Abstract
Spermidine/spermine N1-acetyltransferase (SSAT), the rate-controlling enzyme in the interconversion of spermidine and spermine, is regulated by polyamines and their analogs at many levels of gene expression. Recently, SSAT pre-mRNA has been shown to undergo alternative splicing by inclusion of an exon that contains premature termination codons. In the present study, we show that alterations in the intracellular polyamine level resulted in a change in the relative abundance of SSAT transcripts. Addition of polyamines or their N-diethylated analogs reduced the amount of the variant transcript, whereas polyamine depletion by 2-difluoromethylornithine or MG-132 enhanced the exon inclusion. Experiments performed with protein synthesis inhibitors and siRNA-mediated down-regulation of Upf1 protein verified that the variant transcript was degraded by nonsense-mediated mRNA decay (NMD). Interestingly, several proteins have been shown to regulate their expression by alternative splicing-coupled NMD, termed regulated unproductive splicing and translation (RUST). Our present results suggest that in the case of SSAT, RUST is mediated by polyamines, and this system functions to fine-tune the polyamine metabolism.
Keywords: transgenic mouse, fetal fibroblast, embryonic stem cell, polyamine analogs, 2-difluoromethylornithine, regulated unproductive splicing and translation, nonsense-mediated mRNA decay
INTRODUCTION
Polyamines, spermidine and spermine and their precursor putrescine, are small ubiquitous organic cations that are essential for cell growth and proliferation and for the synthesis of proteins and nucleic acids. Due to their positive charge, polyamines interact with different polyanionic macromolecules such as DNA and RNA. They are involved in the repair of the extracellular matrix, immunity, cell adhesion, and regulation of several ion channels. Polyamine depletion has been shown to inhibit cell proliferation and migration, whereas overaccumulation of polyamines induces cell death and transformation. Therefore the intracellular polyamine pools appear to be tightly regulated by various homeostatic responses including biosynthesis, interconversion, catabolism, and transport (Thomas and Thomas 2003; Moinard et al. 2005).
Spermidine/spermine N1-acetyltransferase (SSAT) is the rate-controlling enzyme in the interconversion of polyamines. The enzyme acetylates spermidine and spermine, which are then either excreted out from the cell or converted back to putrescine or spermidine, respectively, by polyamine oxidase. SSAT is an enzyme with an extremely short half-life (<30 min) showing striking inducibility in response to diverse agents and pathophysiological conditions (Matsui and Pegg 1981). Its activity is known to be increased enormously on an exposure to certain polyamine analogs, such as N1,N11-diethylnorspermine (DENSpm), leading to depletion of higher polyamines and retardation of cell growth (Porter et al. 1991).
SSAT is regulated by polyamines and their analogs, such as DENSpm, at many levels of gene expression, including transcription and stabilization of mRNA (Fogel-Petrovic et al. 1993; Xiao and Casero 1996; Wang et al. 1999). In addition, the analogs have been shown to powerfully stabilize the enzyme protein by preventing its ubiquinylation and subsequent degradation by 26S proteasome (Coleman et al. 2001). Consequently, the half-life of SSAT is prolonged to >12 h, resulting in a superinduction (Parry et al. 1995). Conversely, natural polyamines do not stabilize SSAT as efficiently as alkylated analogs (Fogel-Petrovic et al. 1996). Therefore, the analogs have proven to be valuable tools for studying polyamine metabolism, and some of them may have therapeutic value as anti-cancer drugs (Sharma et al. 1997; Schipper et al. 2000).
SSAT pre-mRNA has been recently found to undergo alternative splicing to yield, along with normal SSAT mRNA, a longer variant (referred here as SSAT-X) by insertion of an additional 110-bp exon between exons 3 and 4. The exon inclusion introduces three in-frame premature termination codons (PTC), thus making the SSAT-X variant a likely target for nonsense-mediated mRNA decay (NMD). Previous studies have shown that SSAT-X mRNA is accumulating upon various factors, including X-ray irradiation (Mita et al. 2004), infection of certain RNA viruses (Nikiforova et al. 2002), iron chelation, and hypoxia (Kim et al. 2005a). The latter study appears to indicate that cells stably overexpressing a cDNA clone of SSAT-X are protected from apoptosis under iron-deficient conditions (Kim et al. 2005a).
In the present work we describe several approaches undertaken to elucidate the physiological role of the alternative transcript. The results demonstrate that (1) SSAT-X mRNA is degraded by NMD, (2) polyamines and their analogs inhibit the exon inclusion, and (3) depletion of cellular spermidine and/or spermine promotes the exon inclusion. Our results suggest that SSAT gene expression is fine-tuned by regulated unproductive splicing and translation (RUST), which is modulated by polyamine levels.
RESULTS
Effect of polyamines and various polyamine analogs on alternative splicing of SSAT
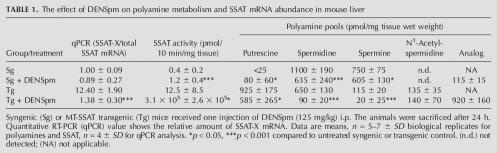
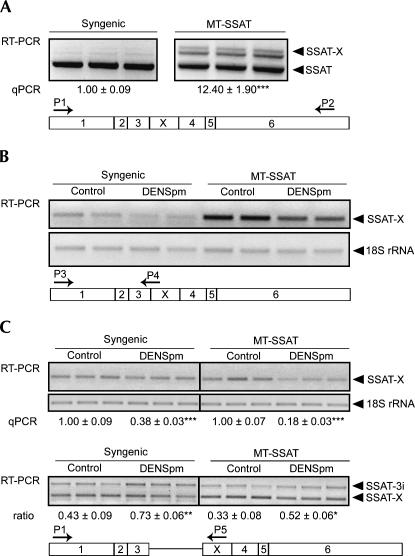
Previous studies have shown that the alternative splice variant of SSAT is expressed at a very low basal level. To discover how overexpression of SSAT would affect the splicing pattern, we used a previously generated transgenic mouse line overexpressing genomic mouse SSAT under the control of a heavy metal-inducible metallothionein I (MT) promoter (Suppola et al. 1999). As shown in Table 1, these animals displayed, even in the absence of heavy metals, significantly reduced levels of spermidine and spermine and overaccumulation of putrescine and N1-acetylspermidine in the liver as compared with their syngenic littermates. These alterations are due to the activation of SSAT and resulting compensatory activation of ornithine decarboxylase, the rate-limiting enzyme of polyamine biosynthesis. Our previous Northern blot experiments had indicated that even though the hepatic SSAT mRNA accumulates to massive levels in noninduced transgenic animals, SSAT activity is only moderately elevated (Suppola et al. 1999). However, Northern blot does not differentiate between the splice variants, as their size difference is only 110 bp. There have been earlier reports indicating the existence of 1.3-kb and 1.5-kb SSAT mRNA species in the MALME-3M cell line in which the size difference between distinct isoforms was due to changes in polyadenylation levels (Fogel-Petrovic et al. 1993; Shappell et al. 1993). Therefore, PCR analyses were carried out to investigate the relative expression levels of SSAT transcripts. Interestingly, RT-PCR analysis showed a very high basal level of SSAT-X variant in the livers of transgenic mice (Fig. 1A). Quantitative PCR indicated that the relative amount of SSAT-X to the total SSAT mRNA was 12-fold higher in the livers of transgenic mice as compared with their syngenic littermates. As shown in Figure 1B, SSAT-X was also present in primary fetal fibroblasts derived from these animals, although the relative amount of SSAT-X was considerably lower than in the liver (1.9-fold higher in transgenic than in syngenic cells). The alternative variant was found in the cytoplasm and was obviously polyadenylated, because the reverse transcription was carried out with oligo-dT primers.
TABLE 1.
The effect of DENSpm on polyamine metabolism and SSAT mRNA abundance in mouse liver
FIGURE 1.
Expression of SSAT transcripts. (A) RT-PCR from the livers of syngenic and MT-SSAT transgenic mice showing both SSAT and SSAT-X (P1/P2). Note that in order to detect SSAT-X, PCR cycles used for syngenic and transgenic samples were 35 and 30, respectively. Also note that the RT-PCR pictures in (A) are not quantitative due to saturation of PCR reaction. The middle-sized product contained both SSAT-X and SSAT as verified by sequencing, and thus was formed during PCR reaction. (B) Cytoplasmic RNA fractions. SSAT-X-specific RT-PCR (P3/P4) from syngenic and MT-SSAT transgenic fetal fibroblasts treated with or without 10 μM DENSpm for 24 h. (C) Nuclear RNA fractions. Cells were treated with or without 10 μM DENSpm for 7 h. (Upper panel) SSAT-X-specific RT-PCR (P3/P4). (Lower panel) exon X-containing transcripts (SSAT-X and intron 3-containing transcript (SSAT-3i) (P1/P5). The ratio of SSAT-3i/SSAT-X was quantified by densitometry. Lanes represent individual samples. 18S rRNA was used as a control. The relative amount of SSAT-X (normalized to total SSAT mRNA) was quantified by quantitative RT-PCR (qPCR). n = 3 ± SD, analyzed in triplicate.
Next, a polyamine analog, DENSpm, was used to further induce the expression of SSAT. Interestingly, RT-PCR specific for SSAT-X variant showed that, after 24-h DENSpm treatment, the amount of SSAT-X was clearly decreased in the cells (Fig. 1B). Based on our findings we speculated that the analog might modulate the alternative splicing of SSAT pre-mRNA. To further test this idea, nuclear RNA fraction was extracted for RT-PCR analysis. The results revealed that DENSpm had essentially the same effect on the SSAT-X amount in the nucleus as in cytoplasm and the mRNA reduction was already apparent within 7 h (Fig. 1C). A third PCR-primer set was used to detect exon X-containing mRNAs (Fig. 1C). These primers recognized not only SSAT-X but also a splicing intermediate containing unspliced intron 3 sequence before exon X. As DENSpm decreased the amount of properly spliced SSAT-X, it also increased the amount of the intermediate form in the nucleus, thus supporting the view that the analog modulates alternative splicing.
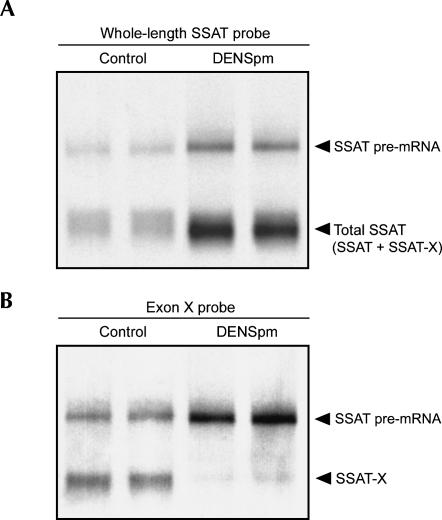
Next, the effect of DENSpm on hepatic SSAT-X mRNA abundance was studied in syngenic and MT-SSAT transgenic mice. As shown in Table 1, the analog accumulated efficiently in the livers of transgenic mice and strikingly induced SSAT activity. However, DENSpm (125 mg/kg i.p.) was poorly accumulated in the livers of syngenic mice and had only a minor effect on enzyme activity. Quantitative PCR analysis showed that the relative amount of SSAT-X mRNA was reduced by 90% in the livers of transgenic mice. The result was verified with Northern blot with probes targeted either to whole SSAT or exon X only. As shown in Figure 2A,B, DENSpm increased both SSAT pre-mRNA and total SSAT mRNA (containing both SSAT and SSAT-X mRNA) steady-state levels but dramatically decreased the amount of SSAT-X variant.
FIGURE 2.
Northern blot analysis of SSAT mRNA expression. MT-SSAT transgenic mice were given one injection of DENSpm (125 mg/kg i.p. in saline). The mice were sacrificed after 24 h and total RNA was extracted from liver. Northern blots were detected with (A) whole-length SSAT probe (exons 1–6) or (B) exon X-specific probe. Lanes represent individual samples. Note that the variants differ only by 110 bp in size and therefore appear as one band when detected with whole-length SSAT probe.
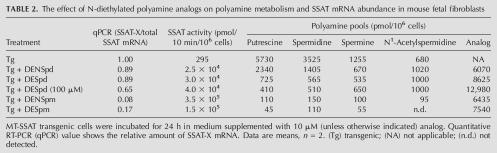
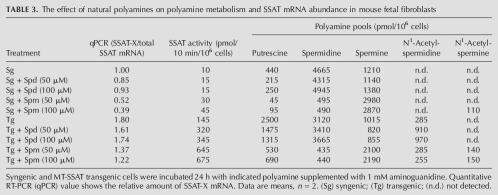
We then tested the effect of various N-diethylated polyamine analogs and natural polyamines on the alternative splicing of SSAT pre-mRNA in primary fetal fibroblasts derived from syngenic or MT-SSAT transgenic mice. The data shown in Table 2 revealed that all tested analogs readily accumulated in the cells within 24 h. Spermine analogs, DENSpm and DESpm, most effectively induced SSAT activity and decreased the relative amount of SSAT-X mRNA by ∼90% and 80%, respectively. Spermidine analogs had similar but less pronounced effect. As shown in Table 3, the natural polyamine, spermine (supplemented with 1 mM aminoguanidine to prevent degradation by serum amine oxidases), also modulated the alternative splicing both in syngenic and transgenic cells while it only slightly induced SSAT activity.
TABLE 2.
The effect of N-diethylated polyamine analogs on polyamine metabolism and SSAT mRNA abundance in mouse fetal fibroblasts
TABLE 3.
The effect of natural polyamines on polyamine metabolism and SSAT mRNA abundance in mouse fetal fibroblasts
SSAT-X is a target for nonsense-mediated mRNA decay
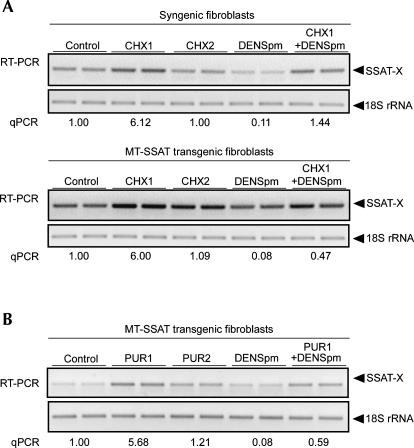
As exon X inclusion introduces premature termination codons, SSAT-X seems to be a perfect candidate for an mRNA surveillance system called NMD, a process requiring ongoing translation (Wagner and Lykke-Andersen 2002). To test whether SSAT-X was a target for NMD, we exposed syngenic or MT-SSAT transgenic fetal fibroblasts to two concentrations of cycloheximide (10 μg/mL and 50 ng/mL), of which only the higher concentration completely inhibits protein synthesis. As indicated in Figure 3A, the higher concentration of the drug for 6 h markedly enhanced the accumulation of the alternatively spliced transcript both in syngenic and transgenic cells while the noninhibitory concentration did not. Similar results were obtained with another inhibitor of protein synthesis, puromycin, at a concentration (100 μg/mL) inhibiting general protein synthesis (Fig. 3B).
FIGURE 3.
Stabilization of SSAT-X mRNA with protein synthesis inhibitors. (A) Syngenic or MT-SSAT transgenic fetal fibroblasts were exposed to cycloheximide (10 μg/mL [CHX1] or 50 ng/mL [CHX2]) for 6 h, pretreated with or without 10 μM DENSpm (added 24 h before CHX). (B) The cells were exposed to puromycin (100 μg/mL [PUR1] or 200 ng/mL [PUR2]) for 6 h with or without 10 μM DENSpm (added 24 h before PUR). Total RNA was extracted and analyzed with SSAT-X-specific primers (P3/P4). Lanes represent individual samples. The relative amount of SSAT-X (SSAT-X/total SSAT) was quantified with qPCR. n = 2, analyzed in triplicate.
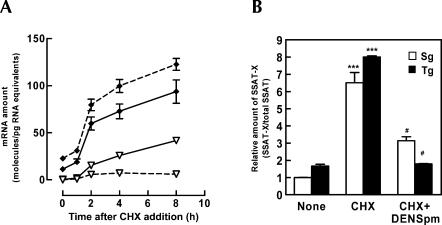
To investigate the effect of DENSpm on the synthesis rate of SSAT-X, we performed a time-course experiment with CHX and DENSpm in the MT-SSAT transgenic fibroblasts. The cells were grown with or without DENSpm for 24 h, after which CHX (10 μg/mL) supplemented with or without DENSpm were added. The relative amounts of SSAT-X and SSAT compared to 18S rRNA control were determined 1, 2, 4, and 8 h after the addition of CHX. We found that CHX treatment not only increased SSAT-X but also SSAT mRNA when compared to the zero time point reference values (eightfold after 8 h) (Fig. 4A). The result implies that the transcription of SSAT is under the control of labile repressor protein, as suggested earlier (Fogel-Petrovic et al. 1996). However, the accumulation of SSAT was marginal when compared to that of SSAT-X. As shown in Figure 4A, before the addition of CHX, the level of SSAT-X amounted to only 4% of that of the regular transcript, whereas at 8 h after the addition of drug, the variant transcript level was increased by 92-fold, to 45% of that of the regular transcript. In contrast, pretreatment of cells with DENSpm almost completely prevented CHX-mediated accumulation of SSAT-X. The results indicate that a significant part of SSAT pre-mRNA generates an unproductive splice variant, whereas DENSpm supplementation directs the splicing to a productive variant. In the same experiment transcription inhibitor actinomycin D was used to determine the half-lives of SSAT-X and SSAT mRNA. Overall, actinomycin D data showed that the half-life of SSAT-X mRNA was significantly lower (4 h) than that of the regular transcript (12 h) (P < 0.0032) (data not shown), thus supporting the view that SSAT-X is an NMD substrate.
FIGURE 4.
(A) Kinetics of SSAT and SSAT-X mRNA accumulation after inhibition of protein synthesis. MT-SSAT transgenic fetal fibroblasts were treated with CHX (10 μg/mL) and 10 μM DENSpm (added 24 h before CHX) (broken line) or with CHX only (solid line). The amount of SSAT (♦) and SSAT-X (▽) mRNA was quantified with qPCR. 18S rRNA was used as control. Values are means ± SD (n = 3, analyzed in duplicate). (B) The cells were exposed to CHX (10 μg/mL) for 8 h with or without 10 μM DENSpm (added 1 h after CHX). The relative amount of SSAT-X (SSAT-X/total SSAT) was quantified with qPCR. Values are means ± SD (n = 3, analyzed in duplicate). ***p < 0.001 compared to untreated control group, #p < 0.001 compared to CHX group.
In the previous experiments, DENSpm was added 24 h before the addition of protein synthesis inhibitors, because it has been found to elicit the maximal effect on SSAT activity and polyamine pools in 48 h. In the next experiment we tested how DENSpm affects alternative splicing after first blocking the NMD pathway and the general protein synthesis with CHX (10 μg/mL). As indicated in Figure 4B, 8-h CHX treatment significantly increased the amount of SSAT-X both in syngenic and transgenic cells, while addition of DENSpm 1 h after CHX markedly decreased the amount of SSAT-X within 7 h of incubation. This result clearly indicates that the effect of DENSpm on the relative ratio of SSAT-X to SSAT is not mediated by enhanced NMD activity but by alternative splicing of SSAT.
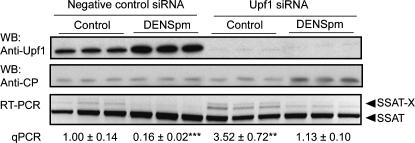
To gain further evidence that SSAT-X is a substrate for NMD, we used RNA interference to silence the expression of the Upf1 protein, which is an essential component of the NMD machinery (He et al. 1993). We used siRNA that was shown to be effective in the previous study (Kim et al. 2005b). As shown in Figure 5, silencing of Upf1 (>90% protein knockdown efficiency, normalized to cyclophilin protein level) significantly increased the relative amount of SSAT-X by 3.5-fold, and addition of DENSpm 24 h after Upf1 or negative control siRNA electroporation reduced SSAT-X accumulation.
FIGURE 5.
Stabilization of SSAT-X mRNA after siRNA-mediated silencing of Upf1 protein. MT-SSAT transgenic fetal fibroblasts were electroporated with negative control or Upf1 siRNA. Ten micromolar DENSpm was added after 24 h and the cells were harvested 72 h after electroporation. Silencing of Upf1 protein was detected from cell lysates by immunoblotting (WB) with anti-Upf1 antibody and anti-cyclophilin (CP) as a control. RT-PCR was carried out with primers P1 and P2. The relative amount of SSAT-X (SSAT-X/total SSAT) was quantified with qPCR. Values are means ± SD (n = 3, analyzed in triplicate). **p < 0.01, ***p < 0.001 compared to negative control group.
Effect of polyamine depletion on the alternative splicing of SSAT
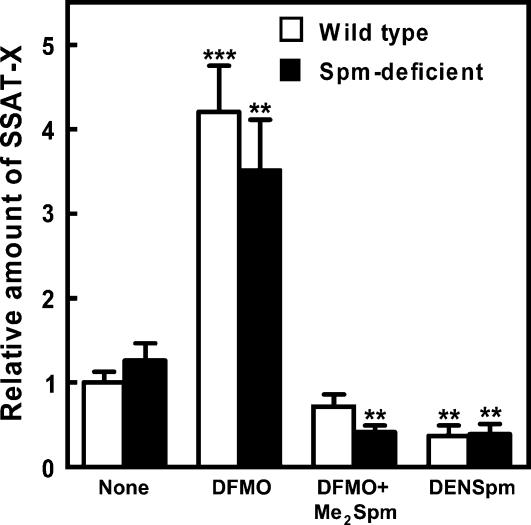
The previous experiments had indicated that spermine and its analogs most effectively prevented the exon inclusion. To further clarify the role of polyamines in the splicing of SSAT pre-mRNA, we used spermine synthase gene-disrupted mouse embryonic stem cells totally lacking spermine. As depicted in Figure 6, spermine deficiency slightly increased the relative amount of the splice variant as measured by quantitative PCR. To further deplete higher polyamines, 2-difluoromethylornithine (DFMO) was added. DFMO is an irreversible inhibitor of ornithine decarboxylase, the rate-controlling enzyme in the biosynthesis of putrescine and spermidine (Metcalf et al. 1978). As shown in Figure 6, accumulation of SSAT-X was enhanced by an exposure to DFMO both in wild-type and spermine-deficient cells. After the treatment with DFMO, the wild-type cells contained only spermine, while the mutant cells contained the same amount of spermidine, but no spermine (data not shown). This result can be understood in terms that a depletion of either of the higher polyamines increases the amount of the alternative splice variant. In fact, we found a close (r 2 = 0.75) and highly significant (p < 0.001) inverse correlation between the amount of SSAT-X transcript and the total amount of spermidine and spermine in cells. As also indicated in Figure 6, an inclusion of polyamine analog, bis-α,ω-methylspermine (Me2Spm) together with DFMO prevented the production of the alternative transcript as likewise did the exposure to DENSpm. Me2Spm is a metabolically stable analog of spermine being capable to fulfill most of the putative functions of natural spermine. Moreover, it is resistant toward serum amine oxidase and thus may be used without aminoguanidine supplement (Lakanen et al. 1992; Yang et al. 1995; Järvinen et al. 2005).
FIGURE 6.
Polyamine depletion increases the relative amount of SSAT-X variant. Wild-type or spermine-deficient embryonic stem cells were treated with 20 μM DENSpm or 1 mM DFMO supplemented with or without 100 μM Me2Spm for 48 h. Quantitative PCR data for SSAT-X expression (means ± SD, n = 3, analyzed in triplicate) are presented as relative to the amount of total SSAT mRNA. **p < 0.01, ***p < 0.001 compared to untreated control group.
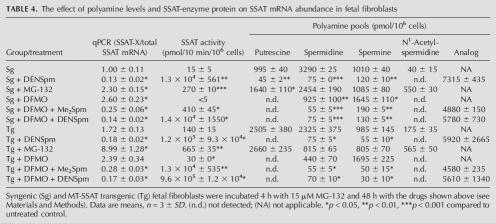
Several proteins have been shown to autoregulate their levels by modulating the alternative splicing of their pre-mRNAs to generate transcripts that are directed to NMD when there is no need for functional protein (Lewis et al. 2003; Wollerton et al. 2004). To investigate the possibility that SSAT protein has a similar autoregulatory loop, we treated syngenic and MT-SSAT transgenic cells with DFMO or MG-132. Both compounds decrease polyamine pools, but have an opposite effect on SSAT activity. DFMO is a specific inhibitor for ODC, having no direct interaction with SSAT, whereas MG-132 inhibits proteasomal degradation of SSAT and thus increases the amount of SSAT enzyme protein. As shown in Table 4, both DFMO and MG-132 increased the relative amount of SSAT-X mRNA, while the drugs had opposite effects on SSAT activity. It was shown earlier that the SSAT enzyme activity correlates directly with protein amount (McCloskey et al. 1999). The data demonstrate that the SSAT protein level does not correlate with the relative abundance of the alternative transcripts.
TABLE 4.
The effect of polyamine levels and SSAT-enzyme protein on SSAT mRNA abundance in fetal fibroblasts
Effect of polyamine level on splicing of other pre-mRNAs
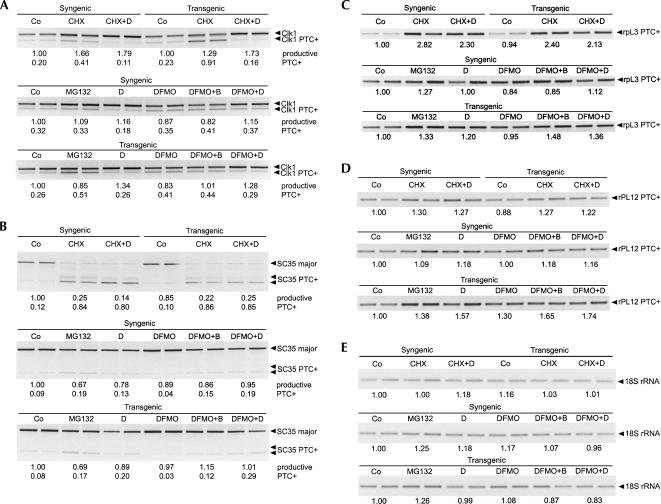
To address how specific the effect of polyamines is on splicing of SSAT, we tested if changes in intracellular polyamine level result in altered ratios of other PTC-containing to non-PTC-containing splice variants. As SSAT-X is induced upon various stress stimuli, it could imply a more general or stress-related effect and not specific regulation. Overall, seven different mouse genes were tested for splice variant expression, but only four of them showed PTC-containing alternative splice variants with the primers used. The genes that were reported to be alternatively spliced in humans, but that were found to have no additional splice variants in mouse fetal fibroblasts as assessed by our primer sets, were activating transcription factor 3 (ATF3), multidrug resistance associated protein 4 (ABCC4), and polypyrimidine tract binding protein 1 (PTB1). The NMD-targeted variants of ribosomal proteins L3 and L12 were found to be expressed at low levels, and therefore PTC-containing variant-specific primers were used. As depicted in Figure 7, RT-PCR analysis showed that CHX treatment increased the amount of all NMD-targeted splice variants, as expected. Changes in polyamine levels by various compounds had some effect on the splicing patterns of SC35, rpL12, and rpL3, but the changes did not correlate with the intracellular polyamine levels. As shown in Figure 7D, NMD-targeted variant of rpL12 was induced by all treatments. Similar induction was seen in rpL3, with the exception of DFMO (Fig. 7C). In the case of SC35, the unproductive variant was increased both by polyamine supplementation (DENSpm, Me2Spm) and depletion (MG-132), while polyamine depletion by DFMO had the opposing effect (Fig. 7B). As shown in Figure 7A, the splicing of Clk1 was found to somewhat correlate with polyamine pools, thus suggesting that polyamines might also regulate genes other than SSAT. Overall the results imply that the alterations in the SSAT-X/SSAT ratio produced by changes in polyamine levels by various compounds are not due to stress.
FIGURE 7.
Expression of PTC-containing splice variants in syngenic and MT-SSAT transgenic mouse fetal fibroblasts. (A) Cdc2-like kinase 1 (Clk1), (B) SC35, (C) rpL3, (D) rpL12, (E) 18S rRNA. RT-PCR shows both productive and PTC-containing unproductive variant of Clk1 and SC35. For rpL3 and rpL12, unproductive variant-specific primers were used. cDNA samples from previous experiments (Fig. 4B, Table 4) were used for RT-PCR, and the relative amounts were analyzed by densitometry with ImageQuant software. Values were normalized to 18S rRNA control values. D = DENSpm; B = Me2Spm.
Semliki Forest virus infection and adenovirus transduction induce the alternative splicing of SSAT
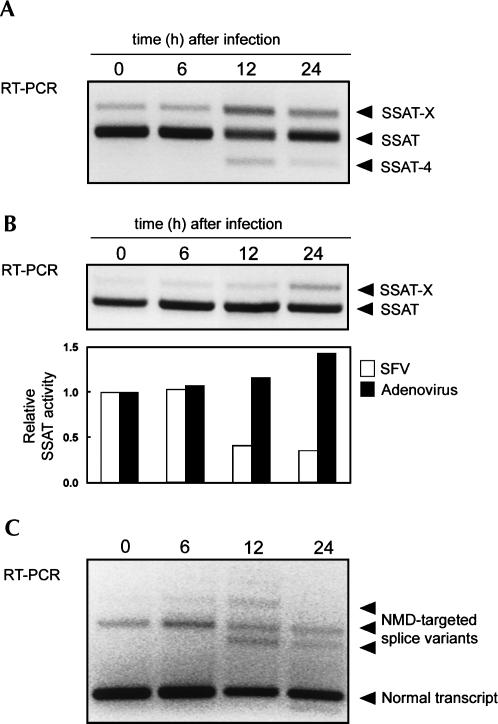
The findings of Nikiforova et al. (2002) indicating that certain RNA viruses induce SSAT-X formation prompted us to examine whether the induction of SSAT-X during virus infection might influence SSAT activity and polyamine pools. The modulation of alternative splicing might be beneficial for virus replication, as polyamines have been shown to influence virus replication (Raina et al. 1981). As shown in Figure 8A, infection of HEK293 cells with Semliki Forest virus (SFV) resulted, in addition to accumulation of the SSAT-X variant, in an appearance of another splice variant, which had skipped exon 4. However, we could not detect any smaller SSAT-like proteins with our SSAT antibodies (data not shown). Cells infected with SFV showed decreased SSAT activity (Fig. 8B, lower panel) and fluctuations in polyamine concentrations (data not shown). At earlier time points spermidine and spermine concentrations decreased, but by 24 h both had increased almost twofold (data not shown).
FIGURE 8.
Infection of HEK293 cells with Semliki Forest virus (SFV) and adenovirus results in induction of SSAT-X variant. (A) RT-PCR showing SSAT splice variants after infection with SFV. (B) Upper panel represents RT-PCR from cells transduced with adenovirus. Lower panel represents relative SSAT activity from SFV-infected and adenovirus-transduced cells. Values are means (n = 2). (C) RT-PCR of ABCC4 splice variants during SFV infection. Normal transcript and NMD-targeted splice variants are shown with arrows. All lanes represent individual samples.
In contrast, cell transduction with slowly replicating adenovirus did not induce accumulation of exon 4-skipped transcript despite accumulation of SSAT-X (Fig. 8B, upper panel). In addition, adenovirus transduction slightly increased SSAT activity. Changes in polyamine concentrations were similar to that of SFV infection, first decreased and then slightly increased after 24 h (data not shown). To determine whether the effect of virus infection on mRNA accumulation was SSAT specific, we examined the expression of ABCC4 splice variants in the course of SFV infection. ABCC4 has been documented to have at least four splice variants in human cells, produced by inclusion of additional exons 1a, 1b, none, or both (Lamba et al. 2003). Using RT-PCR we found that the NMD-targeted transcripts of ABCC4 accumulated during SFV infection (Fig. 8C), thus suggesting that SFV infection also up-regulates nonsense transcripts other than SSAT-X.
DISCUSSION
Previous studies have described an induction of the alternative splice variant of SSAT in various cellular stress conditions, such as Venezuelan equine encephalitis and tick-borne encephalitis virus infection (Nikiforova et al. 2002), X-ray irradiation (Mita et al. 2004), iron deficiency, or hypoxia. The latter study appears to indicate that cells stably overexpressing cDNA clone of the variant transcript are protected from apoptosis under iron-deficient conditions (Kim et al. 2005a). Polyamines have been associated to many cellular functions, and proper maintaining of intracellular polyamine pools is vital for cells. Therefore the appearance of SSAT-X variant by various events causing cellular stress prompted us to study the physiological role for the variant expression.
Our results revealed that the variant transcript is a target for the mRNA degradation system known as NMD, where a premature termination codon triggers an ongoing translation-dependent mRNA degradation (Wagner and Lykke-Andersen 2002). As reported earlier, the degradation of transcripts with premature termination codons by the NMD system is effectively prevented by inhibitors of protein synthesis (Carter et al. 1995). Interestingly, a previous report indicates that inhibition of protein synthesis greatly enhances the accumulation of SSAT-specific mRNA (up to 15-fold in the MALME-3M cell line) as judged by Northern blotting analyses (Fogel-Petrovic et al. 1996). This can be understood in terms that transcription of SSAT is under the control of the labile repressor protein, as suggested earlier by Fogel-Petrovic and colleagues. The repressor protein was recently characterized as IκB (Choi et al. 2006). As shown by our present results, cycloheximide and puromycin caused a massive accumulation of the alternative variant but clearly smaller increase of the regular transcript (Fig. 4A). In addition, our Upf1-knockdown experiment indicated that the variant is a substrate for NMD. Our result is supported by a microarray study, where SSAT message was induced over fourfold after siRNA-mediated down-regulation of Upf1 in mammalian cells (Mendell et al. 2004). For currently unknown reasons, knockdown of Upf1 also slightly increased SSAT mRNA. One possibility is that the accumulation of SSAT-X may stabilize SSAT mRNA when the NMD pathway is inhibited. On the other hand, SSAT enzyme is known to be induced in various conditions of cellular stress, and NMD blockade with subsequent accumulation of premature termination codon-containing transcripts could be one of those factors.
The recent report indicating that certain RNA viruses induce SSAT-X transcript (Nikiforova et al. 2002) fits nicely into the general picture of NMD, as viruses have been shown to shut down the endogenous protein synthesis in the host cell, leading to the stabilization of transcripts targeted to the NMD pathway (Bushell and Sarnow 2002). Indeed, we found that, in addition to SSAT-X, NMD-targeted transcripts of ABCC4 gene were as well stabilized during SFV infection of HEK293 cells, thus indicating that at least SFV infection does not specifically lead to enrichment of SSAT alternative variants. A unique finding was the appearance of transcript that had skipped exon 4. According to our sequence analysis, the exon skipping should not change the reading frame, and the transcript should therefore produce smaller (∼16 kDa) SSAT-like protein under the conditions of SFV infection. Since we did not detect any additional proteins with our SSAT antibodies (results not shown), it is likely that during virus infection host proteins are not translated efficiently, which leads to accumulation of NMD-targeted transcripts. In addition, some of the proteins involved in splicing of RNA may be down-regulated during virus infection, causing an appearance of various splice variants.
A recent report describes detection of SSAT-X-derived peptide from cells stably expressing SSAT-X cDNA (Kim et al. 2005a). Although we did not detect the presence of SSAT-X-derived peptide, there are some examples of NMD-targeted transcripts that can, under some conditions, escape NMD and produce a protein (Danckwardt et al. 2002; Dreumont et al. 2005). If SSAT-X mRNA was not degraded, it should generate a truncated peptide containing only the first 71 N-terminal amino acids. As active SSAT is an oligomeric protein (Libby et al. 1991), this peptide might be able to bind to the enzyme as a defective subunit and inhibit SSAT activity in a dominant-negative fashion. However, transfection of truncated SSAT-X (containing exons 1–3 and the first 26 bp of exon X, and thus not NMD substrate) did not have any effect on SSAT activity or half-life despite producing the predicted peptide. In addition, our experiments with recombinant or synthetic peptide did not reveal any affinity between native SSAT and the 8.3-kDa peptide, at least as judged by activity measurements (results not shown). We believe that Kim and colleagues managed to detect the peptide for two main reasons. First, they used cDNA construct, which is not targeted to NMD. Second, they also used proteasomal inhibitor, MG-132, which prevented the degradation of proteins. Interestingly, a vast number of protein isoforms deposited in SWISS-PROT have been shown to derive from premature termination codon-containing mRNAs (Hillman et al. 2004).
Remarkably, our present results establish that the polyamines and some of their analogs modulate the alternative splicing of SSAT pre-mRNA both in vitro and in vivo. The data presented here show that addition of DENSpm most effectively inhibited the exon inclusion, whereas depletion of the higher polyamines, either by DFMO or MG-132 treatment or by the use of spermine-deficient cells, enhanced the alternative splicing (Fig. 6, Table 4). Our results suggest that the alternative splicing of SSAT is not mediated by the SSAT protein itself but rather by the polyamine level.
The possibility was considered that polyamines and their analogs might modulate NMD activity and have no effect on the splicing reaction. In fact, Figure 5 shows an induction of Upf1 protein in response to 48-h DENSpm treatment in MT-SSAT transgenic cells. However, Figure 4B clearly shows that addition of DENSpm after blocking the NMD with CHX effectively reduced the amount of SSAT-X already within 7 h. Essentially the same result was obtained in a Upf1 knockdown experiment (Fig. 5), indicating that DENSpm affects SSAT pre-mRNA splicing. However, the possibility that DENSpm also modulates NMD activity is not excluded. In addition, the effect of DENSpm on the alternative splicing of other pre-mRNAs is yet to be discovered.
Several genes have recently been shown to generate alternative splice variants that are differentially subjected to NMD (Green et al. 2003; Lewis et al. 2003). For example, the human SR-like protein TRA2-BETA autoregulates its level by inducing inclusion of PTC-containing additional exon when the protein level is increased (Stoilov et al. 2004), whereas PTB promotes skipping of exon 11 (Wollerton et al. 2004). A similar example is TIAR protein regulation, which is not autoregulatory, but involves related TIA-1 protein (Le Guiner et al. 2003). The process, which has been termed regulated unproductive splicing and translation (RUST), fits nicely into the general picture of gene regulation, allowing controlling in a developmental stage- and cell-specific manner (Green et al. 2003; Lewis et al. 2003). On the other hand, a recent study argues against a widespread regulatory role of alternative splicing-coupled NMD by showing that the majority of PTC-containing splice variants are present at uniformly low levels in mammalian cells, independent of the action of NMD (Pan et al. 2006). The study implies that the proportion of genes generating NMD-targeted splice variants that actually are regulated by NMD appears to be relatively small. However, there is an increasing amount of experimental data proving that the negative feedback loop has an important regulatory role in the expression of several genes.
The alternative splicing may thus represent a novel regulatory system of SSAT expression where the processing of pre-mRNA is directed to generate unproductive transcripts under conditions in which there is no need to produce a functional protein. An example of such a condition is transgene-derived massive transcription with no apparent need for functional SSAT, especially as the pools of the higher polyamines are reduced due to SSAT overexpression (Table 1). As indicated in the present results, the relative amount of the alternatively spliced transcript was markedly increased in tissues of transgenic animals in comparison with their wild-type littermates. The steady-state level of SSAT-X may look small in comparison with the regular transcript, but one should take into consideration the fact that the NMD system extremely effectively degrades the unproductive transcripts, as indicated by the rapid accumulation of SSAT-X in the absence of protein synthesis (Figs. 3, 4). Thus, under certain conditions, a major part of the SSAT transcripts may result from unproductive splicing and do not give rise to functional enzyme.
In addition to SSAT, we examined the alternative splicing of a set of genes known to also generate PTC-containing transcripts. Among the genes analyzed by RT-PCR we observed polyamine level-correlating changes in the alternative splicing of Clk1. Clk1 is a kinase that modulates the phosphorylation of SR proteins involved in the regulation of alternative splicing (Duncan et al. 1997). Our result might indicate that polyamines also regulate alternative splicing of genes other than SSAT. However, that does not exclude the RUST-type regulation of SSAT. We used several different cell lines and drugs for manipulation of intracellular polyamine levels, and similar alterations in SSAT-X/SSAT ratio were observed regardless of the method we used. Therefore, it is unlikely that the observed changes were related to the general stress reaction. Polyamines are known to modulate the transcription of several genes, which harbor the polyamine-responsive element (PRE) in their promoter regions, by inducing the expression of polyamine-modulated factor-1, which then binds to PRE in conjunction with Nrf-2 (Wang et al. 1998). Other examples of highly specific interactions between polyamines and polynucleotides apparently are the ribosomal frameshifting induced by the polyamines in decoding ODC antizyme and translational regulation of S-adenosylmethionine decarboxylase mediated by 5′ and 3′ uORFs (Rom and Kahana 1994; Hanfrey et al. 2005). Therefore, it would not be surprising if there were other genes whose splicing is regulated by polyamines. However, further studies are needed to elucidate the exact mechanism of polyamines’ action in alternative splicing.
Due to the complex nature of regulation of SSAT expression, it has been very difficult to elucidate the crucial element in this puzzle. It was shown earlier that polyamines and their analogs positively regulate SSAT gene at the level of mRNA (Fogel-Petrovic et al. 1993; Shappell et al. 1993). Alkylated polyamine analogs powerfully stabilize both the mRNA and enzyme protein resulting in superinduction of SSAT activity (Libby et al. 1989). However, under physiological conditions where only natural polyamines are present, enzyme stabilization does not necessarily play an important role in the regulation of SSAT. As shown in Table 3, natural polyamines spermidine and spermine increased SSAT activity in MT-SSAT transgenic cells only 2.4- and 4.7-fold, respectively, while DENSpm produced a dramatic 85-fold induction (Table 2). Thus, RUST might have a very important role in controlling SSAT activity under physiological conditions.
Taken together, the present results distinctly indicate that polyamines and their analogs modulate the splicing of SSAT pre-mRNA and that SSAT-X transcript is degraded by nonsense-mediated mRNA decay. On the basis of the results described above, we propose that polyamine-regulated unproductive splicing and translation of SSAT represents a novel mechanism to fine-tune the regulation of polyamine metabolism.
MATERIALS AND METHODS
Chemicals and antibodies
N1,N7-Diethylnorspermidine (DENSpd), N1,N8-diethylspermidine (DESpd), N1,N11-diethylnorspermine (DENSpm), and N1,N12-diethylspermine (DESpm) were synthesized essentially as described by Rehse et al. (1990). Bis-α,ω-methylspermine (Me2Spm) was synthesized as described by Grigorenko et al. (2005) and Järvinen et al. (2006). Ornithine decarboxylase inhibitor 2-difluoromethyl-ornithine (DFMO) was from ILEX Oncology Inc. Anti-Upf1 was obtained from Bethyl Laboratories and anti-cyclophilin A from Upstate. Anti-SSAT antibodies against recombinant full-length SSAT 1–171 and recombinant SSAT-X 1–71 or against synthetic peptides ([N-terminal mixture of 1–25 and 26–71]; [C-terminal four overlapping 20 amino acid peptides from exon 6]) were generated in rabbits. All other reagents were from Sigma Aldrich or Merck.
Animals
Transgenic mice from line UKU181 overexpressing mouse genomic SSAT under the control of mouse metallothionein I promoter (Suppola et al. 1999) or their syngenic littermates were used in the experiments. Therefore, all the RT-PCR and qPCR primers described below recognize both the endogenous and transgene-derived mRNAs. Mice received one injection of DENSpm (125 mg/kg i.p. in saline). Mice were sacrificed after 24 h and the tissues used for analyses were frozen in liquid nitrogen. The animals were housed in a 12-h-light/dark-cycle facility with free access to food and water. The Institutional Animal Care and Use Committee of the University of Kuopio and the Provincial Government approved the animal experiments.
Cloning of SSAT/SSAT-X cDNAs and construction of plasmids
The SSAT and SSAT-X cDNAs containing exons 1–6 and 1–6 including X, respectively, were amplified from the pool of first strand cDNA (see RT-PCR in Materials and Methods) using primers 5′-TACGTCGACACGAATGAGGAACCACC-3′ and 5′-CTAGCGGCCGCAGGTTGTCATTGTCTAC-3′. The resultant PCR products were gel-purified, digested with SalI and NotI (underlined sequences in primers) and cloned into the vector pSTEC-1 (a gift from Dr. Curt D. Sigmund, The University of Iowa) in the same restriction sites. Plasmids for transfection were made as follows: the cDNAs for SSAT, SSAT-X, and for the truncated SSAT-X (containing exons 1–3 plus the first 26 nt from exon X) were PCR amplified from the SSAT and SSAT-X cDNA carrying plasmids described above using primers 5′-TACGTCGACATGGCTAAATTTAAGATCCG-3′ and 5′-CTAGCGGCCGCAGGTTGTCATTGTCTAC-3′ (SSAT/ SSAT-X) and 5′-TACGTCGACATGGCTAAATTTAAGATCCG-3′ and 5′-CTAGCGGCCGCTGTACATGGCGAAGCTAG-3′ (truncated SSAT-X). The PCR products were purified by the QIA-quick PCR purification kit (Qiagen), restriction digested with SalI and NotI and cloned into the vector CMV/myc/cyto (Invitrogen) cut with the same enzymes. The structures of all constructed plasmids were confirmed by automated sequencing. Plasmid DNAs were prepared using QIAFilter Maxi kit (Qiagen) following the manufacturer's protocol.
Cell cultures
Syngenic and MT-SSAT transgenic primary fetal fibroblasts were isolated and cultured as described previously (Alhonen et al. 1998), except that the fetuses were taken on day 16 of pregnancy. The cells were seeded at a density of 2 × 106 cells/100 mm tissue culture dish and incubated for 24 h before treatments. Spermidine and spermine were used at 50/100 μM (supplemented with 1 mM aminoguanidine to prevent their oxidation by serum amine oxidase) for 24 h. Bis-α,ω-methylspermine was used at 100 μM (supplemented with 1 or 5 mM DFMO to facilitate the uptake and to remove natural polyamines). After treatments, cells were harvested by trypsinization and counted using a Coulter model ZM electronic cell counter (Coulter Electronics). Mouse embryonic stem cells with targeted disruption of spermine synthase gene were generated and cultured as described by Korhonen et al. (2001).
Inhibitors of protein synthesis
The cells were treated with protein synthesis inhibitors cycloheximide (10 μg/mL or 50 ng/mL) or puromycin (100 μg/mL or 200 ng/mL) with or without 10 μM DENSpm. For half-life measurements, the cells were incubated up to 8 h with transcription inhibitor, Actinomycin D (5 μg/mL).
RNA interference
Small interfering RNAs targeted to mouse Upf1 (sense 5′-GAUGCAGUUCCGUUCCAUCtt-3′, antisense 5′-GAUGGAACGGAACUGCAUCtt-3′) (Kim et al. 2005b) or negative control (cat. no. 4611) were chemically synthesized at Ambion. MT-SSAT transgenic primary fetal fibroblasts were electroporated using ECM 830 (BTX) in electroporation buffer (Ambion) according to the manufacturer's instructions, plated on 6-well plates (225,000 cells/well), and harvested after 72 h. DENSpm (10 μM) was added 24 h after electroporation.
Western blotting
Cells were lysed in a buffer containing 25 mM Tris (pH 7.4), 0.1 mM EDTA, 0.1% Triton-X-100, 1 mM dithiotreitol, and 1× Complete EDTA-Free (Roche). After centrifugation (15 min 10 000g, +4°C) the supernatant was retained. Western blot was performed with standard protocols and anti-SSAT (see Materials and Methods) and anti-Upf1 antibodies. Anti-cyclophilin A antibody was used as loading control.
RT-PCR
Total RNA was extracted with TRIzol Reagent (Invitrogen) and treated with Dnase I (Dna-free, Ambion) according to the manufacturer's instructions. Cytoplasmic RNA was purified with RNeasy as described by the manufacturer (Qiagen). One microgram of Dnase-treated RNA was used for first-strand cDNA synthesis using oligo-dT primers and AMV reverse transcriptase (Promega) in a total volume of 25 μL. A 2-μL aliquot of the first-strand cDNA was used for PCR in a mixture containing 2.5 μL 10× buffer with MgCl2, 0.5 μL dNTP mix (10 mM each), 25 pmol forward and reverse primers, and 1 U DyNAzyme DNA polymerase (Finnzymes) in a total volume of 25 μL. The following primers were used: P1 (exon 1) 5′-AGCCACTGCCTCTGACTG-3′, P2 (exon 6) 5′-CTGCCTCCAAACCACATAC-3′, P3 (exon 1) 5′-TGACATCCTGCGACTGAT-3′, P4 (exon 3/X junction) 5′-CGAAGCTAGAGACTGTAACCTTC-3′, and P5 (exon X) 5′-ATGGCGAAGCTAGAGACTGT-3′. PCR products were isolated from 2% agarose gel and purified with QIAEX II according to the manufacturer's instructions (Qiagen) and sequenced using Thermo Sequenase CY5 Dye Terminator Kit and A.L.F.express DNA Sequencer (Amersham Biosciences). Expression of human ABCC4 splice variants was examined as described (Lamba et al. 2003). Expression of splice variants of several mouse genes was examined with the following primers: activating transcription factor 3 (ATF3) forward 5′-ATGATGCTTCAACATCCAG-3′, reverse 5′-GTGGAAAAGGAGGATTCAG-3′; ribosomal protein L3 (rpL3) forward 5′-GGGCATTGTGGGATATGT-3′, reverse 5′-CCTCAGGAGCAGAGCACA-3′; ribosomal protein (rpL12) forward 5′-GAGGTCAAAGTCGGTGC-3′, reverse 5′-AGACCCAGAGGACCGAT-3′; multidrug resistance associated protein 4 (ABCC4) forward 5′-GAGATGCTGCCGGTGCAC-3′, reverse 5′-CCTTTGAAGCTCCTCTCCGA-3′; SC35 forward 5′-CCAAGTCTCCAGAAGAAGAG-3′, reverse 5′-TAGATGTGCTCACTGTATGCT-3′; polypyrimidine tract binding protein 1 (PTB1) forward 5′-TCTCTGTCCCTAATGTCCAT-3′, reverse 5′-GCGTTCTCCTTCTTATTGAA-3′; and cdc2-like kinase 1 (Clk1) forward 5′-TCAAAGAGAACTTACTGTCCTGAC-3′, reverse 5′-GGTCTGTTGTATTCAAGTGTTCC-3′.
Quantitative RT-PCR
Quantitative RT-PCRs were performed using 6 ng (RNA equivalents) of cDNA as template, and gene specific Assay-by-Design (AbD) probe and primer sets and TaqMan Universal PCR Master Mix with or without AmpErase UNG from Applied Biosystems in TaqMan 7700 (Applied Bio-systems). Running conditions were 2 min at 50°C, 10 min at 95°C, and 40 cycles of 15 sec at 95°C and 1 min at 60°C. The total amount of SSAT mRNA was quantified using an AbD targeted to the junction of exons 4 and 5 (probe (antisense), 5′-CAAAGCCTCTGTAATCAC-3′; forward primer, 5′-TGGATTGGCAAGTTGCTGTATCTT-3′; reverse primer, 5′-GCAACCTGGCTTAGATTCTTCAAAA-3′). The amount of the intronless, exon X-containing SSAT mRNA was quantified using an AbD targeted to the junction of exons 3 and X (probe (sense), 5′-CCCTGAAGGTTACAGTCTC-3′; forward primer, 5′-TGGTTGCAGAAGTGCCTAAAGAG-3′; reverse primer, 5′-CGCCCATCCATGTACACAGAAG-3′). Plasmids containing SSAT and SSAT-X were mixed 1:1 and the resulting mixture was serially diluted to produce standard curves. Data for SSAT-X mRNA are normalized to total SSAT mRNA and presented as relative to the means of untreated groups, unless otherwise indicated. The total SSAT mRNA was chosen for normalization, because it shows the balance between SSAT-X and SSAT mRNA. 18S rRNA was used as control in experiments where SSAT-X and SSAT mRNA amount was shown separately
Northern blot hybridization
Fifteen micrograms of total RNA were electrophoresed in 1.2% agarose gel under denaturing conditions, transferred to positively charged nylon membrane (Roche), and hybridized to digoxigenin-labeled (Roche) single-stranded whole-length SSAT (exons 1–6) or exon X-specific cDNA probe and detected by chemiluminescence.
SSAT activity and polyamine concentrations
Polyamines and their acetylated derivatives were determined using HPLC as described by Hyvönen et al. (1992). Ethylated analogs were measured as described by Kabra et al. (1986). SSAT activity was assayed using a published method (Bernacki et al. 1992).
Semliki Forest virus infection and adenovirus transduction
Human epithelial kidney cell line 293 (ATCC CRL-1573) was used. For viral transductions 4 × 106 cells were plated onto 100-mm plates 24 h prior to transduction. Adenoviral vector Ad5 CMV contains no transgene under CMV promoter. Ad5 CMV was propagated on 293 cells and purified with double CsCl gradients using standard methods and titrated for the amount of viral particles with a spectrophotometer. Functional titer (PFU, 1.35 × 1010) was determined with plaque assay with an overnight infection in 293 cells. An attenuated strain of Semliki Forest virus SFV A7 (74) was produced and titrated in BHK cells as previously described (Tuittila et al. 2000). The titer of the virus stock was 1.2 × 109 plaque forming units /mL. Prior to viral transductions, medium was removed from the plates and viruses were applied onto the plates in appropriate medium containing 10% FCS for SFV and 2% FCS for Ad5 CMV. All transductions were carried out with multiplicity of infection 1.
Statistical analyses
Data are expressed as means ± SD where applicable. Student's t-test, one-way ANOVA and linear regression analysis were used with the aid of a software package, GraphPad Prism 4.03 (GraphPad Software).
ACKNOWLEDGMENTS
We thank Anne Karppinen, Tuula Reponen, and Sisko Juutinen for their skilful technical assistance. This work was supported by grants from the Academy of Finland.
Footnotes
Article published online ahead of print. Article and publication date are at http://www.rnajournal.org/cgi/doi/10.1261/rna.39806.
Abbreviations: SSAT, spermidine/spermine N1-acetyltransferase; MT, metallothionein; NMD, nonsense-mediated mRNA decay; DENSpd, N1,N7-diethylnorspermidine; DESpd, N1N8-diethylspermidine; DENSpm, N1N11-diethylnorspermine; DESpm, N1,N12-diethylspermine; DFMO, 2-difluoromethylornithine; Me2Spm, Bis-α,ω-methylspermine; SFV, Semliki Forest virus; CHX, cycloheximide; PUR, puromycin; Upf1, Up-frameshift protein 1; ABCC4, multidrug resistance associated protein 4; RUST, regulated unproductive splicing and translation; PTC, premature termination codon.
REFERENCES
- Alhonen L., Karppinen A., Uusi-Oukari M., Vujcic S., Korhonen V.P., Halmekytö M., Kramer D.L., Hines R., Jänne J., Porter C.W. Correlation of polyamine and growth responses to N1,N11-diethylnorspermine in primary fetal fibroblasts derived from transgenic mice overexpressing spermidine/spermine N1-acetyltransferase. J. Biol. Chem. 1998;273:1964–1969. doi: 10.1074/jbc.273.4.1964. [DOI] [PubMed] [Google Scholar]
- Bernacki R.J., Bergeron R.J., Porter C.W. Antitumor activity of N, N'-bis(ethyl)spermine homologues against human MALME-3 melanoma xenografts. Cancer Res. 1992;52:2424–2430. [PubMed] [Google Scholar]
- Bushell M., Sarnow P. Hijacking the translation apparatus by RNA viruses. J. Cell Biol. 2002;158:395–399. doi: 10.1083/jcb.200205044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter M.S., Doskow J., Morris P., Li S., Nhim R.P., Sandstedt S., Wilkinson M.F. A regulatory mechanism that detects premature nonsense codons in T-cell receptor transcripts in vivo is reversed by protein synthesis inhibitors in vitro. J. Biol. Chem. 1995;270:28995–29003. doi: 10.1074/jbc.270.48.28995. [DOI] [PubMed] [Google Scholar]
- Choi W., Proctor L., Xia Q., Feng Y., Gerner E.W., Chiao P.J., Hamilton S.R., Zhang W. Inactivation of IκB contributes to transcriptional activation of spermidine/spermine N(1)-acetyltransferase. Mol. Carcinog. 2006 doi: 10.1002/mc.20239. in press. [DOI] [PubMed] [Google Scholar]
- Coleman C.S., Pegg A.E. Polyamine analogues inhibit the ubiquitination of spermidine/spermine N1-acetyltransferase and prevent its targeting to the proteasome for degradation. Biochem. J. 2001;358:137–145. doi: 10.1042/0264-6021:3580137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danckwardt S., Neu-Yilik G., Thermann R., Frede U., Hentze M.W., Kulozik A.E. Abnormally spliced β-globin mRNAs: A single point mutation generates transcripts sensitive and insensitive to nonsense-mediated mRNA decay. Blood. 2002;99:1811–1816. doi: 10.1182/blood.v99.5.1811. [DOI] [PubMed] [Google Scholar]
- Dreumont N., Maresca A., Boisclair-Lachance J.F., Bergeron A., Tanguay R.M. A minor alternative transcript of the fumarylacetoacetate hydrolase gene produces a protein despite being likely subjected to nonsense-mediated mRNA decay. BMC Mol. Biol. 2005;6 doi: 10.1186/1471-2199-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan P.I., Stojdl D.F., Marius R.M., Bell J.C. In vivo regulation of alternative pre-mRNA splicing by the Clk1 protein kinase. Mol. Cell. Biol. 1997;17:5996–6001. doi: 10.1128/mcb.17.10.5996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogel-Petrovic M., Shappell N.W., Bergeron R.J., Porter C.W. Polyamine and polyamine analog regulation of spermidine/spermine N1-acetyltransferase in MALME-3M human melanoma cells. J. Biol. Chem. 1993;268:19118–19125. [PubMed] [Google Scholar]
- Fogel-Petrovic M., Vujcic S., Brown P.J., Haddox M.K., Porter C.W. Effects of polyamines, polyamine analogs, and inhibitors of protein synthesis on spermidine-spermine N1-acetyltransferase gene expression. Biochemistry. 1996;35:14436–14444. doi: 10.1021/bi9612273. [DOI] [PubMed] [Google Scholar]
- Green R.E., Lewis B.P., Hillman R.T., Blanchette M., Lareau L.F., Garnett A.T., Rio D.C., Brenner S.E. Widespread predicted nonsense-mediated mRNA decay of alternatively-spliced transcripts of human normal and disease genes. Bioinformatics. 2003;19(Suppl 1):i118–i121. doi: 10.1093/bioinformatics/btg1015. [DOI] [PubMed] [Google Scholar]
- Grigorenko N.A., Vepsäläinen J., Järvinen A., Keinänen T.A., Alhonen L., Jänne J., Khomutov A.R. New syntheses of α-methyl- and α, α′-dimethylspermine. Bioorg. Khim. 2005;31:200–205. doi: 10.1007/s11171-005-0025-5. [DOI] [PubMed] [Google Scholar]
- Hanfrey C., Elliott K.A., Franceschetti M., Mayer M.J., Illingworth C., Michael A.J. A dual upstream open reading frame-based autoregulatory circuit controlling polyamine-responsive translation. J. Biol. Chem. 2005;280:39229–39237. doi: 10.1074/jbc.M509340200. [DOI] [PubMed] [Google Scholar]
- He F., Peltz S.W., Donahue J.L., Rosbash M., Jacobson A. Stabilization and ribosome association of unspliced pre-mRNAs in a yeast upf1-mutant. Proc. Natl. Acad. Sci. 1993;90:7034–7038. doi: 10.1073/pnas.90.15.7034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillman R.T., Green R.E., Brenner S.E. An unappreciated role for RNA surveillance. Genome Biol. 2004;5 doi: 10.1186/gb-2004-5-2-r8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyvönen T., Keinänen T.A., Khomutov A.R., Khomutov R.M., Eloranta T.O. Monitoring of the uptake and metabolism of aminooxy analogues of polyamines in cultured cells by high-performance liquid chromatography. J. Chromatogr. 1992;574:17–21. doi: 10.1016/0378-4347(92)80093-6. [DOI] [PubMed] [Google Scholar]
- Järvinen A., Grigorenko N., Khomutov A.R., Hyvönen M.T., Uimari A., Vepsäläinen J., Sinervirta R., Keinänen T.A., Vujcic S., Alhonen L., et al. Metabolic stability of α-methylated polyamine derivatives and their use as substitutes for the natural polyamines. J. Biol. Chem. 2005;280:6595–6601. doi: 10.1074/jbc.M412788200. [DOI] [PubMed] [Google Scholar]
- Järvinen A.J., Cerrada-Gimenez M., Grigorenko N.A., Khomutov A.R., Vepsäläinen J.J., Sinervirta R.M., Keinänen T.A., Alhonen L.I., Jänne J.E. α-Methyl polyamines: Efficient synthesis and tolerance studies in vivo and in vitro. First evidence for dormant stereospecificity of polyamine oxidase. J. Med. Chem. 2006;49:399–406. doi: 10.1021/jm050872h. [DOI] [PubMed] [Google Scholar]
- Kabra P.M., Lee H.K., Lubich W.P., Marton L.J. Solid-phase extraction and determination of dansyl derivatives of unconjugated and acetylated polyamines by reversed-phase liquid chromatography: Improved separation systems for polyamines in cerebrospinal fluid, urine and tissue. J. Chromatogr. 1986;380:19–32. doi: 10.1016/s0378-4347(00)83621-x. [DOI] [PubMed] [Google Scholar]
- Kim K., Ryu J.H., Park J.W., Kim M.S., Chun Y.S. Induction of a SSAT isoform in response to hypoxia or iron deficiency and its protective effects on cell death. Biochem. Biophys. Res. Commun. 2005a;331:78–85. doi: 10.1016/j.bbrc.2005.03.121. [DOI] [PubMed] [Google Scholar]
- Kim Y.K., Furic L., Desgroseillers L., Maquat L.E. Mammalian Staufen1 recruits Upf1 to specific mRNA 3′UTRs so as to elicit mRNA decay. Cell. 2005b;120:195–208. doi: 10.1016/j.cell.2004.11.050. [DOI] [PubMed] [Google Scholar]
- Korhonen V.P., Niiranen K., Halmekytö M., Pietilä M., Diegelman P., Parkkinen J.J., Eloranta T., Porter C.W., Alhonen L., Jänne J. Spermine deficiency resulting from targeted disruption of the spermine synthase gene in embryonic stem cells leads to enhanced sensitivity to antiproliferative drugs. Mol. Pharmacol. 2001;59:231–238. doi: 10.1124/mol.59.2.231. [DOI] [PubMed] [Google Scholar]
- Lakanen J.R., Coward J.K., Pegg A.E. α-Methyl polyamines: Metabolically stable spermidine and spermine mimics capable of supporting growth in cells depleted of polyamines. J. Med. Chem. 1992;35:724–734. doi: 10.1021/jm00082a013. [DOI] [PubMed] [Google Scholar]
- Lamba J.K., Adachi M., Sun D., Tammur J., Schuetz E.G., Allikmets R., Schuetz J.D. Nonsense mediated decay downregulates conserved alternatively spliced ABCC4 transcripts bearing nonsense codons. Hum. Mol. Genet. 2003;12:99–109. doi: 10.1093/hmg/ddg011. [DOI] [PubMed] [Google Scholar]
- Le Guiner C., Gesnel M.C., Breathnach R. TIA-1 or TIAR is required for DT40 cell viability. J. Biol. Chem. 2003;278:10465–10476. doi: 10.1074/jbc.M212378200. [DOI] [PubMed] [Google Scholar]
- Lewis B.P., Green R.E., Brenner S.E. Evidence for the widespread coupling of alternative splicing and nonsense-mediated mRNA decay in humans. Proc. Natl. Acad. Sci. 2003;100:189–192. doi: 10.1073/pnas.0136770100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libby P.R., Bergeron R.J., Porter C.W. Structure-function correlations of polyamine analog-induced increases in spermidine/spermine acetyltransferase activity. Biochem. Pharmacol. 1989;38:1435–1442. doi: 10.1016/0006-2952(89)90182-2. [DOI] [PubMed] [Google Scholar]
- Libby P.R., Ganis B., Bergeron R.J., Porter C.W. Characterization of human spermidine/spermine N1-acetyltransferase purified from cultured melanoma cells. Arch. Biochem. Biophys. 1991;284:238–244. doi: 10.1016/0003-9861(91)90291-p. [DOI] [PubMed] [Google Scholar]
- Matsui I., Pegg A.E. Effect of inhibitors of protein synthesis on rat liver spermidine N-acetyltransferase. Biochim. Biophys. Acta. 1981;675:373–378. doi: 10.1016/0304-4165(81)90028-3. [DOI] [PubMed] [Google Scholar]
- McCloskey D.E., Coleman C.S., Pegg A.E. Properties and regulation of human spermidine/spermine N1-acetyltransferase stably expressed in Chinese hamster ovary cells. J. Biol. Chem. 1999;274:6175–6182. doi: 10.1074/jbc.274.10.6175. [DOI] [PubMed] [Google Scholar]
- Mendell J.T., Sharifi N.A., Meyers J.L., Martinez-Murillo F., Dietz H.C. Nonsense surveillance regulates expression of diverse classes of mammalian transcripts and mutes genomic noise. Nat. Genet. 2004;36:1073–1078. doi: 10.1038/ng1429. [DOI] [PubMed] [Google Scholar]
- Metcalf B.W., Bey P., Danzin C., Jung M.J., Casara P., Vevert J.P. Catalytic irreversible inhibition of mammalian ornithine decarboxylase (E.C. 4.1.1.17) by substrate and product analogues. J. Am. Chem. Soc. 1978;100:2551–2553. [Google Scholar]
- Mita K., Fukuchi K., Hamana K., Ichimura S., Nenoi M. Accumulation of spermidine/spermine N1-acetyltransferase and alternatively spliced mRNAs as a delayed response of HeLa S3 cells following X-ray irradiation. Int. J. Radiat. Biol. 2004;80:369–375. doi: 10.1080/09553000410001695886. [DOI] [PubMed] [Google Scholar]
- Moinard C., Cynober L., de Bandt J.P. Polyamines: Metabolism and implications in human diseases. Clin. Nutr. 2005;24:184–197. doi: 10.1016/j.clnu.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Nikiforova N.N., Velikodvorskaja T.V., Kachko A.V., Nikolaev L.G., Monastyrskaya G.S., Lukyanov S.A., Konovalova S.N., Protopopova E.V., Svyatchenko V.A., Kiselev N.N., et al. Induction of alternatively spliced spermidine/spermine N1-acetyltransferase mRNA in the human kidney cells infected by venezuelan equine encephalitis and tick-borne encephalitis viruses. Virology. 2002;297:163–171. doi: 10.1006/viro.2002.1456. [DOI] [PubMed] [Google Scholar]
- Pan Q., Saltzman A.L., Kim Y.K., Misquitta C., Shai O., Maquat L.E., Frey B.J., Blencowe B.J. Quantitative microarray profiling provides evidence against widespread coupling of alternative splicing with nonsense-mediated mRNA decay to control gene expression. Genes & Dev. 2006;20:153–158. doi: 10.1101/gad.1382806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parry L., Balana Fouce R., Pegg A.E. Post-transcriptional regulation of the content of spermidine/spermine N1-acetyltransferase by N1N12-bis(ethyl)spermine. Biochem. J. 1995;305:451–458. doi: 10.1042/bj3050451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter C.W., Ganis B., Libby P.R., Bergeron R.J. Correlations between polyamine analogue-induced increases in spermidine/spermine N1-acetyltransferase activity, polyamine pool depletion, and growth inhibition in human melanoma cell lines. Cancer Res. 1991;51:3715–3720. [PubMed] [Google Scholar]
- Raina A., Tuomi K., Mäntyjärvi R. Roles of polyamines in the replication of animal viruses. Med. Biol. 1981;59:428–432. [PubMed] [Google Scholar]
- Rehse K., Puchert E., Leissring S. Antiaggregatory and anticoagulant effects of oligoamines. 12. Alkyl- and arylalkyl-derivatives of putrescine, spermidine and spermine. Arch. Pharm. (Weinheim) 1990;323:287–294. doi: 10.1002/ardp.19903230507. [DOI] [PubMed] [Google Scholar]
- Rom E., Kahana C. Polyamines regulate the expression of ornithine decarboxylase antizyme in vitro by inducing ribosomal frame-shifting. Proc. Natl. Acad. Sci. 1994;91:3959–3963. doi: 10.1073/pnas.91.9.3959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schipper R.G., Deli G., Deloyer P., Lange W.P., Schalken J.A., Verhofstad A.A. Antitumor activity of the polyamine analog N(1), N(11)-diethylnorspermine against human prostate carcinoma cells. Prostate. 2000;44:313–321. doi: 10.1002/1097-0045(20000901)44:4<313::aid-pros8>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Shappell N.W., Fogel-Petrovic M.F., Porter C.W. Regulation of spermidine/spermine N1-acetyltransferase by intracellular polyamine pools. Evidence for a functional role in polyamine homeostasis. FEBS Lett. 1993;321:179–183. doi: 10.1016/0014-5793(93)80103-2. [DOI] [PubMed] [Google Scholar]
- Sharma A., Glaves D., Porter C.W., Raghavan D., Bernacki R.J. Antitumor efficacy of N1,N11-diethylnorspermine on a human bladder tumor xenograft in nude athymic mice. Clin. Cancer Res. 1997;3:1239–1244. [PubMed] [Google Scholar]
- Stoilov P., Daoud R., Nayler O., Stamm S. Human tra2-β1 autoregulates its protein concentration by influencing alternative splicing of its pre-mRNA. Hum. Mol. Genet. 2004;13:509–524. doi: 10.1093/hmg/ddh051. [DOI] [PubMed] [Google Scholar]
- Suppola S., Pietilä M., Parkkinen J.J., Korhonen V.P., Alhonen L., Halmekytö M., Porter C.W., Jänne J. Overexpression of spermidine/spermine N1-acetyltransferase under the control of mouse metallothionein I promoter in transgenic mice: Evidence for a striking post-transcriptional regulation of transgene expression by a polyamine analogue. Biochem. J. 1999;338:311–316. [PMC free article] [PubMed] [Google Scholar]
- Thomas T., Thomas T.J. Polyamine metabolism and cancer. J. Cell. Mol. Med. 2003;7:113–126. doi: 10.1111/j.1582-4934.2003.tb00210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuittila M.T., Santagati M.G., Roytta M., Maatta J.A., Hinkkanen A.E. Replicase complex genes of Semliki Forest virus confer lethal neurovirulence. J. Virol. 2000;74:4579–4589. doi: 10.1128/jvi.74.10.4579-4589.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner E., Lykke-Andersen J. mRNA surveillance: The perfect persist. J. Cell Sci. 2002;115:3033–3038. doi: 10.1242/jcs.115.15.3033. [DOI] [PubMed] [Google Scholar]
- Wang Y., Xiao L., Thiagalingam A., Nelkin B.D., Casero R.A., Jr. The identification of a cis-element and a trans-acting factor involved in the response to polyamines and polyamine analogues in the regulation of the human spermidine/spermine N1-acetyltransferase gene transcription. J. Biol. Chem. 1998;273:34623–34630. doi: 10.1074/jbc.273.51.34623. [DOI] [PubMed] [Google Scholar]
- Wang Y., Devereux W., Stewart T.M., Casero R.A., Jr. Cloning and characterization of human polyamine-modulated factor-1, a transcriptional cofactor that regulates the transcription of the spermidine/spermine N(1)-acetyltransferase gene. J. Biol. Chem. 1999;274:22095–22101. doi: 10.1074/jbc.274.31.22095. [DOI] [PubMed] [Google Scholar]
- Wollerton M.C., Gooding C., Wagner E.J., Garcia-Blanco M.A., Smith C.W. Autoregulation of polypyrimidine tract binding protein by alternative splicing leading to nonsense-mediated decay. Mol. Cell. 2004;13:91–100. doi: 10.1016/s1097-2765(03)00502-1. [DOI] [PubMed] [Google Scholar]
- Xiao L., Casero R.A., Jr. Differential transcription of the human spermidine/spermine N1-acetyltransferase (SSAT) gene in human lung carcinoma cells. Biochem. J. 1996;313:691–696. doi: 10.1042/bj3130691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Xiao L., Berkey K.A., Tamez P.A., Coward J.K., Casero R.A., Jr. Significant induction of spermidine/spermine N1-acetyltransferase without cytotoxicity by the growth-supporting polyamine analogue 1,12-dimethylspermine. J. Cell. Physiol. 1995;165:71–76. doi: 10.1002/jcp.1041650109. [DOI] [PubMed] [Google Scholar]