Abstract
Melanoma cell migration along the outside of vessels has been termed “extravascular migratory metastasis” (EVMM), as distinct from intravascular dissemination. Previous studies in both human and experimental melanoma models have shown angiotropism of melanoma cells, suggesting EVMM. Our objectives are to study the mechanism of dissemination of human melanoma cells in the chick chorioallantoic membrane (CAM) and to compare the histopathology in the CAM with that of patients with in transit and other cutaneous melanoma metastases.
Human and murine melanoma cells were inoculated onto the CAM and observed over a 10-day period for tumor dissemination. Both human melanoma specimens from 26 patients and melanoma cells growing on the CAM showed the presence of tumor cell angiotropism at the invasive front of the tumor and at some distance from the tumor mass. In addition, a clear progression of melanoma cells spreading on the CAM was observed along the abluminal surface of vessels, where they occupied a perivascular location. By day 10 after injection, small micrometastases had developed along vessels, in a pattern similar to that in transit and other cutaneous melanoma metastases. In addition, the results suggested that the number of micrometastases directly correlated with increasing tumor volume. Taken together, these data suggest that the CAM is a relevant model for studying tumor cell dissemination, and that EVMM may be a mechanism by which some melanoma cells spread to nearby and even distant sites.
Keywords: angiotropism, extravascular migratory metastasis (EVMM), in transit melanoma metastases, chick chorioallantoic membrane (CAM), human melanoma cells
The fundamental problem of cancer is the propensity of tumor cells to metastasize,1 and this is especially problematic with malignant melanoma.2 Metastasis is defined by end points, that is, metastatic lesions detected in specific organs distant from a primary tumor. The sequential steps in the process of metastasis are believed to include the following: escape of cells from the primary tumor, intravasation (entry of cells into the lymphatic or blood circulation), survival and transport in the circulation, arrest in distant organs, extravasation (escape of cells from the circulation), and growth of cells to form secondary tumors in the new organ environment.3 Experimental and clinical evidence support some of these steps, but the complex in vivo nature of the process has not resulted in a full understanding.4-8
As distinct from intravascular dissemination, extravascular migratory metastasis (EVMM) is a potential additional mechanism of melanoma spread, in which tumor cells migrate along the outside of vessels.9,10 In transit melanoma metastases are defined as metastases occurring on the skin between the primary tumor and the regional lymph node.2 Such “in transit” tumors, including epidermotropic and regional lymph node metastases, may be compatible with extravascular step-by-step tumor cell dissemination.
Using electron microscopy and immunohistochemistry, our studies have demonstrated, in both human melanoma and experimental melanoma models, a tumor-endothelial cell interaction which we have termed the angio-tumoral complex.11-13 In the angio-tumoral complex, angiotropic melanoma cells occupy a pericytic location on the outside of the endothelium without any evidence of intravasation. In addition, we have shown that angiotropism in human melanoma, the histopathologic counterpart of the angio-tumoral complex, could be a prognostic factor predicting risk for metastasis.14 Recent experimental studies strongly suggest a correlation of angiotropism of melanoma cells with EVMM. These studies, including cocultures of melanoma cells with capillarylike structures in vitro and with rat aortic rings ex vivo, have demonstrated the migration of angiotropic melanoma cells along the vascular channels, supporting the concept of EVMM.15,16 The analysis of melanoma tumors developed on the chick chorioallantoic membrane (CAM) demonstrated the presence of angiotropic melanoma cells cuffing the outside of vessels around the tumor in a pattern similar to the angio-tumoral complex.16
In the present study, we have studied over time the growth and dissemination of human melanoma cells expressing green fluorescent protein (GFP) on the CAM. The migration of tumor cells on the CAM from the primary tumor has been quantitated. Histopathology of the CAM has been compared with the histopathology of 26 specimens of human cutaneous in transit and other melanoma metastases. The results suggest that this CAM assay is a relevant model for studying tumor cell dissemination, and that EVMM may be a mechanism by which some cells spread to nearby and even distant organs.
MATERIALS AND METHODS
Human Melanoma Specimens
Sixteen in transit, 7 epidermotropic (non in transit), and 3 local satellite human melanoma metastasis specimens were retrieved from the personal consultation files (R.L.B), the pathology files of Memorial Sloan Kettering Cancer Center, and the pathology files of Global Pathology Laboratory Services. The specimens were fixed in 10% formalin, embedded in paraffin, sectioned at 5 μm, and stained with hematoxylin and eosin (H&E) for pathologic observation.
Double Immunostaining
For double immunostaining, slides were first deparaffinized and heated in a pressure cooker for 20 minutes (antigen retrieval). After blocking endogenous peroxidase activity, slides were incubated for 30 minutes with mouse monoclonal anti-CD31 (Dako Cytomation, Carpinteria, CA). They were then rinsed, incubated for 30 minutes with labeled polymer (horseradish peroxidase-conjugated goat antimouse immunoglobulins), washed, and exposed to diaminobenzadine (DAB) until brown color developed. Slides were then washed, endogenous alkaline phosphatase activity blocked, and then incubated for 30 minutes with mouse monoclonal anti-melan-A (Dako Cytomation, Carpinteria, CA). After washing, the cells were incubated for 30 minutes with labeled polymer (alkaline phosphatase conjugated to goat antimouse immunoglobulins), washed, and exposed to fast red until red color developed. After washing, they were counterstained with light green and coverslipped with a water-soluble mounting medium.
GFP Melanoma Cells
C8161, a highly invasive and spontaneously metastatic human melanoma cell line,17 was transfected with the enhanced GFP as previously described.18 Cells were maintained in a 1:1 mixture of Dulbecco modified Eagle medium (DMEM) and Ham’s F-12 medium (DME-F12, Gibco BRL) supplemented with 5% fetal bovine serum (Invitrogen) and 1 mg/mL puromycin (Gibco BRL). The cells were cultured at 371C in a humidified, 5% CO2/95% air atmosphere.
B16F10 melanoma cells19 were transfected with the enhanced GFP as previously described.16 Cells were grown in DMEM (Gibco BRL Life Technologies), containing 10% fetal bovine serum (HyClone, Logan, Utah), 100 U/mL penicillin, 100 mg/mL streptomycin, and nonessential amino acids solution (Gibco BRL Life Technologies). The cells were maintained at 371Cina humidified, 5% CO2/95% air atmosphere.
In Vivo CAM Assay
We used a modified CAM assay as previously described.20,21 Briefly, fertilized white leghorn eggs were incubated in a humidified, 371C incubator for a total of 20 days. At day 10, a small hole was drilled on the top of the egg above the area of greatest vasculature, causing the CAM to detach from the shell membrane. GFP melanoma cells (105 B16 and 106 C8161), in a volume of 50 μL of complete corresponding cell culture media plus 50 μL of Matrigel, were inoculated onto the dropped CAM with a pipet tip. The half of the CAM above the long circumference containing the tumor inoculate was designated as the “upper CAM”, whereas the opposite half was designated as the “lower CAM” as recently described.20 The holes were sealed with tape and the eggs were returned to the incubator. To observe cell growth and dissemination over time, eggs were opened everyday after inoculation, that is, from day 1 to day 10 after inoculation (5 eggs for each time point). The CAM was opened along the long circumference, the upper CAM was collected, and was directly observed under the fluorescent microscope. Briefly, we have used fluorescence microscopy to examine the fresh CAM, that is, before any fixation or sectioning. This preparation allows one to obtain striking images of the relationship between fluorescent GFP cells and microvessels. It is even possible to observe on the fresh CAM fluid and cellular movement within these vessels, as recently described.16
For specimens obtained at day 10 after inoculation, tumor volume on the CAM (l×w×h) was measured with a caliper. The number of vessels cuffed by the fluorescent melanoma cells in 5 consecutive fields around the tumor mass was counted. To quantitate the migration of tumor cells from the tumor, the lower CAM that lines the cavity of the eggshell was lifted and immediately observed. The number of tumor cells or tumor aggregates was counted. After dissection, tumor and nontumor areas were fixed in 10% formalin for histopathologic observation. The experiment was repeated twice.
Statistical Analysis
Student t-test (P<0.05) was used to compare the differences between the calculated means.
RESULTS
Histopathology of Human Melanoma Metastases
In 23 of the total 26 melanoma metastases studied, angiotropism of melanoma cells was observed in some portion of the metastasis. Thirteen out of 16 in transit metastases (Fig. 1), all 7 epidermotropic metastases, and 2 of 3 satellite metastases showed angiotropism. In general, the in transit metastases were small and some were epidermotropic (Figs. 1A-C) whereas others involved solely the reticular dermis and possibly the subcutaneous fat (Figs. 1D-F). Melanoma cells cuffed the external surfaces of microvessels within the metastasis (Figs. 1B, C), at the peritumoral interface of the metastasis, or in immediate proximity to the main portion of the metastasis, in a pattern analogous to the angio-tumoral complex (Figs. 1E, F). The melanoma cells were present in 1 or more layers and occasionally in small aggregates juxtaposed to the external vascular wall. There was no evidence of intravascular involvement (intravascular invasion) in any of the 26 human melanoma metastases. Simultaneous (double) immunostaining of 5 specimens with S100 protein and CD31 highlighted melanoma cell angiotropism, that is, melanoma cells expressing S100 were observed along the external surfaces of microvessels labeling with CD31 (Fig. 1F).
FIGURE 1.
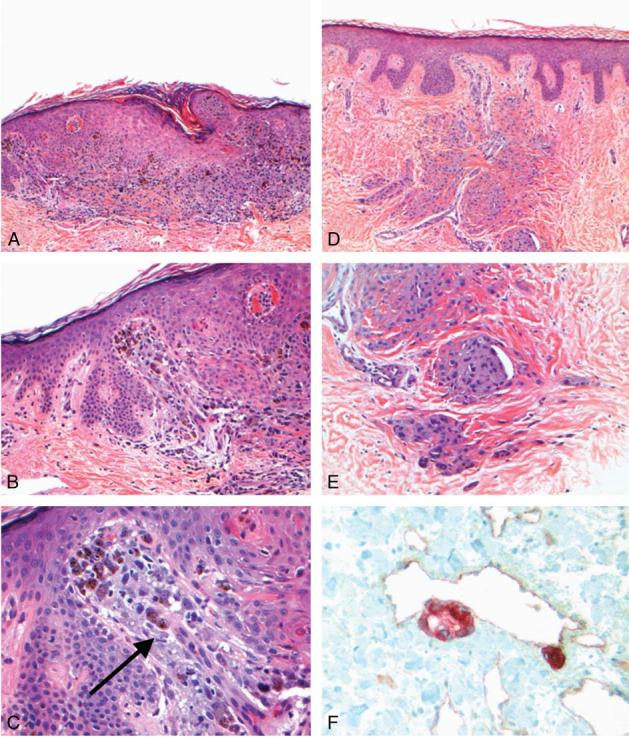
Histopathology of human melanoma metastases. A, In transit metastasis. Melanoma cells showing epidermotropism and mimicking a primary melanoma in this field [hematoxylin and eosin (H&E),× 40]. B, In transit metastasis. Note melanoma cells arrayed about a microvessel in the papillary dermis (H&E,× 200). C, In transit metastasis. There is a striking angiotropism of melanoma cells (arrow) along the external surface of the microvessel in (B) (H&E,× 400). D, In transit metastasis. Melanoma cells are arranged in angiotropic aggregates about microvessels in the dermis (H&E,× 40). E, In transit metastasis. Melanoma cells arranged along microvessels most likely migrating distally from the central portion of the metastasis (H&E,×200). F, In transit metastasis. Note the angiotropism of melanoma cells about the microvessel at the periphery of metastasis, highlighted by double immunostaining. Melan-A (red chromagen fast red) is expressed by melanoma cells and CD31 by microvessel (brown chromagen DAB) (immunohistochemistry with methyl green,× 400).
Tumor Growth and Spread on the CAM
We analyzed over time the spreading of tumor cells from inoculates grown on the CAM. Between days 2 and 4 after cell injection, melanoma cells were observed spreading along or in the immediate proximity of vessels, exhibiting a primary angiotropism (Figs. 2A, B). Between days 5 and 7 after cell injection, a tumor mass had formed. Vessels around the tumor mass were cuffed by melanoma cells lying along the vessel surface, where they also formed small tumor masses (Figs. 2C, D). Between days 8 and 10 after injection, the tumor had enlarged in size, progressively entrapping portions of vessels along which they had progressed (Figs. 2E-H). At day 10, melanoma cells had developed highly vascularized, grossly visible solid tumors at the inoculation site. The C8161 human melanoma tumors were beige, whereas the B16 ones were black (Fig. 3E). Larger numbers of angiotropic tumor cells were observed along vessels surrounding the tumor (Figs. 2G, H).
FIGURE 2.
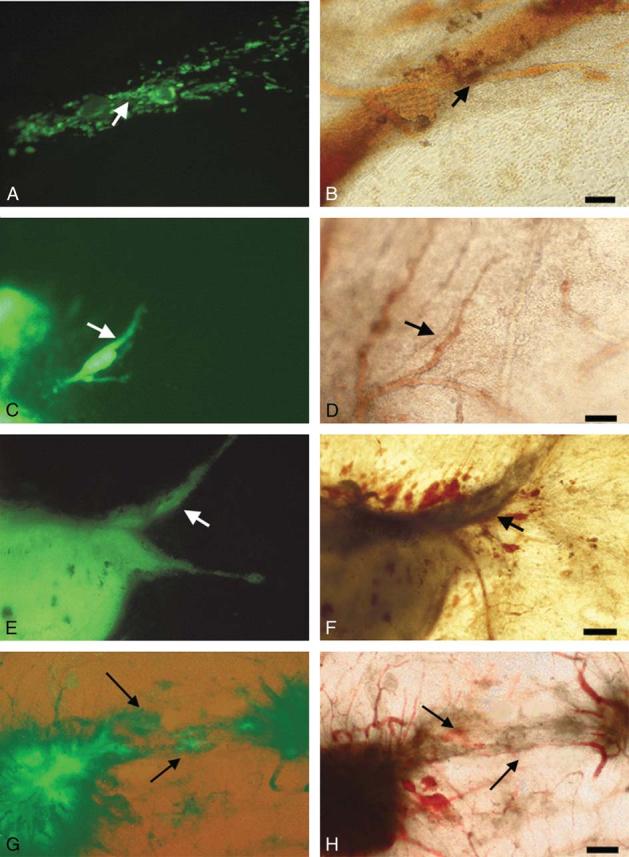
Migration and growth of GFP C8161 human melanoma cells on the chick CAM over a 10-day interval. A, C, E, and G demonstrate fluorescence microscopy, and B, D, F, and H demonstrate corresponding fields with conventional microscopy. A and B, 3 days after inoculation. Melanoma cells (fluorescent in A) are observed spreading along or in the immediate proximity of vessels (arrows), exhibiting a primary angiotropism. Bar = 25 μm. C and D, 6 days after inoculation. Melanoma cells begin to form a tumor mass, and some tumor cells clearly spread along the external surface of a vessel (arrows in C and D), occupying a pericytelike location. Bar = 50 μm. E and F, 10 days after inoculation. A tumor mass has developed at the site of injection, entrapping progressively portions of vessels on which it has grown. Around the tumor, observable vessels are cuffed by tumor cells (arrows). Bar = 50 μm. G and H, 10 days after inoculation. Two tumor masses are linked by vessels. The vessels (arrow in H) are progressively entrapped by tumor cells (arrow in G) growing along these vessels. Bar = 100 μm.
FIGURE 3.
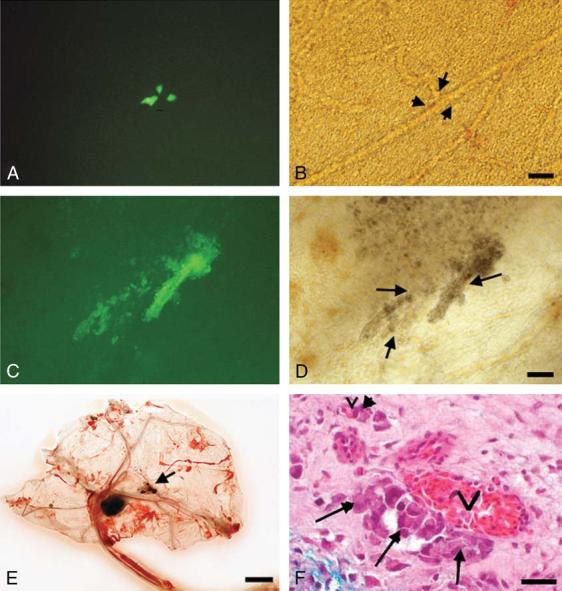
Migration and growth of GFP melanoma cells on the chick CAM, 10 days after inoculation. A and C demonstrate fluorescence microscopy, and B and D demonstrate corresponding fields with conventional microscopy. A and B, C8161 human melanoma cells form micrometastases (fluorescent in A), along the vascular network (arrows in B) 3 cm from the main tumor. Bar = 25 μm. C and D, B16 murine melanoma cells (fluorescent in C) spread along vessels (arrows in D). Note the brown pigmentation of B16 melanoma cells in D. E, B16 murine melanoma tumor. The tumor measures 7 μm in diameter. Note the black microtumors (arrow). Bar = 10 mm. F, Histopathology of the CAM, 2 cm from the main tumor. C8161 melanoma cells (arrows) occupy a pericytelike location around vessels (v). Note the absence of tumor cells in the vessel lumen. Bar = 25 μm.
Detailed examination of the lower CAM allowed one to follow the progressive dissemination of melanoma cells along vessels at the single cell level. From day 1 to day 5, no distant tumor cells were noted on the lower CAM. From day 6 to day 10 after inoculation, melanoma cells and small tumor masses were regularly found spreading along the vascular network on the lower CAM (20 to 35 mm away from the tumor), resembling in transit micrometastases (Figs. 3A-D).
At day 10, the average tumor volume was 74.6 mm3 for the human C8161 melanoma tumor and 96 mm3 for the murine B16F10 melanoma tumors (Fig. 4A). The average number of micrometastases on the lower half of the CAM was 8.7 for the human C8161 melanoma tumor and 7.8 for the murine B16F10 melanoma tumors (Fig. 4B), which was not significantly less than for the C8161 melanoma tumors (P>0.05). In both the C8161 and B16 melanoma tumors, the results strongly suggest that the number of micrometastases directly correlated with increasing tumor volume (Fig. 4C).
FIGURE 4.
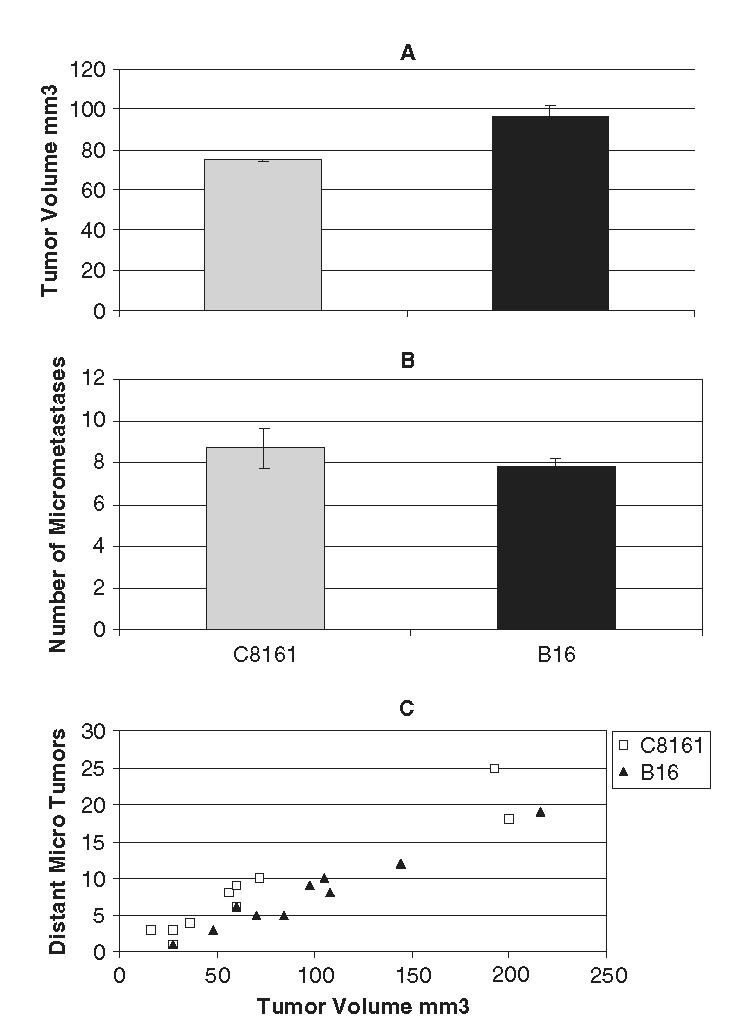
Tumor volume and micrometastases at day 10. A, Tumor volume (upper CAM). At day 10, the mean tumor volume was 74.6 mm3 for the human C8161 melanoma tumor and 96 mm3 for the murine B16F10 melanoma tumors. B, Number of micrometastases on the lower CAM. The mean number of micrometastases on the lower half of the CAM was 8.7 for the human C8161 melanoma tumor and 7.8 for the murine B16F10 melanoma tumors, which was not significantly less than that for the C8161 melanoma tumors (P > 0.05). C, Correlation between tumor volume and number of micrometastases. In both the C8161 and B16 melanoma tumors, the results suggested that the number of micrometastases directly correlates with tumor volume.
The CAM preparation allowed the direct visualization of fluid and cellular movement inside vessels, confirming that the GFP tumor cells observed along the vessels were clearly not part of the circulating cells. The results were similar for both C8161 human melanoma cells and B16 murine melanoma cells, except that B16 cells showed pigmented cells and significant necrosis (Figs. 3C, D). Histopathology confirmed the presence of tumor cells along the abluminal surface of vessels and absence of tumor cells within the vascular lumina (Fig. 3F). It also confirmed the presence of micrometastases along vessels on the lower CAM, away from the main tumor.
DISCUSSION
Both human melanoma specimens and GFP-labeled human and murine melanoma cells growing on the CAM have morphologic similarities, that is, the presence of angiotropism of tumor cells at the invasive front of the tumor or at some distance from the tumor mass. In addition, it was possible to observe a clear progression of melanoma cells spreading on the CAM along the abluminal surface of vessels, where they occupied a pericytic location. On day 10 after injection, small micrometastases had developed along vessels on the lower CAM, in a pattern similar to human satellite and in transit melanoma metastases. In addition, the correlation between tumor volume on the CAM and number of micrometastases on the lower CAM suggests that this model has similarities with human melanoma, wherein Breslow tumor thickness is the most reliable prognostic marker for future tumor dissemination (Fig. 4C).
The present observations indicate that the CAM assay is a relevant model for studying tumor dissemination and its relationship with the vascular network. These results suggest a progression of melanoma cells along the abluminal, mesenchymal surface of vessels at the advancing front of the primary tumor (Figs. 2C-F), and support the hypothesis of EVMM. However, further studies are needed to better elucidate the mechanism of melanoma migration from the upper CAM to the lower CAM, that is, traveling 20 to 35 mm. The observation of angiotropic melanoma cells in the lower CAM does not prove that they have remained entirely external to the vessel lumina during their migration. Tumor cells have been reported to migrate at rates of 0.1 to 2 μm/min,22 which would result in 5.2 to 105 cm/y. Such velocities are compatible with the time intervals noted between the recognition of the primary tumor and the formation of obvious metastases in human cancer progression. Considering the recent proposal of amoeboid migration, tumor cells could even achieve velocities 10 to 30 times greater than those in the mesenchymal mechanism of migration,22,23 potentially explaining very rapid dissemination of tumor cells in some instances. With respect to the potential rates of migration mentioned above, angiotropic melanoma cells could conceivably migrate 20 mm outside the vessel lumina in the CAM assay anywhere between 5 hours 30 minutes, and 245 days.
Angiotropism of melanoma cells has been rarely reported until now and only anecdotally. We have reported 7 additional cases,21 and more recently 36 cases of primary angiotropic melanoma.24 This feature is generally observable at the advancing front or some distance (1 to 3 mm) away from the primary tumor. However, the present results in the CAM assay suggest that an invasive tumor may result from primary tumor nests linked by microvessels and merging after some tumor cells have migrated along these vessels, leading to a larger tumor mass (Figs. 2G, H). Therefore, this primary angiotropism becomes enmeshed in the growing tumor, making it difficult to distinguish such vessels from those entrapped in the tumor mass, as previously described in human tumors.14 The migration and growth of tumor cells over time on the CAM suggests such tumor progression (Figs. 2A-H). To try to define a marker of angiotropism in human melanoma, we had shown in former studies that the b2 laminin chain exhibited a consistent positivity in an angiocentric pattern around microvessels.9 Given the role of laminin in migration and metastasis,1,9 its presence between the angiotropic tumor cells and the adjacent vessel suggests that laminin could be an important ligand in this process. Further studies to detect additional markers are currently in progress.
The EVMM proposed for melanoma has strong analogies with the migration of neoplastic glial cells invading the nervous system, as we have recently demonstated.25 Glial tumor cells can rapidly migrate along tracts, such as the vascular basement membrane, and invasive glioma cells can be found several centimeters away from the main tumor mass,26 as observed in in transit, epidermotropic, and other melanoma micrometastases.2
Other anatomic structures are known to be involved in tumor migration, for example, the glial limitans externa in glioma27 or nerves and epidermal appendages in melanoma,2 and perineural invasion in prostate cancer and pancreatic carcinoma.28,29 This kind of migration through or within the mesenchyme has striking analogies with the migration occurring during embryogenesis.23,27,30,31 Metastasizing cells may acquire motile properties, and embark upon an extensive continuous migration through the body, that is, EVMM, to reach their ultimate specific secondary sites in a pattern having analogies with embryonic cells migration.
In conclusion, the similarities observed between the spread of melanoma cells along vessels in the CAM assay and human in transit and other melanoma metastases suggest that EVMM may be a mechanism by which some melanoma cells spread to nearby and even distant organs. Understanding the molecular basis of EVMM may be of critical importance for the identification and evaluation of new therapeutic approaches to melanoma.
ACKNOWLEDGMENT
The authors thank Joseph Olczyk for his technical assistance.
Footnotes
Supported in part by a grant from Global Pathology Laboratory Services, FL.
REFERENCES
- 1.Engbring JA, Kleinman HK. Extracellular matrix and malignancy. J Pathol. 2003;200:465–470. doi: 10.1002/path.1396. [DOI] [PubMed] [Google Scholar]
- 2.Barnhill RL. Pathology of Melanocytic Nevi and Malignant Melanoma. 2nd ed Springer; New York: 2004. [Google Scholar]
- 3.Tuszynski GP, Wang TN, Berger D. Adhesive proteins and the hematogenous spread of cancer. Acta Haematol. 1997;97:29–39. doi: 10.1159/000203657. [DOI] [PubMed] [Google Scholar]
- 4.Chambers AF, Matrisian LM. Changing views of the role of matrix metalloproteinases in metastasis. J Natl Cancer Inst. 1997;89:1260–1270. doi: 10.1093/jnci/89.17.1260. [DOI] [PubMed] [Google Scholar]
- 5.Recamier JCA. Recherche sur le Traitement du Cancer. Gabon; Paris: 1829. [Google Scholar]
- 6.Lugassy C, Escande JP. The haematogenous theory of metastasis: Recamier did not propose it. Virchows Arch. 1997;431:371. doi: 10.1007/s004280050113. [DOI] [PubMed] [Google Scholar]
- 7.Wilder RJ. The historical development of the concept of metastasis. J Mt Sinai Hosp. 1956;23:728–734. [PubMed] [Google Scholar]
- 8.Billroth T. Lectures on Surgical Pathology and Therapeutics: A Handbook for Students and Practitioners. The New Syndeham Society; London: 1878. p. 355. [Google Scholar]
- 9.Lugassy C, Shahsafaei A, Bonitz P, et al. Tumor microvessels in melanoma express the beta-2 chain of laminin. Implications for melanoma metastasis. J Cutan Pathol. 1999;26:222–226. doi: 10.1111/j.1600-0560.1999.tb01834.x. [DOI] [PubMed] [Google Scholar]
- 10.Lugassy C, Barnhill RL, Christensen L. Melanoma and extravascular metastasis. J Cutan Pathol. 2000;27:281. doi: 10.1034/j.1600-0560.2000.027009481.x. [DOI] [PubMed] [Google Scholar]
- 11.Lugassy C, Eyden BP, Christensen L, et al. Angio-tumoral complex in human malignant melanoma characterised by free laminin: ultrastructural and immunohistochemical observations. J Submicrosc Cytol Pathol. 1997;29:19–28. [PubMed] [Google Scholar]
- 12.Lugassy C, Dickersin GR, Christensen L, et al. Ultrastructural and immunohistochemical studies of the periendothelial matrix in human melanoma: evidence for an amorphous matrix containing laminin. J Cutan Pathol. 1999;26:78–83. doi: 10.1111/j.1600-0560.1999.tb01806.x. [DOI] [PubMed] [Google Scholar]
- 13.Lugassy C, Christensen L, Le Charpentier M, et al. Ultrastructural and immunohistological observations concerning laminin in B16 melanoma: is an amorphous form of laminin promoting a non hematogenous migraton of tumor cells. J Submicrosc Cytol Pathol. 1998;30:137–144. [PubMed] [Google Scholar]
- 14.Barnhill RL, Dy K, Lugassy C. Angiotropism in cutaneous melanoma: a prognostic factor strongly predicting risk for metastasis. J Invest Dermatol. 2002;119:705–706. doi: 10.1046/j.1523-1747.2002.01871.x. [DOI] [PubMed] [Google Scholar]
- 15.Lugassy C, Kleinman HK, Fernandez PM, et al. Human melanoma cell migration along capillary-like structures in vitro: a new dynamic model for studying extravascular migratory metastasis. J Invest Dermatol. 2002;119:703–704. doi: 10.1046/j.1523-1747.2002.01857.x. [DOI] [PubMed] [Google Scholar]
- 16.Lugassy C, Kleinman HK, Engbring JA, et al. Angiotropism and pericyte-like location of GFP melanoma cells: ex vivo and in vivo studies of extravascular migratory metastasis. Am J Pathol. 2004;164:1191–1198. doi: 10.1016/S0002-9440(10)63207-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Welch DR, Bisi JE, Miller BE, et al. Characterization of a highly invasive and spontaneously metastatic human malignant melanoma cell line. Int J Cancer. 1991;47:227–237. doi: 10.1002/ijc.2910470211. [DOI] [PubMed] [Google Scholar]
- 18.Goldberg SF, Harms JF, Quon K, et al. Metastasis-suppressed C8161 melanoma cells arrest in lung but fail to proliferate. Clin Exp Metastasis. 1999;17:601–607. doi: 10.1023/a:1006718800891. [DOI] [PubMed] [Google Scholar]
- 19.Engbring JA, Hoffman MP, Karmand AJ, et al. The B16F10 cell receptor for a metastasis-promoting site on laminin-1 is a heparan sulfate/chondroitin sulfate containing-proteoglycan. Cancer Res. 2002;62:3549–3554. [PubMed] [Google Scholar]
- 20.Ossowski L, Reich E. Experimental model for quantitative study of metastasis. Cancer Res. 1980;40:2300–2309. [PubMed] [Google Scholar]
- 21.Barnhill RL, Sagebiel RW, Lugassy C. Angiotropic melanoma: report of seven additional cases. J Cutan Pathol. 2000;27:548–12. [Google Scholar]
- 22.Friedl P, Wolf K. Tumour-cell invasion and migration: diversity and escape mechanisms. Nat Rev Cancer. 2003;3:362–374. doi: 10.1038/nrc1075. [DOI] [PubMed] [Google Scholar]
- 23.Friedl P. IL-21 Cutting and funneling: novel pathways in melanoma invasion and migration. Pigment Cell Res. 2003;16:581. [Google Scholar]
- 24.Barnhill RL, Lugassy C. Angiotropic malignant melanoma and extra vascular migratory metastasis: description of 36 cases with emphasis on a new mechanism of tumor spread. Pathology. 2004;36:485–490. doi: 10.1080/00313020412331282708. [DOI] [PubMed] [Google Scholar]
- 25.Lugassy C, Haroun RI, Brem H, et al. Pericytic-like angiotropism of glioma and melanoma cells. Am J Dermatopathol. 2002;24:473–478. doi: 10.1097/00000372-200212000-00003. [DOI] [PubMed] [Google Scholar]
- 26.Wesseling P, Ruiter DJ, Burger PC. Angiogenesis in brain tumors; pathobiological and clinical aspects. J Neurooncol. 1997;32:253–265. doi: 10.1023/a:1005746320099. [DOI] [PubMed] [Google Scholar]
- 27.Clark WH, Jr, Elder DE, Van Horn M. The biologic forms of malignant melanoma. Hum Pathol. 1986;17:443–450. doi: 10.1016/s0046-8177(86)80032-6. [DOI] [PubMed] [Google Scholar]
- 28.Yi SQ, Miwa K, Ohta T, et al. Innervation of the pancreas from the perspective of perineural invasion of pancreatic cancer. Pancreas. 2003;27:225–229. doi: 10.1097/00006676-200310000-00005. [DOI] [PubMed] [Google Scholar]
- 29.Rubin MA, Bassily N, Sanda M, et al. Relationship and significance of greatest percentage of tumor and perineural invasion on needle biopsy in prostatic adenocarcinoma. Am J Surg Pathol. 2000;24:183–189. doi: 10.1097/00000478-200002000-00003. [DOI] [PubMed] [Google Scholar]
- 30.Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2:442–454. doi: 10.1038/nrc822. [DOI] [PubMed] [Google Scholar]
- 31.Perris R, Perissinotto D. Role of the extracellular matrix during neural crest cell migration. Mech Dev. 2000;95:3–21. doi: 10.1016/s0925-4773(00)00365-8. [DOI] [PubMed] [Google Scholar]


