chen, D. Clark, V. Tembe, O. Lomovskaya, and M. Dudley, Abstr. 39th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 1271, p. 328, 1999). This AUC/MIC ratio has also been shown to be predictive of outcomes in fluconazole clinical trials (1, 14, 21; C. J. Clancy, C. A. Kauffman, A. Morris, M. L. Nguyen, D. C. Tanner, D. R. Snydman, V. L. Yu, and M. H. Nguyen, Progr. Abstr. 36th Annu. Meet. Infect. Dis. Soc. Am., abstr. 98, p. 93, 1998).
Similar in vivo studies with other drugs within the triazole class have not been reported. In the present study we have characterized the PK/PD parameter that is predictive of efficacy of a new triazole, ravuconazole, in a neutropenic murine model of disseminated candidiasis. Furthermore, we have determined the magnitude of the PK/PD parameter required to achieve efficacy for numerous strains of C. albicans with various azole susceptibilities in order to provide a framework for the rational development of dosing regimens as well as the development of preliminary in vivo breakpoints for ravuconazole.
MATERIALS AND METHODS
Organisms.
Eight clinical isolates of C. albicans (designated K-1, 412, 580, 98-17, 98-234, 2512, 2438, and 2183) were utilized. The isolates were chosen to include both fluconazole-susceptible and -resistant strains. Isolates were also chosen based upon a relatively similar degree of virulence in this animal model as determined by amount of growth in the kidneys of untreated animals over 24 h (data not shown). C. albicans K-1 and 580 were isolated from patients with systemic candidiasis. The other isolates were from mucosal infections. Isolates 412, 2307, 2512, and 2183 were kindly provided by J. Lopez-Ribot et al. (15). Three of the isolates provided by Lopez-Ribot are fluconazole resistant. The resistance mechanism(s) responsible for reduced susceptibility has been previously characterized and is reported in Table 1. The organisms were maintained, grown, subcultured, and quantified on Sabouraud dextrose agar (SDA) slants (Difco Laboratories, Detroit, Mich.). Twenty-four hours prior to study, organisms were subcultured at 35°C.
TABLE 1.
Comparative efficacies of ravuconazole against eight strains of C. albicans in a neutropenic murine model
| C. albicans strain | Ravuconazole
|
Fluconazole MIC (μg/ml) | Comment | ||||
|---|---|---|---|---|---|---|---|
| MIC (μg/ml) | ED50 (mg/kg) | 24-h AUC (mg · h/liter) | 24-h AUC/MIC ratio | Free-drug 24-h AUC/MIC ratioa | |||
| K-1 | 0.03 | 48.9 | 16.4 | 547 | 23.7 | 0.25 | |
| 412 | 0.016 | 27.0 | 9.25 | 578 | 24.3 | 0.5 | |
| 98-17 | 0.12 | 204 | 60.2 | 502 | 21.1 | 16 | |
| 98-234 | 0.12 | 125.6 | 38.2 | 318 | 13.1 | 32 | |
| 580 | 0.06 | 73.1 | 25.0 | 416 | 17.8 | 4 | |
| 2512 | 0.03 | 34.2 | 11.7 | 390 | 16.4 | 32 | ↑MDR, ↑ERG11 |
| 2307 | 0.016 | 40.8 | 14.0 | 873 | 36.7 | >128 | ↑CDR, ↑ERG11 |
| 2183 | 0.06 | 41.8 | 14.3 | 238 | 10.0 | >128 | ↑CDR |
P = 0.43.
Antifungal.
Ravuconazole was obtained as a powder from Bristol-Myers Squibb Pharmaceuticals (92.7% purity). The powder was stored desiccated at −4°C. Drug solutions were prepared on the day of study by dissolving the powder in dimethyl sulfoxide and uniformly suspended and diluted further in 0.5% sodium carboxymethyl cellulose.
In vitro susceptibility testing.
MICs were determined using the NCCLS M27-A method (17). Determinations were performed in duplicate on two separate occasions. Final results are expressed as the geometric means of these results.
Animals.
Six-week old ICR/Swiss specific-pathogen-free female mice weighing 23 to 27 g were used for all studies (Harlan Sprague-Dawley, Indianapolis, Ind.). Animals were maintained in accordance with the American Association for Accreditation of Laboratory Care criteria (19). All animal studies were approved by the Animal Research Committee of the William S. Middleton Memorial VA Hospital.
Infection model.
Mice were rendered neutropenic (polymorphonuclear leukocytes < 100/mm3) by injecting cyclophosphamide (Mead Johnson Pharmaceuticals, Evansville, Ind.) intraperitoneally 4 days (150 mg/kg of body weight) and 1 day (100 mg/kg) before infection. Absolute white blood cell and neutrophil counts were monitored every 24 h (q24h) throughout the period of study with a Coulter counter and peripheral blood smears, respectively. Neutrophil counts remained at or below 100/mm3 throughout the study.
Organisms were subcultured on SDA 24 h prior to infection. The inoculum was prepared by placing three to five colonies into 5 ml of sterile pyrogen-free 0.9% saline warmed to 35°C. The final inoculum was adjusted to 0.6 transmittance at 530 nm. Fungal counts of the inoculum determined by viable counts on SDA were 106 CFU/ml.
Disseminated infection with the Candida organisms was achieved by injection of 0.1 ml of inoculum via lateral tail vein 2 h prior to start of drug therapy. At the end of the study period animals were sacrificed by CO2 asphyxiation. After sacrifice the kidneys of each mouse were immediately removed and placed in sterile 0.9% saline at 4°C. The homogenate was then serially diluted 1:10, and aliquots were plated on SDA for viable fungal colony counts after incubation for 24 h at 35°C. The lower limit of detection was 100 CFU/ml. Results were expressed as the mean CFU/kidneys for two mice (four kidneys).
Pharmacokinetics.
Single-dose pharmacokinetics of ravuconazole were determined in individual neutropenic infected ICR/Swiss mice following oral gavage administration of 10, 40, and 160 mg/kg administered in 0.2-ml volumes. Samples were analyzed by both high-performance liquid chromatography (HPLC) and microbiologic assays. Protein binding studies utilized previously described ultrafiltration methods (6). For the microbiologic assay, Candida kefyr ATCC 46764 was used as the assay organism in YNB agar supplemented with glucose and trisodium citrate (29). Groups of three halothane anesthetized mice were sampled three times by retrooribital puncture, and blood was collected in heparinized capillary tubes (Fisher Scientific, Pittsburgh, Pa.). The volume collected with each sample ranged from 30 to 50 μl. Less than 5% of the total mouse blood volume was collected from any individual animal. The samples were collected at 4- to 6-h intervals over 24 h. Capillary tubes were immediately centrifuged (model MB centrifuge; International Equipment Co.) at 10,000 × g for 5 min. The serum samples (10 μl each) were then placed in the agar wells. Assays of serum and standard curves were performed on the same day. Intraday variation was less than 7%. The lower limit of detection for this assay was 0.25 μg/ml.
Because the lower dose (10 mg/kg) achieved levels below the limit of detection for this bioassay, we repeated determinations of kinetics at both the 40- and 10-mg/kg dose levels utilizing HPLC analysis performed at Bristol-Myers Squibb. Groups of three halothane-anesthetized mice were sampled by intracardiac puncture, and serum was pooled at each time point. Individual animals were immediately euthanized via CO2 asphyxiation after blood sampling. The number and timing of samples were similar to those described for the microbiologic assay. The pooled blood was placed in 1.5-ml tubes (Fisher Scientific) and immediately centrifuged (model MB; International Equipment Co.) at 10,000 × g for 5 min. The serum was subsequently removed and stored at −80°C. Serum drug levels were determined by HPLC assay at Bristol-Myers Squibb. Intraday coefficient of variation was less than 5%. The lower level of detection for this assay was 50 ng/ml. Pharmacokinetic constants including elimination half-life and concentration at time zero were calculated via nonlinear least-squares techniques (MINSQ; Micromath Inc., Salt Lake City, Utah). The AUC was calculated by the trapezoidal rule. For treatment doses for which no kinetics were determined, pharmacokinetic parameters were linearly extrapolated from the values obtained in the above kinetic studies.
In vivo time kill and PAFE.
Infection in neutropenic mice was produced as described above. Two hours after infection with C. albicans K-1, mice were treated with single oral doses of ravuconazole (10 and 40 mg/kg). Groups of two treated and control mice were sacrificed at sampling time intervals ranging from q6h to q12h. Control growth was determined with four sampling points over 24 h. Ravuconazole-treated animals were sampled nine times over 48 h. Kidneys were removed at each time point and processed immediately for CFU determination as outlined above. The time that serum levels of ravuconazole remain above the MIC of the organism following the three doses was calculated from the pharmacokinetic data. Both total and free drug concentrations were utilized for kinetic calculations. The postantifungal effect (PAFE) was calculated by determining the time it took for controls to increase 1 log10 CFU/kidneys (C) and subtracting this from the amount of time it took organisms from the treated animals to grow 1 log10 CFU/kidney (T) after levels in serum fell below the MIC of the organism: postantibiotic effect (PAE) = T − C (5).
Pharmacodynamic parameter determination.
Neutropenic mice were infected with C. albicans K-1 2 h prior to start of therapy. Thirty-six dosing regimens were chosen to determine the impact of dose level, dosing interval, and treatment duration on ravuconazole efficacy. These 36 regimens are comprised of six total dose levels (0.625, 2.5, 10, 40, 160, and 640 mg/kg/24 h), five dosing intervals (q6h, q12h, q18h, q24h, and q36h), and two treatment durations (24 and 72 h). This wide variety of regimens was used to minimize the interdependence among the three pharmacodynamic parameters studied and also to describe the complete dose response relationship. Groups of two mice were treated with each dosing regimen. Drug was administered in 0.2-ml volumes. Mice were sacrificed at the end of therapy, and kidneys were removed for CFU determination as described above. Untreated control mice were sacrificed just before treatment and at the end of the experiment. Efficacy was defined as the change in log10 CFU/kidneys over the study period and was calculated by subtracting the mean log10 CFU/kidneys in treated mice from the mean number of CFU from kidneys of two mice at the end of therapy in untreated animals.
Pharmacodynamic parameter magnitude determination.
Studies similar to those described above were performed with seven additional strains of C. albicans (98-17, 98-234, 412, 580, 2512, 2307, and 2183). Attempts were made to choose organisms with various susceptibilities to ravuconazole. However, the range of ravuconazole MICs in our stock of organisms varied only eightfold. This group of organisms includes both fluconazole-susceptible, fluconazole-susceptible dose-dependent, and fluconazole-resistant strains. Dosing studies were designed to vary the magnitude of the pharmacodynamic parameters. The six total dose levels varied from 0.625 to 640 mg/kg/72 h. Doses were fractionated into three doses (q24h) for the 3-day study period. Groups of two mice were again used for each dosing regimen. At the end of study, mice were euthanized and kidneys immediately processed for CFU determination.
Data analysis.
A sigmoid dose-effect model was used to measure the in vivo potency of ravuconazole. The model is derived from the Hill equation: E = (Emax × DN)/(ED50N + DN), where E is the observed effect (change in log10 CFU/kidneys compared with untreated controls at the end of the treatment period), D is the cumulative dose, Emax is the maximum effect, ED50 is the dose required to achieve 50% of Emax, and N is the slope of the dose-effect relationship. The correlation between efficacy and each of the three parameters (percent of time above the MIC, 24-h AUC/MIC ratio, and peak/MIC ratio) studied was determined by nonlinear least-squares multivariate regression analysis (Sigma Stat; Jandel Scientific Software, San Rafael, Calif.). The coefficient of determination (R2) was used to estimate the percent of variance in the change of log10 CFU/kidneys over the treatment period for the different dosing regimens that could be attributed to each of the pharmacodynamic parameters. Calculations were performed using both total and free drug concentrations.
To allow a more meaningful comparison of potency among the dosing regimens studied, we calculated the dose required to produce the ED50 over the treatment period. The ED50 was chosen as an endpoint to allow comparison with similar studies performed with fluconazole (1). If the doses needed to achieve these benchmarks increased significantly as the dosing interval was lengthened from q6h through q72h regimens, the time that serum levels remained above the MIC was the parameter predictive of efficacy. On the other hand, if the doses necessary to reach these outcomes decreased with dosing interval lengthening, then the parameter associated with these outcomes was be the peak serum level. If the doses remained similar independent of changes in the dosing interval, then the AUC was predictive of efficacy.
The ED50 was determined for the q24h dosing regimen for each of the eight strains. The magnitude of the pharmacodynamic parameter predictive of the efficacy of ravuconazole was then calculated for each of the eight organisms studied to determine if a similar parameter magnitude was associated with efficacy as determined by these indices. Again, both total and free drug concentrations were considered. The significance of differences among these values was determined by ANOVA (Sigma Stat; Jandel Scientific Software). A two-tailed P of <0.05 was considered to be statistically significant.
RESULTS
In vitro susceptibility testing.
The 48-h MICs for the eight C. albicans organisms studied varied eightfold (range, 0.016 to 0.12 μg/ml). The fluconazole MICs for this group of organisms varied more than 500-fold (range, 0.25 to >128 μg/ml).
Pharmacokinetics.
The serum time course of ravuconazole in infected neutropenic mice following oral doses of 10, 40, and 160 mg/kg are shown in Fig. 1. Peak serum levels and the AUC increased in a relatively linear fashion with dose escalation. Peak levels were achieved within the first two sampling times for each of the doses and ranged from 0.36 ± 0.01 to 4.37 ± 0.64 μg/ml (means ± standard deviations). The elimination half-life ranged from 3.9 to 4.8 h. The AUC, as determined by the trapezoidal rule, ranged from 3.4 to 48 mg · h/liter with the lowest and highest doses, respectively. Serum levels produced by the 40-mg/kg dose as measured by bioassay and HPLC methods were similar (P = 1.0). Protein binding in mouse serum was 95.8% at concentrations of 100 and 400 μg/ml. The lower limit of detection for the bioassay precluded study of lower concentrations.
FIG. 1.
Serum ravuconazole concentrations after administration of oral doses of 10, 40, and 160 mg/kg in neutropenic infected mice. Each symbol represents the geometric mean ± standard deviation (error bars) of the levels in the sera of three mice.
In vivo PAE.
Following tail vein inoculation of 106 CFU/ml, the C. albicans burden in the kidneys of untreated mice increased 2.67 ± 0.24 log10 CFU/kidneys over 24 h. Control growth of one log10 CFU/kidneys in untreated mice was achieved in 6.4 h. No drug carryover was observed in treatment groups. Based upon the above pharmacokinetics, the two doses of ravuconazole studied (10 and 40 mg/kg) would produce levels of total drug in serum above the MIC for the Candida organism (0.03 μg/ml) for 16 and 27 h, respectively. Free drug level time above MIC for these dose levels would be considerably shorter (0 and 9.4 h, respectively). Neither dose produced a net reduction in organisms when compared to numbers at the start of therapy. Growth curves for both the control group as well as those following the single doses of ravuconazole are shown in Fig. 2. Ravuconazole suppressed regrowth of organisms at each of the doses studied. Considering total drug levels, organism regrowth was suppressed for 9.8 and 2.9 h with the 10- and 40-mg/kg doses, respectively. The PAFE was much longer when free drug levels were considered, 15.1 and 12.1 h, respectively.
FIG. 2.
In vivo PAE following ravuconazole doses of 10 and 40 mg/kg against C. albicans K-1 in neutropenic infected mice. Each symbol represents the mean ± standard deviation (error bars) for two mice. The widths of the hollow horizontal bars represents the time that total serum drug levels exceeded the MIC. The widths of the solid horizontal bars represents the time that free serum drug levels exceeded the MIC.
Pharmacodynamic parameter determination.
At the start of therapy kidneys had 3.40 ± 0.25 log10 CFU/kidneys. After 24 and 72 h the organisms grew 3.42 ± 0.43 and 2.76 ± 0.26 log10 CFU/kidneys in untreated mice, respectively. Drug carryover was not observed in any of the samples. Over the range of ravuconazole doses studied there was no killing of organisms when compared to organism burden in kidneys at the start of therapy. However, when compared to burden at the end of the study period in untreated animals, maximal organism reduction with the various dosing intervals ranged from 2.40 ± 0.10 to 3.62 ± 0.13 log10 CFU/kidneys. The dose-response curves for each of the six dosing regimens are shown in Fig. 3. As the dosing interval was shortened the dose-response curves retain a similar shape indicating similar efficacy (Table 2). There was not a significant difference among the doses necessary to produce the ED50 (Table 2) with each of the dose levels (range, 25 ± 15 to 100 ± 51; P = 0.42). Results were clearly dependent upon dose level and not dosing interval, suggestive that the AUC of exposure best determines outcome.
FIG. 3.
Relationship between the 24-h total dose and the change in log10 CFU per kidney using C. albicans K-1 over the treatment period for ravuconazole administered at different dosing intervals in a neutropenic murine model of disseminated candidiasis. Each symbol represents the mean ± standard deviation (error bars) for two mice. The hollow symbols represent the 24-h treatment durations. The solid symbols represent the 72-h treatment duration. The dashed horizontal line represents the number of CFU at the start of therapy.
TABLE 2.
Impact of six dosing intervals and two durations of treatment on ravuconazole efficacy against C. albicans
| Dosing regimen | ED50 ± SE (mg/kg/24 h)a |
|---|---|
| q36h for 72 h | 100 ± 51 |
| q24h for 72 h | 45 ± 44 |
| q18h for 72 h | 64 ± 21 |
| q6h for 24 h | 25 ± 15 |
| q12h for 24 h | 81 ± 60 |
| q24h for 24 h | 32 ± 13 |
P = 0.42
The relationships between microbiologic effect and each of the pharmacodynamic parameters—percent of time above the MIC, AUC/MIC ratio, and peak level/MIC ratio, is shown in Fig. 4 (total drug). The data regressed with the AUC in relation to the MIC had the strongest relationship, with an R2 of 91%. However, in these studies the data that regressed with peak/MIC ratio also had a strong relationship (peak/MIC, R2 = 85%). time above the MIC had the least correlation with treatment efficacy, whether total or free drug levels were considered R2 = 47 and 65%, respectively. That the peak/MIC ratio was also important in this model is a reflection of the strong interrelationship between these concentration-associated parameters.
FIG. 4.
Relationship between total drug time above the MIC (T>MIC), AUC/MIC ratio, peak/MIC ratio, and the change in log10 CFU per kidney. Each symbol represents data for two mice. The dashed horizontal line represents the number of CFU at the start of therapy. R2 is the coefficient of determination.
Correlation of the magnitude of the pharmacodynamic parameter with efficacy.
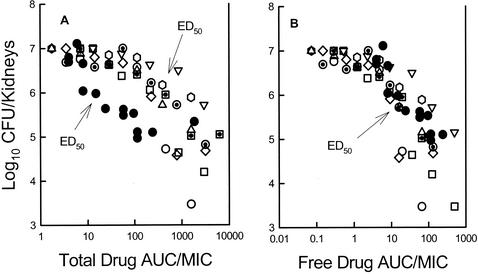
Each of the eight C. albicans strains grew similarly in the animals. At the start of therapy fungal burdens in the kidneys were between 3.05 ± 0.14 and 3.71 ± 0.32 log10 CFU/kidneys. The range of organism growth in control animals was 2.75 ± 0.30 to 4.20 ± 0.11 log10 CFU/kidneys. The relationship between the ravuconazole free drug 24 h AUC/MIC ratios and efficacy with the eight strains is displayed in Fig. 5. The relationships among the treatment groups was strong (R2 = 85.2%). The ravuconazole dose necessary to achieve 50% of the maximal effect varied 7.6-fold (range, 27 to 204 mg/kg) (Table 1). However, the 24-h AUC/MIC ratios representative of these doses varied only 3.6-fold (total drug AUC/MIC ratio, range 238 to 873; free drug AUC/MIC ratio range, 10 to 36). There was not a significant difference among these AUC/MIC ratios (P = 0.43).
FIG. 5.
(A) Relationship between total drug 24-h AUC/MIC ratio and the change in log10 CFU per kidney after 3 days of treatment for ravuconazole against eight C. albicans organisms. Each hollow symbol represents data for two ravuconazole-treated mice. Each solid symbol represents data for two fluconazole-treated mice (from reference 1). (B) Relationship between the free-drug 24-h AUC/MIC ratio and the change in log10 CFU per kidney after 3 days of treatment for ravuconazole against eight C. albicans organisms. Each hollow symbol represents data for two ravuconazole-treated mice. Each solid symbol represents data for two fluconazole-treated mice. (Reprinted from reference 1.)
DISCUSSION
There are several new triazole compounds under development (26). Animal infection models have demonstrated the potency of these new triazole compounds against a variety of Candida spp. (10, 26). However, these investigations have not determined the in vivo PK/PD parameter and parameter magnitude associated with treatment outcome with these drugs. The present studies were designed to see if the PK/PD characteristics of the new triazole, ravuconazole, were similar to those observed with fluconazole (1, 16).
The time course of antifungal activity of fluconazole against C. albicans has been well described (1, 11, 16). Studies have demonstrated concentration-independent organism killing, but prolonged inhibitory effects after drug levels have fallen below the MIC (PAFE) (1). The single-dose in vivo studies with fluconazole yielded PAFEs in the range of 4 h to more than 20 h. The present studies also found prolonged PAFE durations when free drug levels were considered (12 to 18 h). As discussed in prior publications, these in vivo determinations cannot differentiate between persistent growth suppression due to initial concentrations above the MIC in serum and those potentially due to sub-MIC effects. This is likely why in vivo triazole studies demonstrate prolonged PAE durations and in vitro PAE studies do not. This is particularly evident with the lowest dose of ravuconazole studied, where prolonged growth suppression was observed despite the fact that free drug levels did not exceed the MIC. Another potential limitation of these investigations is the correlation of kinetics in the serum with outcomes in the kidneys. This could be particularly troublesome for comparison of two drugs with different degrees of renal clearance. However, similar observations with numerous antimicrobials have demonstrated that serum levels are a good surrogate of tissue concentrations in the interstitial space. Unfortunately, drug concentrations in the kidney determined with current tissue homogenate methods would be contaminated by urine, blood, intracellular, and extracellular fluids (9). Unlike the tissue concentration methods using filaments or microdialysis, a similar method for studying drug concentrations in visceral organs is not available (23).
Multiple dosing interval studies with fluconazole have shown that outcome is dependent upon the total amount of drug (AUC) and not the dosing interval. The present analysis looked at outcomes of ravuconazole therapy with a total dose range of more than 1,000-fold, five dosing intervals, and two treatment durations. These investigations with ravuconazole also observed treatment outcomes dependent most upon the total amount of drug or AUC.
The concordance of PK/PD parameter magnitudes among animal species and in humans has been demonstrated for a variety of antibacterials (4). This should not be surprising given that PK/PD parameters can correct for differences in pharmacokinetics among animal species. Furthermore, the drug receptors for antimicrobials are in the pathogen and therefore are similar in all animals. Studies with numerous antimicrobials have also shown that the magnitude of the PK/PD parameter required for efficacy is similar for drugs within the same class provided free-drug concentrations are considered and is similar in the treatment of organisms with reduced-susceptibility (2, 4). Thus, the results of studies from these experimental models have been shown to be useful for the design of dosing regimens in humans and for the more rational development of in vivo susceptibility breakpoints (8, 18).
In vivo observations with fluconazole found an AUC/MIC ratio in the range of 20 to 25 produced the ED50 against both fluconazole-susceptible and -resistant strains. Similar AUC/MIC ratios were also found to be predictive of fluconazole clinical trial outcomes (14; Clancy et al., Progr. Abstr. 36th Annu. Meet. Infect. Dis. Soc. Am.). In these studies we also chose to utilize the dose necessary to produce the ED50 to allow comparison of this data with the fluconazole data.
One major difference between ravuconazole and fluconazole is the degree of protein binding (26). Fluconazole has a low degree of protein binding in all species studied (10%). Because of this low degree of protein binding across animal species, total drug levels were utilized for PK/PD parameter calculations in the fluconazole publication (1). Each of the newer triazole compounds has a much higher degree of protein binding. Because of this discrepancy the present studies attempted to determine the impact of protein binding on treatment outcome. In general, it is accepted that only free drug is pharmacologically active. This is related both to the limited ability of protein bound drug to diffuse across tissue and cellular membranes to reach the drug target. The impact of protein binding upon antimicrobial agents have been most clearly shown for antibacterials (5). These observations are perhaps most clearly demonstrated by the studies of Kunin et al. with a variety of beta-lactam antibiotics with various degrees of protein binding (12, 13). Previous in vivo studies have not considered the impact of azole protein binding. Recent PK/PD evaluation of a new sordarin antifungal demonstrated that in vitro-in vivo correlations were strongest when free drug levels were utilized for the in vivo drug concentration time course (3). Several in vitro investigations have attempted to discern the impact of protein binding discrepancies among various azole compounds (24, 25, 30). Study findings have been mixed, with some investigations suggesting free-drug levels are a better predictor of potency when comparing compounds, while others suggest that the relationship is poor. Methods utilized in these investigations have been dissimilar, varying in the type of protein and the amount of animal serum utilized. Our protein binding determinations were performed in mouse serum collected from neutropenic, infected animals attempting to closely mimic the binding that would occur in treatment studies. These studies of organisms for which the MICs varied nearly eightfold suggested that when free-drug ravuconazole concentrations are considered, treatment efficacy is similar to that observed with fluconazole (Table 2 and Fig. 5).
Ravuconazole kinetics in humans at doses of 400 mg daily would produce free-drug AUC values of 1.12 μg · h/ml (M. Marino, V. Mummaneni, J. Norton, O. Hadjilambris, and P Pierce, Abstr. 41st Intersci. Conf. Antimicrob. Agents Chemother., abstr. J1622, 2001). If one considers the strong correlation between the fluconazole PK/PD analysis in this infection model and outcomes in clinical trials, one would predict that these ravuconazole dosing regimens would be successful in treatment of C. albicans organisms for which the MICs are as high as 0.05 (free drug AUC/MIC ratio 20). The MIC surveillance of C. albicans has reported MIC90s of 0.015 (20). Although most azole resistance mechanisms do appear to produce a class effect, the differential in vitro potency between fluconazole and ravuconazole would likely allow one to successfully treat patients with infections with these more resistant pathogens as well.
REFERENCES
- 1.Andes, D., and M. L. van Ogtrop. 1999. Characterization and quantitation of the pharmacodynamics of fluconazole in a neutropenic murine model of disseminated candidiasis model. Antimicrob. Agents Chemother. 43:2116-2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andes, D., and W. A. Craig. 1998. In vivo activities of amoxicillin and amoxicillin-clavulanate against Streptococcus pneumoniae: application to breakpoint determinations. Antimicrob. Agents Chemother. 42:2375-2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aviles, P., C. Falcoz, M. J. Guillen, R. San Roman, H. Gomez de Las Heras, and D. Gargallo-Viola. 2001. Correlation between in vitro and in vivo activities of GM 237354, a new sordarin derivative, against Candida albicans in an in vitro pharmacokinetic-pharmacodynamic model and influence of protein binding. Antimicrob. Agents Chemother. 45:2746-2754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Craig, W. A. 1998. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin. Infect. Dis. 26:1-12. [DOI] [PubMed] [Google Scholar]
- 5.Craig, W. A., and S. Gudmundsson. 1996. Postantibiotic effect, p. 296-329. In V. Lorian (ed.), Antibiotics in laboratory medicine, 4th ed. Williams and Wilkins, Baltimore, Md.
- 6.Craig, W. A., and B. Suh. 1996. Protein binding and the antimicrobial effects: methods for the determination of protein binding, 4th ed., p. 367-402. Williams and Wilkins, Baltimore, Md.
- 7.Craig, W. A., and D. Andes. 1996. Pharmacokinetics and pharmacodynamics of antibiotics in otitis media. Pediatr. Infect. Dis. J. 15:255-259. [DOI] [PubMed] [Google Scholar]
- 8.Dowell, S. F., J. C. Butler, G. S. Giebink, et al. 1999. Acute otitis media: management and surveillance in an era of pneumococcal resistance—a report from the Drug-Resistant Streptococcus pneumoniae Working Group. Pediatr. Infect. Dis. J. 18:1-9. [PubMed] [Google Scholar]
- 9.Fish, D. N., M. H. Gotfried, L. H. Danziger, and K. A. Rodvold. 1994. Penetration of clarithromycin into lung tissues from patients undergoing lung resection. Antimicrob. Agents Chemother. 38:876-878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hata, K., J. Kimura, H. Miki, T. Toyosawa, T. Nakamura, and K. Katsu. 1996. In vitro and in vivo antifungal activities of ER-30346, a novel oral triazole with a broad antifungal spectrum. Antimicrob. Agents Chemother. 40:2237-2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klepser, M. E., E. J. Wolfe, R. N. Jones, C. H. Nightengale, and M. A. Pfaller. 1997. Antifungal pharmacodynamic characterization of fluconazole and amphotericin B tested against Candida albicans. Antimicrob. Agents Chemother. 41:1392-1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kunin, C. M. 1965. The importance of serum protein binding in determining antimicrobial activity and concentration in serum. Clin. Pharmacol. Ther. 7:168-179. [DOI] [PubMed] [Google Scholar]
- 13.Kunin, C. M., W. A. Craig, M. Kornguth, and R. Monson. 1973. Influence of binding on the pharmacological activity of antibiotics. Ann. N. Y. Acad. Sci. 226:214-224. [DOI] [PubMed] [Google Scholar]
- 14.Lee, S. C., C. P. Fung, J. S. Huang, C. J. Tsai, K. S. Chen, N. L. Chen, L. C. See, and W. B. Shieh. 2000. Clinical correlates of antifungal macrodilution susceptibility test results for non-AIDS patients with severe candida infections treated with fluconazole. Antimicrob. Agents Chemother. 44:2715-2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lopez-Ribot, J. L., R. K. McAtee, L. N. Lee, W. R. Kirkpatrick, T. C. White, D. Sanglard, and T. F. Patterson. 1998. Distinct patterns of gene expression associated with development of fluconazole resistance in serial Candida albicans isolates from human immunodeficiency virus-infected patients with oropharyngeal candidiasis. Antimicrob. Agents Chemother. 42:2932-2937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Louie, A., G. L. Drusano, P. Banerjee, Q. F. Liu, W. Liu, M. Kaw, H. Shayegani, H. Taber, and M. H. Miller. 1998. Pharmacodynamics of fluconazole in a murine model of systemic candidiasis. Antimicrob. Agents Chemother. 42:1105-1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.National Committee for Clinical Laboratory Standards. 1997. Reference method for broth dilution antifungal susceptibility testing for yeast. Approved standard M27-A. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 18.National Committee for Clinical Laboratory Standards. 2000. Development of in vitro susceptibility testing criteria and quality control parameters. Approved guidelines, 2nd ed. Document M23-A2. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 19.National Research Council Committee on the Care and Use of Laboratory Animals of the Institute of Laboratory Animal Resources, Commission on Life Sciences. 1996. Guide for the care and use of laboratory animals. National Academy Press, Washington, D.C.
- 20.Pfaller, M. A., R. N. Jones, G. V. Doern, A. C. Fluit, J. Verhoef, H. S. Sader, S. A. Messer, A. Houston, S. Coffman, and R. J. Hollis. 1999. International surveillance of blood stream infections due to Candida species in the European SENTRY Program: species distribution and antifungal susceptibility including the investigational triazole and echinocandin agents. Diagn. Microbiol. Infect. Dis. 35:19-25. [DOI] [PubMed] [Google Scholar]
- 21.Rex, J. H., M. A. Pfaller, J. N. Galgiani, M. S. Bartlett, A. Espinel-Ingroff, M. A. Ghannoum, M. Lancaster, F. C. Odds, M. G. Rinadli, T. J. Walsh, and A. L. Barry for the NCCLS Subcommittee on Antifungal Susceptibility Testing. 1997. Development of interpretive breakpoints for antifungal susceptibility testing: conceptual framework and analysis of in vitro and in vivo correlation data for fluconazole, itraconazole, and candida infections. Clin. Infect. Dis. 24:235-247. [DOI] [PubMed] [Google Scholar]
- 22.Rogers, T. E., and J. N. Galgiani. 1986. Activity of fluconazole (UK 49,858) and ketoconazole against Candida albicans in vitro and in vivo. Antimicrob. Agents Chemother. 30:418-422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ryan, D. M., B. Hodges, G. R. Spencer, and S. M. Harding. 1982. Simultaneous comparison of three methods for assessing ceftazidime penetration into extravascular fluid. Antimicrob. Agents Chemother. 22:995-998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schafer-Korting, M., H. C. Korting, W. Rittler, and W. Obermuller. 1995. Influence of serum protein binding on the in vitro activity of antifungal agents. Infection 23:292-297. [DOI] [PubMed] [Google Scholar]
- 25.Schafer-Korting, M., H. C. Korting, F. Amman, R. Peuser, and A. Lukacs. 1991. Influence of serum protein binding on the in vitro activity of antifungal activity: results of a dynamic in vitro study. Antimicrob. Agents Chemother. 35:2053-2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sheehan, D. J., C. A. Hitchcock, and C. M. Sibley. 1999. Current and emerging azole antifungal agents. Clin. Microbiol. Rev. 12:40-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Van't Wout, J., H. Mattie, and R. van Furth. 1989. Comparison of the efficacies of amphotericin B, fluconazole, and itraconazole against systemic Candida albicans infection in normal and neutropenic mice. Antimicrob. Agents Chemother. 33:147-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vogelman, B., S. Gudmundsson, J. Leggett, J. Turnidge, S. Ebert, and W. A. Craig. 1988. Correlation of antimicrobial pharmacokinetic parameters with therapeutic efficacy in an animal model. J. Infect. Dis. 158:831-847. [DOI] [PubMed] [Google Scholar]
- 29.Warnock, D. W., E. M. Johnson, and D. A. White. 1999. Antifungal drug measurements, p. 221-233. In D. S. Reeves, R. Wise, J. M. Andrews, L. O. White, and D. Speller (ed.), Clinical antimicrobial assays. Oxford University Press, Oxford, United Kingdom.
- 30.Zhanel, G. G., D. G. Saunders, D. J. Hoban, and J. A. Karlowsky. 2001. Influence of human serum on antifungal pharmacodynamics with Candida albicans. Antimicrob. Agents Chemother. 45:2018-2022. [DOI] [PMC free article] [PubMed] [Google Scholar]