Abstract
The purpose of this paper is to establish the pharmacokinetics and safety of escalating, once-daily doses of daptomycin, a novel lipopeptide antibiotic active against gram-positive pathogens, including those resistant to methicillin and vancomycin. This phase 1, multiple-dose, double-blind study involved 24 healthy subjects in three dose cohorts (4, 6, and 8 mg/kg of body weight) who were randomized to receive daptomycin or the control at a 3:1 ratio and administered the study medication by a 30-min intravenous infusion every 24 h for 7 to 14 days. Daptomycin pharmacokinetics was assessed by blood and urine sampling. Safety and tolerability were evaluated by monitoring adverse events (AEs) and laboratory parameters. Daptomycin pharmacokinetics was linear through 6 mg/kg, with a slight (∼20%) nonlinearity in the area under the curve and trough concentration at the highest dose studied (8 mg/kg). The pharmacokinetic parameters measured on the median day of the study period, (day 7) were half-life (∼9 h), volume of distribution (∼0.1 liters/kg), systemic clearance (∼8.2 ml/h/kg), and percentage of the drug excreted intact in urine from 0 to 24 h (∼54%). Daptomycin protein binding (mean amount bound, 91.7%) was independent of the drug concentration. No gender effect was observed. All subjects who received daptomycin completed the study. The frequencies and distributions of treatment-emergent AEs were similar for the subjects who received daptomycin and the control subjects. There were no serious AEs and no pattern of dose-related events. The pharmacokinetics of once-daily administration of daptomycin was linear through 6 mg/kg. For all three doses, plasma daptomycin concentrations were consistent and predictable throughout the dosing interval. Daptomycin was well tolerated when it was administered once daily at a dose as high as 8 mg/kg for 14 days.
Daptomycin is a novel lipopeptide antibiotic that possesses several pharmacologic advantages over other gram-positive antimicrobial agents (3, 12). It is a fermentation product of Streptomyces roseosporus that was discovered in the early 1980s (12). Daptomycin exhibits potent in vitro bactericidal activity against most clinically relevant strains of gram-positive bacteria, including antimicrobial-resistant pathogens (5). The MICs at which 90% of the microbes tested are inhibited are ≤1 μg/ml, with the exception of those for enterococci, which are approximately 2 to 4 μg/ml (5, 7, 8, 10-13). The spontaneous acquisition of resistance to daptomycin is rare in gram-positive bacteria in vitro, and the emergence of resistance to daptomycin is rare during clinical trials.
Daptomycin appears to bind to the gram-positive bacterial cell membrane by its fatty acid tail, followed by calcium-dependent insertion (6). The resulting decrease in membrane potential causes cell death by rapidly stopping DNA, RNA, and protein synthesis (1). Because daptomycin does not appear to penetrate into cells, the interactions with the plasma membrane are most likely the basis for its pharmacologic actions.
Following successive doses at least 72 h apart, the pharmacokinetics (PK) of daptomycin was linear over the range of 0.5 to 6 mg/kg (14). Following the administration of 14C-labeled daptomycin, 83% of the administered radiolabel was recovered in excreta, with the greatest fraction (78%) appearing in the urine. Specific analysis of daptomycin (microbiologic assay) in both plasma and urine indicated that inactive metabolic products were present in urine but that only daptomycin was present in plasma.
The initial clinical studies with daptomycin conducted prior to 1999 did not establish an acceptable dosing regimen. A dose of 3 mg of daptomycin per kg of body weight every 12 h (q12h) appeared effective against moderate infections (e.g., infections of skin and skin structure) but was less effective than conventional therapy against more serious infections (e.g., staphylococcal endocarditis). The incidence of adverse effects associated with 3 mg/kg q12h (total daily dose, 6 mg/kg) was low and comparable with that of conventional therapy (12). However, in phase 1 studies that evaluated 4 mg/kg q12h (total daily dose, 8 mg/kg), two of five subjects experienced adverse skeletal muscle effects after days 7 and 12, respectively. This myopathy was characterized by elevations in serum creatine phosphokinase (CPK) values to >10 times the upper limit of normal (ULN), and these elevations were associated with muscle discomfort and weakness in the extremities. All findings reversed promptly and fully within a few days of stopping study medication.
In subsequent animal studies, Oleson et al. (9) demonstrated that in dogs the incidence and severity of skeletal muscle effects associated with a given total daily dose of daptomycin were substantially increased by dose fractionation and were decreased if the entire dose was administered once daily. This phase 1 study was designed to assess the PK, safety, and tolerability of daptomycin administered once daily at 4, 6, and 8 mg/kg to healthy subjects.
MATERIALS AND METHODS
Subjects and study design.
The clinical phase of this study was conducted at Innovative Clinical Solutions, Ltd. (Ft. Lauderdale, Fla.), and complied with all relevant federal and institutional policies. Healthy male and nonpregnant female subjects, ≥21 and ≤45 years of age, were eligible for the study if they weighed <120 kg; had normal hematologic, hepatic, and renal functions; had normal serum CPK levels; had no history of neurologic disease, muscular disease, active immune disorders (not including common allergies), or clinically significant abnormalities; were not actively abusing drugs and/or alcohol; had no clinically significant electrocardiographic abnormalities; and were not positive for hepatitis or human immunodeficiency virus antibody. All subjects were recruited from a database maintained by the clinical site, and they were enrolled within 14 days of dosing. All subjects provided written informed consent. Subjects were excluded from the study if they had a history of active neurologic disease, muscular disease, or immune disorders; had developed an acute infection requiring antibiotic therapy within 30 days of the start of the study; had used any investigational drug within 30 days prior to the start of the study; had taken prescription medication within 30 days (not including birth control) or over-the-counter-medications within 5 days of the start of the study; had received intramuscular injections or had taken nutritional supplements within 15 days of the start of the study; or had taken anabolic steroids or donated blood within 30 days of the start of the study.
This was a multiple-dose, double-blind, single-center study that randomized subjects into three dose cohorts (8 subjects per cohort; 24 subjects total). Within each cohort, six subjects were randomized to receive daptomycin and two received 0.9% normal saline (control). Cohort 1 received 4 mg of daptomycin or the control per kg for 7 days, cohort 2 received 6 mg of daptomycin or the control per kg for 7 days, and cohort 3 received 8 mg of daptomycin or the control per kg for 14 days. The duration of dosing for cohort 3 was longer to gain additional safety data. All subjects received study medication as a single, daily, 30-min intravenous infusion q24h and were monitored for 3 days (approximately eight half-lives [t1/2]) after the last infusion. Each subject participated in only a single cohort and received only one dose regimen. All subjects in a lower-dose cohort were required to complete both treatment and follow-up before treatment of the next-higher-dose cohort was initiated.
PK parameters and analyses.
The PK of daptomycin was determined by analysis of daptomycin levels in blood and urine samples obtained throughout the study duration. Day 1 was defined as the day the first dose was administered, and the beginning of the infusion was defined as time zero. Blood was collected for plasma assays at baseline (time zero) and at 0.25, 0.5, 1.0, 1.5, 2.5, 4.5, 6.5, 8.5, 10.5, 12.5, and 24.5 h. Samples were collected on days 1 and 7 for all cohorts and also on day 14 for cohort 3. Blood samples for trough levels were obtained at h 24.5 on days 2 through 7 (all cohorts) and days 10 and 14 (predose) for cohort 3. Blood samples were also collected during follow-up on days 8 through 10 (cohorts 1 and 2) and days 15 through 17 (cohort 3). Urine samples were collected on day 1 for all cohorts at the following intervals: 0, 0 to 2.5, 2.5 to 4.5, 4.5 to 6.5, 6.5 to 8.5, 8.5 to 10.5, 10.5 to 12.5, and 12.5 to 24.5 h. Urine was collected from each subject for each collection interval. Serum samples for the determination of protein binding were collected at the end of the infusion (0.5 h), and at 2.5 and 8.5 h from the start of the infusion.
The PK parameters determined included the maximum concentration of drug in plasma (Cmax), time to maximum concentration of drug in plasma, areas under the concentration-time curve from 0 h to infinity (AUC0-∞, day 1) and from 0 to 24 h (AUC0-24, days 7 and 14), t1/2, clearance (CL), terminal exponential apparent volume of distribution (V), mean residence time (day 1), renal CL (CLR), accumulation index, and the fraction of the first dose excreted intact in urine over the dosing interval (i.e., 24 h after the first dose).
Plasma daptomycin concentration-time data were analyzed by conventional noncompartmental PK analysis (4) with model 202 of WinNonlin Pro (version 3.1; Pharsight Corp., Mountain View, Calif.). The AUCs were determined by the linear trapezoidal rule (4). The Cmax was the highest concentration determined by inspection of the data, and the time to maximum concentration of the drug in plasma was the time of occurrence of Cmax. To obtain the first-dose AUC0-∞, the AUC from time zero to the last relevant time point was measured as described above and the AUC to infinity was extrapolated by dividing the concentration in plasma of the last data point by λz (rate constant associated with terminal elimination phase). This constant, λz for all data sets, was determined from regression analysis of semilogarithmic plasma daptomycin concentration-time data from 4.5 to 24 h (six data points, assuming all data were available) after initiation of the infusion. Unbound CLR was calculated by dividing the total CLR (cumulative drug excreted in urine [0 to 24 h]/AUC0-24) by the fraction unbound in serum. The t1/2 in plasma was defined as 0.693/λz.
Determination of daptomycin concentrations and protein binding.
The concentrations of daptomycin in plasma and urine were determined by high-performance liquid chromatography with UV detection. This method has been validated for daptomycin over the concentration range of 3 to 500 μg/ml, with a lower limit of quantitation equal to the lowest calibration level of 3 μg/ml.
Daptomycin and an internal standard (ethyl paraben in methanol) were isolated from plasma by using protein precipitation with methanol. Following centrifugation of the plasma, the methanol phase was transferred to autosampler vials with glass inserts. Daptomycin was directly detected in urine samples after the addition of the internal standard in methanol. The mobile phase consisted of 90% mobile phase A (acetonitrile-0.5% NH4H2PO4, 34:66[vol/vol]) and 10% mobile phase B (acetonitrile-0.5% NH4H2PO4, 20:80 [vol/vol]) at a flow rate of 1.5 ml/min. A Phenomenex IB-SIL 5 C8 guard column (30 by 4.6 mm) was used with a Phenomenex IB-SIL 5 C8 column (250 by 4.6 mm). A Waters 2487 UV detector set at 214 nm was used to detect daptomycin (retention time, 15 min) and the internal standard (retention time, 7 min). The total run time was 30 min.
Quality control standards were prepared at final concentrations of 3.0, 7.50, 75.0, and 499 μg/ml. The 3.0-μg/ml pool (limit of quantitation) had an intra-assay coefficient of variation of 3.51% and a −9.21% difference from the theoretical value. The remaining quality control pools had intra-assay coefficients of variation ranging from 1.7 to 2.95% and percentages of difference within 2.12% of theoretical values. Interassay precision and accuracy for the 3.0-μg/ml pool were 4.8% and a −3.8% difference from the theoretical value. The remaining quality control pools had interassay coefficients of variation ranging from 1.86 to 2.07% and percentages of difference within 1.16% of theoretical values. Mean absolute recovery for daptomycin (7.5 μg/ml) was 92.4%, with a coefficient of variation of 1.66%. For the 499-μg/ml quality control sample, the mean recovery of daptomycin was 95.0%, with a coefficient of variation of 1.53%. The recovery of the internal standard was 103%, with a coefficient of variation of 0.82%.
Serum protein binding of daptomycin was determined by equilibrium dialysis coupled with an analytical liquid chromatograph-mass spectrophotometer method for measuring daptomycin levels in serum and buffer. Samples were extracted by solid-phase extraction by using Waters Oasis HLB 3-cc cartridges and were subsequently analyzed by liquid chromatography-mass spectrophotometry by using a Zorbax RX C8 column (2.1 by 150 mm, 5 μm) with a Quattro LC (Micromass Inc., Beverly, Mass.) detection system against a standard daptomycin concentration range of 0.2 to 200 μg/ml. A quadratic weighted regression analysis was used, and the coefficient of determination for the regression was >0.9930. The coefficient of variation of the back-calculated calibration standards was ≤9.0%. The coefficient of variation of the back-calculated quality control samples ranged from 1.0 to 15.1%.
Safety evaluations.
All subjects who received one or more doses of daptomycin or the control were included in the analysis of drug safety. Treatment-emergent adverse events (AEs), clinical laboratory parameters (hematology, clinical chemistry, prothrombin time or partial thromboplastin time, and urinalysis, evaluated on days 1, 7, and 10 for cohorts 1 and 2 and on days 1, 7, 14, and 17 for cohort 3), vital signs (checked daily), and 12-lead electrocardiographic recordings (days 1, 4, 7, and 10 for cohorts 1 and 2 and days 1, 4, 7, 14, and 17 for cohort 3) were used to assess safety. Subjects also received physical and neurologic examinations on day 1 (all cohorts) and day 10 (cohorts 1 and 2) or days 14 (physical examination only) and 17 (cohort 3). Serum CPK levels were monitored daily (normal range, 29 to 190 IU/liter). AEs were graded according to the World Health Organization toxicity grading scale and were each assigned a preferred term and body system from the Medical Dictionary for Regulatory Activities (MedDRA) coding dictionary.
RESULTS
Subjects.
A total of 24 healthy men and women with a mean age of 35 years (range, 25 to 43 years) were enrolled in the study. The demographic parameters are summarized in Table 1. All subjects completed the study through follow-up except for one control subject in cohort 2 who discontinued after receiving a single dose of study medication and subsequently experiencing a mild rash and mild pruritus, assessed as possibly treatment related.
TABLE 1.
Subject characteristics
| Dose (mg/kg) | Treatment group (no. of subjects) | Mean age (yr [range]) | No. (%) of subjects of indicated sex | No. (%) of subjects of indicated race | Mean wt (kg [range]) | Mean ht (cm [range]) |
|---|---|---|---|---|---|---|
| 4 | Control (2) | 37.5 (37-38) | 2 females (100) | 2 Hispanics (100) | 64.5 (56.9-72.0) | 163.8 (154.9-172.7) |
| Daptomycin (6) | 32.7 (28-38) | 4 males (67) | 6 Hispanics (100) | 69.8 (55.0-85.0) | 164.5 (147.3-177.8) | |
| 2 females (33) | ||||||
| 6 | Control (2) | 34 (31-37) | 1 male (50) | 1 white (50) | 70.3 (63.2-77.3) | 167.6 (154.9-180.3) |
| 1 female (50) | 1 Hispanic (50) | |||||
| Daptomycin (6) | 35.7 (25-43) | 3 males (50) | 6 Hispanics (100) | 69.6 (52.7-81.4) | 165.1 (154.9-182.9) | |
| 3 females (50) | ||||||
| 8 | Control (2) | 32.5 (32-33) | 1 male (50) | 2 Hispanics (100) | 66.6 (52.7-80.5) | 165.1 (162.6-167.6) |
| 1 female (50) | ||||||
| Daptomycin (6) | 36.8 (29-42) | 3 males (50) | 1 white (17) | 71.3 (65.9-79.1) | 171.0 (160.0-180.3) | |
| 3 females (50) | 1 black (17) | |||||
| 4 Hispanics (66) |
PK.
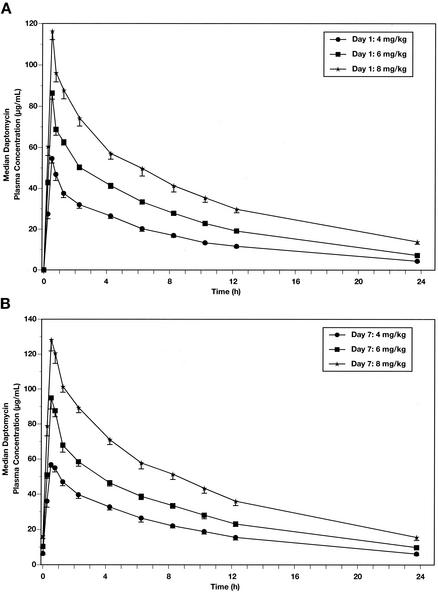
The mean plasma daptomycin concentrations on days 1 and 7 for each dose cohort are shown in Fig. 1. The Cmax at each dose level occurred at the end of the infusion. The mean Cmaxs on days 1 and 7 for the 4- and 6-mg/kg doses were dose proportional; for the 8-mg/kg dose, Cmaxs were approximately 2.2 times greater than those at 4 mg/kg, indicating a slight nonlinearity (∼20%) at dose levels above 6 mg/kg. Box plots of daptomycin trough concentrations (day 4 and onward, number of data points at each dose, 30) as a function of dose amount are shown in Fig. 2. The median values at 4 and 6 mg/kg were 6.37 and 9.13 μg/ml, respectively, consistent with dose proportionality over this dose range. At 8 mg/kg, the median trough plasma daptomycin concentration increased to 15.3 μg/ml, again demonstrating a slight (∼20%) nonlinearity.
FIG. 1.
Plasma drug concentration-versus-time plots for once-daily dosing of daptomycin on day 1 (A) and day 7 (B). Data are shown for 4, 6, and 8 mg/kg.
FIG. 2.
Box plots of daptomycin trough plasma concentrations (day 4 onward) as a function of dose. The plots extend from the 25th percentile to the 75th percentile, and the median value is indicated by a horizontal line across the box. The fences extend to the 10th and 90th percentiles. Minimum and maximum data points are indicated (black circles) if they are different from any of the values in the indicated percentiles. The number of data points for each dose was 30.
Clinically relevant PK parameters of daptomycin are summarized in Table 2. Daptomycin exhibited a long t1/2 (∼9 h), a low V (∼0.1 liter/kg), and an accumulation index of ∼1.2, indicating minor (20%) drug accumulation across the dose ranges studied when administered once daily. The drug is primarily excreted in the urine, with ∼50% of the administered dose being excreted in 24 h as unchanged drug. The CLR of the total drug was less than the systemic CL; the CLR of unbound daptomycin was less than the average glomerular filtration rate of 120 ml/min.
TABLE 2.
Mean PK values for daptomycin
| Day | Dose (mg/kg) | Parameter (mean ± SD)
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cmax (μg/ml) | AUC0−∞a (μg · h/ml) | t1/2 (h) | CL (ml/h/kg) | CLR,Ub (ml/h/kg) | V (liter/kg) | Rcc | Fud | Fe (%)e | ||
| 1 | 4 | 54.6 ± 5.4 | 425 ± 58 | 7.4 ± 0.9 | 9.6 ± 1.3 | 84.6 ± 33.6 | 0.101 ± 0.013 | 0.08 ± 0.02 | 53 ± 10.8 | |
| 6 | 86.4 ± 7.1 | 705 ± 67 | 7.8 ± 1.0 | 8.6 ± 0.8 | 59.5 ± 16.7 | 0.096 ± 0.009 | 0.07 ± 0.01 | 47.4 ± 11.5 | ||
| 8 | 116.3 ± 10.1 | 1,127 ± 161 | 9.6 ± 1.1 | 7.2 ± 1.1 | 50.0 ± 5.2 | 0.099 ± 0.014 | 0.09 ± 0.01 | 52.1 ± 5.2 | ||
| 7 | 4 | 57.8 ± 3.0 | 494 ± 75 | 8.1 ± 1.0 | 8.3 ± 1.3 | 62.9 ± 18.5 | 0.096 ± 0.009 | 1.4 ± 0.2 | 0.08 ± 0.01 | |
| 6 | 98.6 ± 12.0 | 747 ± 91 | 8.9 ± 1.3 | 8.1 ± 1.0 | 59.8 ± 13.8 | 0.104 ± 0.013 | 1.2 ± 0.1 | 0.08 ± 0.02 | ||
| 8 | 133.0 ± 13.5 | 1,130 ± 117 | 9.0 ± 1.2 | 7.1 ± 0.8 | 40.9 ± 6.28 | 0.092 ± 0.012 | 1.2 ± 0.1 | 0.09 ± 0.01 | ||
| 14 | 8 | 129.5 ± 14.5 | 1,090 ± 114 | 8.9 ± 0.8 | 7.4 ± 0.8 | 44.7 ± 9.8 | 0.095 ± 0.013 | 1.2 ± 0.1 | 0.09 ± 0.01 | |
For day 1, AUC0-∞; for days 7 and 14, AUC0-24.
CLR,U, unbound, CLR.
Rc, accumulation index.
Fu, unbound fraction in serum.
Fe, percentage of dose recovered unchanged in urine from 0 to 24 h after the first dose.
Safety and tolerability.
Daptomycin was well tolerated at all doses tested. All 24 subjects received at least one dose of the study drug and were included in the safety analysis. All subjects completed the study except for the control subject in cohort 2 mentioned above.
AEs were reported by 17 of 24 (71%) subjects, including 5 of 6 (83%) who received control infusions and 12 of 18 (67%) who received daptomycin. Table 3 summarizes all events by body system and treatment group, regardless of causality. All the AEs were mild in intensity, none was characterized as serious, and most resolved spontaneously without intervention. The relatively high incidence of AEs in healthy subjects is consistent with the close monitoring in a phase 1 investigational unit. The frequencies of events were similar across all treatment groups; note that the rates in the treatment group that received 8 mg of daptomycin per kg have not been adjusted for the seven additional days of observation. Three of the four injection site events reported for this group occurred on or after day 7; none was considered to be related to the study medication.
TABLE 3.
Subjects with AEs, by body system
| Type of AE | No. of subjects (%) receivinga:
|
|||
|---|---|---|---|---|
| Daptomycin
|
Control | |||
| 4 mg/kg q24h for 7 days | 6 mg/kg q24h for 7 days | 8 mg/kg q24h for 14 days | ||
| Any event | 3 (50) | 3 (50) | 6 (100) | 5 (83) |
| Gastrointestinal | 0 | 0 | 5 (83) | 2 (33) |
| Skin | 0 | 0 | 0 | 3 (50) |
| Nervous system | 1 (17) | 1 (17) | 1 (17) | 1 (17) |
| Laboratory investi- gations | 1 (17) | 0 | 1 (17) | 1 (17) |
| Administration site | 0 | 0 | 4 (67) | 0 |
| Infections | 1 (17) | 0 | 0 | 1 (17) |
| Otherb | 0 | 2 (33) | 2 (33) | 0 |
The total number of subjects in each group was six. Values are numbers of subjects who experienced an event at least once.
Other body systems each reported once included respiratory, renal, and general disorders and complications from surgical procedures.
The most commonly reported AE was constipation (gastrointestinal system), which was noted in two (33%) control subjects and four (22%) daptomycin subjects; none of these events was considered study drug related. Three subjects, all in the control group, reported AEs related to the skin. Three subjects (one who received the control and two who received daptomycin) were noted to have elevations in serum CPK values. All of these elevations were mild in intensity (peak CPK, 350 to 477 IU/liter; at most two and one-half times the ULN) and brief in duration (2 days), and none was associated with symptoms of muscle discomfort or muscle weakness. For both daptomycin subjects, treatment was continued and CPK levels returned to within normal limits. These elevations in CPK were less than those observed in subjects who had engaged in downhill running (2) and, thus, were not considered to be clinically significant. Indeed, the daptomycin recipient who experienced the highest CPK elevation (477 IU/liter on day 11) had performed intense physical activity the previous day that was assessed as the likely cause of his brief, asymptomatic CPK increase. Specifically, on day 10 this subject had rowed to his moored sailboat in inclement weather to secure it against an approaching hurricane. As noted, his CPK levels returned to the normal range within 2 days while he continued to receive 8 mg of daptomycin per kg q24h.
For two control subjects (33%) and two daptomycin subjects (11%), AEs were assessed as possibly treatment related. Among the control subjects, these events included a rash (resulting in discontinuation on day 1 as noted above) and an episode of headache on day 3. Both of the daptomycin subjects who experienced events that were considered possibly treatment related were in cohort 1; the events included insomnia and CPK elevation.
There were no clinically significant treatment-emergent changes in vital signs or in results of hematology, clinical chemistry, or urinalysis studies.
DISCUSSION
This study examined the PK of daptomycin in healthy subjects given 4, 6, or 8 mg of the drug per kg q24h over 7 to 14 days. Across this dose range, plasma daptomycin concentrations were generally consistent and predictable, and PK parameters following multiple-dose treatment were in agreement with earlier single-dose data (14). Taken together, these results suggest that patients receiving these regimens will have reliable concentrations in plasma.
For all three doses of daptomycin studied, the Cmaxs and AUCs were ∼20% greater at steady state (day 7) than following a single dose (day 1). This accumulation is consistent with the observed t1/2 (∼9 h) and the dosing interval (24 h). At the highest dose studied (8 mg/kg), several parameters (e.g., Cmax, AUC, and unbound CLR) demonstrated slight (∼20%) nonlinearity relative to results at the lower doses (4 and 6 mg/kg). Plasma daptomycin levels attained after multiple dosing remained consistent and predictable. The nonlinearity observed was considered minor and not clinically relevant for dosing regimens in this range.
The level of plasma protein binding of daptomycin was consistent across dose levels, with the unbound fraction representing 8.33% ± 1.64% (mean ± standard deviation); the drug was primarily excreted unchanged by the kidneys (14). As a consequence of these features, the level of CL from plasma was relatively low (∼7 to 9 mg/h/kg) relative to hepatic blood flow (∼1,500 ml/h/kg), and the t1/2 was relatively long (∼9 h). Daptomycin has a small V (∼0.1 liter/kg), which suggests that the drug remains primarily in the plasma and interstitial fluid. These data are consistent with in vitro observations that daptomycin does not penetrate the mammalian cell membrane (unpublished observations).
The PK results in this study are similar to those from earlier studies in which a microbiologic assay was used to determine plasma daptomycin levels in healthy subjects following single and multiple intravenous doses (H. R. Black, data on file, Cubist Pharmaceuticals, Inc., Lexington, Mass.) (14). Of note, our subjects were primarily Hispanic, whereas the earlier subjects were primarily Caucasian, which suggests that ethnic differences represent only a minor source of variability in the PK of daptomycin. This conclusion is consistent with the distribution and elimination characteristics of daptomycin (i.e., it does not penetrate cells, is eliminated mainly by glomerular filtration, and is not metabolized by the cytochrome P-450 system). A population PK analysis of both healthy and infected subjects is in progress to further define the influence of demographic and clinical factors on the PK of daptomycin.
Daptomycin was well tolerated in the regimens evaluated. The only subject in this study who discontinued treatment prematurely was receiving saline. The frequency and distribution of treatment-emergent AEs in daptomycin subjects were comparable to those in the control subjects. All reported events were mild, and most resolved without specific intervention. No serious AEs were reported. There was no pattern of dose-related events, such as acute infusion reactions and nephrotoxicity, which has been encountered in other classes of antibiotics (e.g., glycopeptides and aminoglycosides).
Preclinical studies indicate that skeletal muscle is the primary target of dose-related toxicity for daptomycin. In an earlier, phase 1, dose escalation trial, two of five subjects receiving daptomycin at 4 mg/kg q12h had acute CPK elevations (more than 10 times the ULN), associated with muscle weakness and myalgia, after 7 to 12 days of treatment (12). These effects reversed rapidly and completely following discontinuation of the drug. Subsequent studies of dogs indicated that, for a given total daily dose of daptomycin, the frequency and severity of muscle effects were increased with dose fractionation and decreased with once-daily dosing (9).
In the present trial, there were no events consistent with a clinical picture of daptomycin-related myopathy; all six subjects who received 8 mg/kg q24h successfully completed the planned 14-day course. Elevations in CPK were encountered in one control subject and in two daptomycin subjects. These elevations were modest (less than 2.5 times the ULN), transient (2 days), and not associated with any muscle symptoms. Of particular note, the elevations in CPK in the daptomycin subjects, including one subject receiving 8 mg/kg q24h, resolved while they continued to receive study medication. Thus, there was no clinical or pharmacologic evidence that the elevations observed represented an effect of daptomycin.
CPK is released from skeletal muscle after a wide range of physical, biochemical, and biological injuries. Serum CPK elevations can be seen with bacterial infections of skin and soft tissue; with generalized viral infections, including influenza; with intramuscular injections of medications; and with direct trauma, either accidental or surgical. Routine, vigorous physical activity (e.g., running) can also result in increased serum CPK levels (2). Available information suggests that such activity may have contributed to the CPK rise observed in the daptomycin subject in cohort 3.
The PK of daptomycin raises the possibility that total daily exposure at steady state may contribute to the apparent difference in muscle effects among subjects receiving the same total daily dose (8 mg/kg) through two different regimens (4 mg/kg q12h and 8 mg/kg q24h). Given a t1/2 of 9 h and a dosing interval of 12 h, the estimated accumulation factor at steady state would be 1.7, compared with an accumulation factor of 1.2 with once-daily dosing. Thus, the same total dose in two divided doses is associated with a total daily exposure that is 42% higher than that for once-daily dosing (exposures, 13.6 versus 9.6 mg/kg/day, respectively). However, these estimates are primarily applicable in the presence of normal renal function, and muscle effects may also be significantly affected by dosing interval.
In summary, daptomycin exhibits predictable PK over multiple-dose regimens of 4, 6, and 8 mg/kg q24h and was well tolerated at the highest dose for up to 14 days. Pharmacodynamic studies with animal models indicate that daptomycin has a concentration-dependent mode of action and that efficacy is best correlated with the Cmax and/or the AUC rather than with the proportion of time that the concentration exceeds the MIC. In vitro studies document rapid concentration-dependent bactericidal activity. Once-daily dosing is also more convenient to administer and facilitates patient compliance. Other potentially relevant characteristics of daptomycin include its distinct mechanism of action and its in vitro activity against organisms resistant to currently available antibiotics. Once-daily regimens of daptomycin are being evaluated in clinical trials for the treatment of serious infections due to gram-positive pathogens.
Acknowledgments
This study was supported by and conducted by Cubist Pharmaceuticals, Inc.
We gratefully acknowledge the assistance of Maria Gutierrez and the staff at Innovative Clinical Solutions, Ltd., where the clinical portion of this study was conducted; PPD Development and Avantix Laboratories, which performed the high-performance liquid chromatography and liquid chromatography-mass spectrometry-mass spectrometry analyses; and Harold Boxenbaum for his assistance in the analysis of the PK data.
REFERENCES
- 1.Allen, N. E., W. E. Alborn, Jr., and J. N. Hobbs, Jr. 1991. Inhibition of membrane potential-dependent amino acid transport by daptomycin. Antimicrob. Agents Chemother. 35:2639-2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Byrnes, W. C., P. M. Clarkson, J. S. White, S. S. Hsieh, P. N. Frykman, and R. J. Maughan. 1985. Delayed onset muscle soreness following repeated bouts of downhill running. J. Appl. Physiol. 59:710-715. [DOI] [PubMed] [Google Scholar]
- 3.Cassell, G. H., and J. Mekalanos. 2001. Development of antimicrobial agents in the era of new and reemerging infectious diseases and increasing antibiotic resistance. JAMA 285:601-605. [DOI] [PubMed] [Google Scholar]
- 4.Gibaldi, M., and D. Perrier. 1982. Pharmacokinetics, 2nd ed. Marcel Dekker, New York, N.Y.
- 5.Hanberger, H., L. E. Nilsson, R. Maller, and B. Isaksson. 1991. Pharmacodynamics of daptomycin and vancomycin on Enterococcus faecalis and Staphylococcus aureus demonstrated by studies of initial killing and postantibiotic effect and influence of Ca2+ and albumin on these drugs. Antimicrob. Agents Chemother. 35:1710-1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lakey, J. H., and M. Ptak. 1988. Fluorescence indicates a calcium-dependent interaction between the lipopeptide antibiotic LY146032 and phospholipid membranes. Biochemistry 27:4639-4645. [DOI] [PubMed] [Google Scholar]
- 7.Lamp, K. C., M. J. Rybak, E. M. Bailey, and G. W. Kaatz. 1992. In vitro pharmacodynamic effects of concentration, pH, and growth phase on serum bactericidal activities of daptomycin and vancomycin. Antimicrob. Agents Chemother. 36:2709-2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Louie, A., A. L. Baltch, W. J. Ritz, R. P. Smith, and M. Asperilla. 1993. Comparison of in vitro inhibitory and bactericidal activities of daptomycin (LY 146032) and four reference antibiotics, singly and in combination, against gentamicin-susceptible and high-level-gentamicin-resistant enterococci. Chemotherapy 39:302-309. [DOI] [PubMed] [Google Scholar]
- 9.Oleson, F. B., Jr., C. L. Berman, J. B. Kirkpatrick, K. S. Regan, J.-J. Lai, and F. P. Tally. 2000. Once-daily dosing in dogs optimizes daptomycin safety. Antimicrob. Agents Chemother. 44:2948-2953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rybak, M. J., E. Hershberger, T. Moldovan, and R. G. Grucz. 2000. In vitro activities of daptomycin, vancomycin, linezolid, and quinupristin-dalfopristin against staphylococci and enterococci, including vancomycin-intermediate and -resistant strains. Antimicrob. Agents Chemother. 44:1062-1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Snydman, D. R., N. V. Jacobus, L. A. McDermott, J. R. Lonks, and J. M. Boyce. 2000. Comparative in vitro activities of daptomycin and vancomycin against resistant gram-positive pathogens. Antimicrob. Agents Chemother. 44:3447-3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tally, F. P., M. Zeckel, M. M. Wasilewski, C. Carini, C. L. Berman, G. L. Drusano, and F. B. Oleson, Jr. 1999. Daptomycin: a novel agent for gram-positive infections. Expert Opin. Investig. Drugs 8:1223-1238. [DOI] [PubMed] [Google Scholar]
- 13.Vance-Bryan, K., T. A. Larson, J. C. Rotschafer, and J. P. Toscano. 1992. Investigation of the early killing of Staphylococcus aureus by daptomycin by using an in vitro pharmacodynamic model. Antimicrob. Agents Chemother. 36:2334-2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Woodworth, J. R., E. H. Nyhart, Jr., G. L. Brier, J. D. Wolny, and H. R. Black. 1992. Single-dose pharmacokinetics and antibacterial activity of daptomycin, a new lipopeptide antibiotic, in healthy volunteers. Antimicrob. Agents Chemother. 36:318-325. [DOI] [PMC free article] [PubMed] [Google Scholar]