Abstract
1263W94 [maribavir; 5,6-dichloro-2-(isopropylamino)-1, β-l-ribofuranosyl-1-H-benzimidazole], a novel benzimidazole compound, has been demonstrated to potently and selectively inhibit human cytomegalovirus replication in vitro and to have favorable safety profiles in animal species. Two phase I trials evaluated the safety and pharmacokinetics of escalating single doses of 1263W94 in 13 healthy subjects (dose, 50 to 1,600 mg) and 17 human immunodeficiency virus (HIV)-infected subjects (dose, 100 to 1,600 mg). No severe safety concerns were observed in the evaluation of adverse events, vital signs, electrocardiograms, and clinical laboratory tests following administration of a single dose of 1263W94. The most frequently reported adverse events in both populations were taste disturbance (80%) and headache (53%). 1263W94 was rapidly absorbed following oral administration, with peak concentrations in plasma (Cmax) occurring 1 to 3 h after dosing. The increases in the Cmax of 1263W94 and the area under the concentration-time curve from time zero to infinity (AUC0-∞) for 1263W94 were dose dependent; Cmax increased slightly less than proportionally to the dose, and AUC0-∞ increased slightly more than proportionally to the dose. 1263W94 was rapidly eliminated, with a mean half-life in plasma of 3 to 5 h; the half-life was independent of the dose level. Less than 2% of the 1263W94 dose administered was eliminated unchanged in urine. The principal metabolite of 1263W94 was 4469W94 (which is derived by N-dealkylation of 1263W94 via CYP3A4), which accounted for 30 to 40% of the dose in urine. Greater than 98% of the 1263W94 in plasma is bound to proteins, and the extent of binding appears to be constant over the dose range of 200 to 1,600 mg. In the trial with HIV-infected subjects, consumption of a high-fat meal decreased the 1263W94 AUC0-∞ and Cmax in plasma by ∼30%.
Human cytomegalovirus (HCMV) is a ubiquitous β-herpesvirus that infects approximately 40 to >90% of the population worldwide (8). The virus establishes latency, and infection is usually benign or asymptomatic in immunocompetent individuals. However, in immunocompromised individuals, such as AIDS patients and organ transplant recipients, HCMV infection is associated with severe morbidity and mortality (3). In individuals with human immunodeficiency virus (HIV) type 1 infection, activation of latent HCMV infection typically leads to HCMV retinitis. In organ transplant patients, HCMV disease is systemic and can result in rejection of the transplanted organ, opportunistic infections, end-organ disease, and death. Finally, HCMV infection in neonates can lead to mental retardation, permanent hearing loss, and death (4).
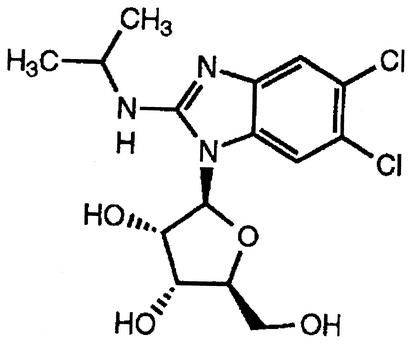
Several therapies are approved for the treatment and/or prevention of HCMV disease in the United States. The disadvantages of the available anti-HCMV medications include treatment-limiting toxicities, such as bone marrow suppression and nephrotoxicity, limited penetration to target sites, and poor oral bioavailability, necessitating intravenous administration of some compounds. The ideal drug would be well tolerated and have high levels of potency and oral bioavailability, a good safety profile for long-term use, and no drug interactions (5). Moreover, the ideal drug would have a novel mechanism of action, with no cross-resistance to other anti-HCMV agents. 1263W94 [maribavir; 5,6-dichloro-2-(isopropylamino)-1, β-l-ribofuranosyl-1-H-benzimidazole] (Fig. 1), a novel benzimidazole compound, has been demonstrated to potently and selectively inhibit HCMV replication in vitro and to have favorable safety profiles and oral bioavailability in animal species. In vitro studies have shown that 1263W94 is effective against clinical isolates resistant to ganciclovir or foscarnet (1). 1263W94 has a novel mechanism of action, which is mediated through inhibition of the protein kinase activity of the UL97 gene product (1; P. Sethna, M. Davis, C. Talarico, W. Miller, K. Blackburn, W. Burkhart, M. Moyer, K. Biron, and R. Harvey, unpublished data).
FIG. 1.
Chemical structure of 1263W94 (molecular weight, 376.24).
1263W94 has been shown to have minimal cytotoxicity against bone marrow progenitors and various human leukemic cell lines in vitro (1, 2) and limited toxicity in rats and monkeys (6). 1263W94 was rapidly and well absorbed in animals, with absolute oral bioavailabilities of ∼90% in rats and ∼50% in monkeys (6). 1263W94 is eliminated primarily via biliary excretion in rats and monkeys, with evidence of enterohepatic recirculation, and >90% of an intravenous or oral dose was recovered as unchanged 1263W94 in the feces of rats (6). Renal and metabolic clearances are minor elimination pathways, with N-dealkylated (at the C-2 amine) and glucuronidated metabolites found in rats and monkeys. However, an in vitro study of 1263W94 in human liver microsomes suggests that CYP3A4 is the primary enzyme responsible for 1263W94 metabolism (N-dealkylation), which forms 4469W94 in humans (6). 1263W94 is extensively bound to plasma proteins in vitro, primarily to albumin (98% in humans, 84% in monkeys, 88% in rats, and 85% in mice) (6). In monkeys, the level of penetration of 1263W94 into the brain is variable, and the distribution of 1263W94 into cerebrospinal fluid and the aqueous and vitreous humors is limited, which may partly be attributable to the extensive plasma protein binding of 1263W94 (6).
This report describes the results of two phase I trials of 1263W94, one with healthy subjects and the other with HIV-infected subjects, that evaluated the safety and pharmacokinetics of single escalating oral doses of 1263W94. The effect of a high-fat meal on the pharmacokinetics of 1263W94 in plasma was also evaluated in the trial with HIV-infected subjects.
(This work was presented in part at the 36th Interscience Conference on Antimicrobial Agents and Chemotherapy, New Orleans, La., September 1996, and at the 4th Conference on Retroviruses and Opportunistic Infections, Washington, D.C., January 1997.)
MATERIALS AND METHODS
Study population. (i) Healthy subjects.
Healthy male subjects (age, 18 to 45 years; weight, within 15% of ideal body weight) who did not smoke and who tested negative for drug abuse (urine was tested for cannabinoids, amphetamines, benzodiazepines, cocaine, and opiates) were eligible for enrollment. Written informed consent was obtained from all subjects, and the study was approved by the independent Glaxo Wellcome Ethics Review Committee and conducted at the Glaxo Wellcome Clinical Pharmacology Unit, Northwick Park Hospital, Harrow, United Kingdom, in 1996. Subjects were excluded from enrollment if they had participated in an investigational trial or had donated a unit of blood or plasma within 3 months prior to study entry, had used a regular course of medications within 1 month of entry, had a history of abnormal bleeding disorder or anemia, or had a history of alcohol or illicit drug abuse.
(ii) HIV-infected subjects.
HIV-infected (determined by HIV antibody enzyme-linked immunosorbent assay) male subjects (age, 18 to 55 years; weight, within 15% of ideal body weight; CD4+ count, <150 cells/mm3 within 30 days of study entry and stable on concurrent therapies for HIV infection) were eligible for enrollment. Written informed consent was obtained from all subjects, and the study was approved by the Riverside Research Ethics Committee and conducted by Clinical Evaluation Ltd., Chelsea & Westminster Hospital, London, United Kingdom, in 1996. Subjects were excluded from enrollment if they had active opportunistic infections including HCMV disease or visual symptoms suggestive of HCMV disease; had used approved or investigational anti-HCMV drugs within 3 months prior to study entry; had participated in an investigational trial within 1 month prior to study entry; had active obstructive hepatobiliary disease or cirrhosis; had malabsorption syndrome or other gastrointestinal dysfunction; had a history of abnormal bleeding disorder or anemia; had a hemoglobin level <10 g/dl; had a neutrophil count <700 cells/mm3; had a platelet count <50,000 cells/mm3; had aspartate transaminase, alanine transaminase, or alkaline phosphate levels more than three times the upper limit of normal; had a total bilirubin level >1.75 mg/dl; or had an estimated creatinine clearance <50 ml/min.
Study design.
Both phase I trials were double-blind, randomized, placebo-controlled, dose-escalation studies evaluating the tolerability and pharmacokinetics of single oral doses of 1263W94 given at doses of 50, 100, 200, 400, 800, and 1,600 mg to 12 healthy subjects or at doses of 100, 400, 800, and 1,600 mg to 16 HIV-infected subjects. In the trial with healthy subjects, each subject received a total of five escalating doses of 1263W94, supplied as 100-mg capsules, and one dose of matching placebo; and for each dose level, 10 subjects were randomized to receive 1263W94 and 2 subjects were randomized to receive placebo. In the trial with HIV-infected subjects, each subject received three doses of 1263W94 and one dose of placebo; based on a four-period, incomplete randomized block design; and for each dose level, 12 subjects were randomized to receive 1263W94 and 4 subjects were randomized to receive placebo. All doses of study medications were administered under fasting conditions (fasting from 8 h prior to dosing until 4 h after dosing), with a 1-week washout interval between doses. Following administration of the 1,600-mg dose in the trial with HIV-infected subjects, the study was unblinded and the 12 subjects who received 400 mg of 1263W94 under fasting conditions also completed a fifth dosing occasion in which they received a 400-mg dose with a high-fat meal (fat, 67 g; carbohydrate, 58 g; protein, 33 g).
Blood and urine sampling.
On all occasions in both trials, blood samples were collected in Monovet tubes with EDTA anticoagulant immediately prior to dosing and at 0.25, 0.50, 0.75, 1, 1.5, 2, 3, 4, 5, 6, 8, 10, 12, 16, and 24 h following the administration of each dose for analysis of the total 1263W94 and 4469W94 concentrations in plasma and for analysis of the unbound 1263W94 and 4469W94 concentrations in plasma following administration of the 200-, 800-, and 1,600-mg doses. For determination of the total 1263W94 and 4469W94 concentrations in plasma, 4-ml blood samples were kept at 4°C and centrifuged at 2,000 × g for 10 min within 30 min of collection to separate the plasma, which was then stored frozen at −20°C until it was analyzed. For determination of the unbound drug concentrations in plasma, 7-ml blood samples were kept at room temperature and within 15 min of collection were centrifuged at 2,000 × g for 10 min to separate the plasma. An aliquot (2 ml) of the plasma was stored frozen at −20°C for analysis of the total drug concentrations. The remaining plasma was transferred into an Amicon Centrifree micropartition unit and centrifuged at 2,000 × g for 20 min at room temperature. The ultrafiltrate was then stored in a Teflon tube at −20°C for analysis of the unbound drug concentrations.
Urine samples were collected predosing and over the intervals of 0 to 4, 4 to 8, 8 to 12, and 12 to 24 h after dosing. The total volume of urine for each collection interval was measured, and a portion (10 ml) was stored frozen at −20°C until it was analyzed.
Safety evaluation.
In both trials, the safety and tolerability of single escalating doses of 1263W94 were evaluated on the basis of reports of adverse experiences, vital signs, and clinical laboratory tests (hematology, serum chemistry, and urinalysis) and the results of physical examination. Clinical laboratory tests were done prior to dosing and 24 h after dosing. Electrocardiography (ECG) with a 12-lead electrocardiograph was performed for the healthy subjects prior to the administration of each dose of study drug, at 1, 2, 4, 8, and 24 h after dosing, and at the follow-up visit. In addition, continuous monitoring by ECG was performed for 4 h following the administration of each dose of study drug.
Plasma assays.
The total (protein-bound and unbound) concentrations of 1263W94 (molecular weight, 376.2) and its N-dealkylated metabolite, 4469W94 (molecular weight, 334.2), in plasma were determined by an isocratic reversed-phase high-pressure liquid chromatography (HPLC) method with UV detection (at λ equal to 222 nm). The two compounds were isolated from plasma (0.5 ml) by using solid-phase extraction cartridges (500 mg of C18 sorbant, 3 ml) eluted with methanol. An aliquot of the extract was then injected onto an HPLC column (Symmetry C18, 5 μm, 150 by 4 mm; Waters) eluted with a mobile phase of acetonitrile-methanol-water (32/3/65) at 0.8 ml/min and 38°C. The rates of recovery of 1263W94 and 4469W94 from plasma were >80%, and the bias of the assay was <5%. The standard curve was linear from 50 to 4,500 ng/ml, with a lower limit of quantitation of 50 ng/ml for both analytes. The within- and between-assay variabilities were <7% for 1263W94 and <10% for 4469W94. The unbound concentrations of 1263W94 and 4469W94 in the ultrafiltrate of the plasma (160 μl) were determined by applying a system of automated trace enrichment prior to injection onto the HPLC column. Briefly, the analytes were retained by a precolumn (Symmetry C18, 20 by 4 mm; Waters) before being backflushed onto the analytical column (ABZ C18, 5 μm, 150 by 4.6 mm; Supelco). A simpler mobile phase (acetonitrile-water at 15/85 for the precolumn and 40/60 for the analytical column) was used to elute the analytes at 1 ml/min. The standard curve was linear from 30 to 800 ng/ml and from 30 to 250 ng/ml for unbound 1263W94 and unbound 4469W94, respectively. The bias of the assay was <10%, and the intra- and interday variabilities of the assay were <12% for each analyte.
Urine assays.
The concentrations of 1263W94 and 4469U89 in urine were determined by a reversed-phase HPLC-mass spectrometric (MS)-MS method. The two compounds were isolated from urine (0.4 ml) by solid-phase (C18 sorbant) extraction procedures and then separated with an HPLC column (PrimeSphere, 5 μm, C8, 30 by 4.6 mm; Phenomenex) eluted with a mobile phase of 0.1% formic acid in acetonitrile-water (25/75) at 1 ml/min and 40°C. Detection was accomplished with a PE-SCIEX triple-quadrapole mass spectrometer with a turbo ion spray for monitoring of molecular ions. The standard curve was linear from 0.05 to 8 μg/ml for 1263W94 and from 1 to 40 μg/ml for 4469W94. The bias of the assay was <8% for both compounds, and the assay variabilities were <10% for 1263W94 and <15% for 4469W94.
Pharmacokinetic analysis.
The plasma concentration-time data for 1263W94 and 4469W94 were analyzed by noncompartmental pharmacokinetic methods with the WinNonlin (version 1.5) program (Pharsight Corporation, Mountain View, Calif.). The peak concentration in plasma (Cmax) and the time to Cmax (Tmax) were obtained from direct inspection of the plasma concentration-time data. Estimates of the apparent half-life (t1/2) were calculated as ln (2)/λz, where λz is a first-order rate constant determined by the slope of the linear regression line of the apparent terminal linear portion of the log concentration-versus-time curve. Generally, the time points used for determination of λz were automatically selected by the WinNonlin program. The area under the concentration-time curve (AUC) from time zero to time t (AUC0-t; where t is the last time point with a measurable drug concentration) was calculated by the linear trapezoidal method. The AUC from time zero to infinity (AUC0-∞) was then determined as AUC0-t + Clast/λz, where Clast is the last measurable drug concentration. The apparent clearance from plasma (CL/F) for 1263W94 was calculated as the dose divided by AUC0-∞. The cumulative percentages of the dose recovered in urine (over 24 h) as unchanged 1263W94 and as 4469W94 (corrected for molecular weight) were determined. Renal clearance was calculated as the cumulative amount of 1263W94 or 4469W94 recovered in urine over the sampling period (24 h) divided by the AUC from 0 to 24 h (AUC0-24) for the relevant compound.
Statistical analysis.
To determine the dose proportionality of 1263W94 pharmacokinetic parameter estimates, the Cmax and AUC0-∞ data were fit to a power model, Cmax (or AUC0-∞) = a · doseb, where a is the intercept and b is the slope, by using the MIXED procedure (SAS Institute, Cary, N.C.), with subject used as a random effect in the model. The parameter estimate is dose proportional if the slope is not significantly different from 1.0. Dose proportionality was tested separately for healthy subjects and HIV-infected subjects.
The effect of food on the pharmacokinetics of total 1263W94 in plasma was assessed by comparing loge-transformed Cmax and AUC0-∞ data on a pairwise basis. The ratio of the test dose (400 mg in the fed state) versus the reference dose (400 mg in the fasted state) geometric least-squares (GLS) means and the resultant 90% confidence interval (CI) was calculated by using the MIXED procedure. The effect of food was determined by the deviation of the GLS mean ratio from 1.0. The median Tmax and associated 95% CI were calculated for each of the treatments by the Wilcoxon signed-rank test. Tmaxs between the test treatment and the reference treatment were compared by the Wilcoxon rank sum test.
RESULTS
Subject characteristics and accountability. (i) Healthy subjects.
Thirteen healthy male subjects (2 black, 11 Caucasian; age range, 19 to 43 years; mean age, 30 years; median age, 31 years; weight range, 61.9 to 89.7 kg; mean weight, 79.0 kg; median weight, 79.6 kg) participated in this trial. Eleven of the 12 subjects originally enrolled completed the study as planned. One subject withdrew from the study for personal reasons after receiving the second dose (placebo). The replacement subject started at the 200-mg dosing period and completed the study through the 1,600-mg dosing period.
(ii) HIV-infected subjects.
Seventeen HIV-infected white male subjects (age range, 30 to 53 years; mean age, 39 years; median age, 38 years; weight range, 60.6 to 90.1 kg; mean weight, 72.8 kg; median weight, 68.8 kg; CD4+ count range, <10 to 187 cells/mm3; mean CD4+ count, 36 cells/mm3; median CD4+ count, 18 cells/mm3) participated in this study. Fifteen of the 16 subjects originally enrolled in the study completed the four dosing escalation periods by receiving three active doses and one placebo. One subject withdrew from the study for personal reasons after receiving the second dose (400 mg of 1263W94). The replacement subject completed the parts of the study with doses of 400, 800, and 1,600 mg in a fasting state and a dose of 400 mg with food. Ten of the 12 subjects who received 400 mg of 1263W94 under fasting conditions completed the fifth dosing period, in which they received a 400-mg dose with a standard high-fat breakfast. One subject failed to attend the study during the fifth dosing period, and one subject was withdrawn following receipt of the 1,600-mg dose period due to a non-drug-related serious adverse event (SAE). All subjects received a number of concomitant medications during the study period, including acyclovir (n = 11), co-trimoxazole (n = 10), diazepam, temazepam, or alprazolam (n = 10), lamivudine (n = 8), zidovudine (n = 7), fluconazole (n = 5), rifabutin (n = 4), ketoconazole (n = 2), clarithromycin (n = 2), and others (n ≤ 2), including 49 compounds in one of the following classes: anti-infectives, anti-inflammatory agents (steroidal and nonsteroidal), anticonvulsants, antidepressants, antipsychotics, antihistamines, and vitamins, as well as warfarin, ranitidine, omeprazole, lactulose, and loperamide.
Safety evaluation.
There were no reports of SAEs in the trial with healthy subjects. Two subjects enrolled in the trial with HIV-infected subjects experienced SAEs that the principal investigator did not consider attributable to the study drug. The first subject was admitted to the hospital with severe abdominal pain, vomiting, and fever 11 days after receiving a 1,600-mg dose of 1263W94. The subject remained in the hospital for 3 days. The abdominal pain resolved after 6 days, the vomiting resolved after a few minutes, and the fever resolved after 1 day. Five days following discharge from the hospital, the subject completed the study period with the last dose (400 mg with food). The second subject was admitted to the hospital with fever, cough, nausea, mouth ulcers, sore throat, dysphagia, leg pain, constipation, and neutropenia approximately 2 weeks after receiving a 1,600-mg dose of 1263W94. The subject underwent an ophthalmologic examination 4 days after hospitalization and was diagnosed with HCMV retinitis. All of these adverse events (AEs) except the mouth ulcers, nausea, and HCMV retinitis resolved by the end of the study.
In the trial with healthy subjects, a total of 51 AEs were reported by the 13 subjects. The AEs reported by at least two subjects receiving any treatment included taste disturbance, headache, dizziness, and herpes simplex of the lips and are summarized by treatment in Table 1. Forty-two AEs were considered by the investigator to be attributable to the study drug. All AEs except for four experiences of severe bitter taste in the mouth (one after receiving the placebo and three after receiving 1,600 mg of 1263W94) were mild to moderate in intensity. All AEs had resolved by the end of the study.
TABLE 1.
AEs reported by at least two subjects on any treatmenta
| Subject group and AE | No. (%) of subjects
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Placebo | 50 mg | 100 mg | 200 mg | 400 mg (fasting) | 400 mg (fed) | 800 mg | 1,600 mg | |
| Healthy subjects | ||||||||
| Taste disturbance | 2 (17) | 0 | 2 (20) | 3 (30) | 6 (60) | 8 (80) | 8 (80) | |
| Headache | 0 | 2 (20) | 0 | 0 | 0 | 1 (10) | 3 (30) | |
| Dizziness | 0 | 0 | 0 | 0 | 2 (20) | 0 | 0 | |
| Herpes simplex of lips | 0 | 0 | 0 | 0 | 2 (20) | 0 | 0 | |
| HIV-infected subjects | ||||||||
| Taste disturbance | 3 (19) | 1 (8) | 3 (23) | 4 (40) | 5 (42) | 8 (67) | ||
| Headache | 2 (13) | 3 (25) | 2 (15) | 1 (10) | 4 (33) | 4 (33) | ||
| Tiredness/drowsiness | 0 | 2 (17) | 0 | 2 (20) | 2 (17) | 3 (25) | ||
| Fever | 0 | 0 | 1 (8) | 0 | 1 (8) | 2 (17) | ||
| Abdominal pain | 2 (13) | 0 | 0 | 0 | 0 | 0 | ||
| Sore throat | 1 (6) | 0 | 0 | 0 | 0 | 2 (17) | ||
Among the healthy subjects, data for the placebo group are for a total of 12 subjects and a total of 10 subjects in each dosing group. Among the HIV-infected subjects, data for the placebo group and for the groups receiving doses of 100, 400 (fasting), 400 (fed), 800, and 1,600 mg are for a total of 16, 12, 13, 10, 12, and 12 subjects, respectively.
In the trial with HIV type 1-infected subjects, a total of 109 AEs were reported by 17 subjects, including 17 events reported by 9 subjects while receiving the placebo and 4 events reported by 3 subjects prior to dosing or during the washout period. AEs reported by at least two subjects receiving any treatment included taste disturbance, headache, tiredness or drowsiness, fever, abdominal pain, and sort throat and are summarized by treatment in Table 1. Fifty-five AEs were considered to be attributable to the study drug. The overall incidence of AEs increased with increasing dose. All AEs were mild to moderate in intensity, except for the events associated with the two SAEs described above.
The only AEs consistently reported more frequently after administration of 1263W94 rather than placebo were taste disturbance and headache, which were reported by 83 and 53% of both populations combined, respectively. Taste disturbance was characterized by a bitter, metallic, chemical, or altered taste in the mouth that occurred with a dose-related frequency. Other drug-related AEs reported in both studies included dizziness or faintness (17% of the two groups of subjects combined), drowsiness or tiredness (30% of the two groups of subjects combined, 15% of healthy subjects, and 41% of HIV-infected subjects), and diarrhea (13% of the two groups of subjects combined).
There were no findings of clinically significant abnormalities or changes from the baseline for vital signs, clinical laboratory values, or ECG assessments among the healthy subjects. Similarly, among the HIV-infected subjects, except for one case of neutropenia, there were no drug-related or clinically significant changes from the baseline in vital signs or clinical laboratory values; the case of neutropenia was reported with the SAE described above. ECG assessments were not included in the trial with HIV-infected subjects. In both trials, hemoglobin levels in some subjects were slightly reduced toward the end of the study, which was possibly due to frequent blood sampling.
Pharmacokinetic evaluation.
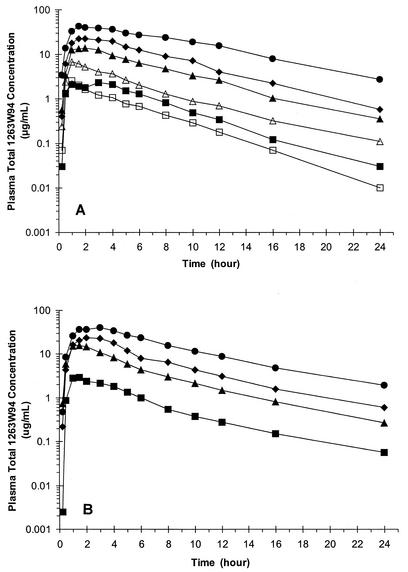
The pharmacokinetic profiles of 1263W94 appeared to be similar between the healthy and HIV-infected subjects over the dose range of 100 to 1,600 mg (Fig. 2; Table 2). 1263W94 was rapidly absorbed, achieving quantifiable concentrations in plasma by the first sampling time (15 min). 1263W94 Cmaxs occurred between 1 and 3 h after dosing across all dose levels, although in some subjects Cmaxs occurred as late as 5 or 6 h, especially after the administration of high doses. Administration of 1263W94 following consumption of a standard high-fat meal delayed the 1263W94 Tmax by approximately 2 h and decreased the level of 1263W94 exposure in plasma (AUC0-∞ and Cmax) by approximately 30% (Table 3).
FIG. 2.
(A) Mean total 1263W94 concentration in plasma-time curves following oral administration of single escalating doses in healthy subjects. □, 50-mg dose; ▪, 100-mg dose; ▵, 200-mg dose; ▴, 400-mg dose; ⧫, 800-mg dose; •, 1,600-mg dose. (B) Mean total 1263W94 concentration in plasma-time curves following oral administration of single escalating doses in HIV-infected subjects. ▪, 100-mg dose; ▴, 400-mg dose; ⧫, 800-mg dose, •, 1,600-mg dose.
TABLE 2.
Pharmacokinetic parameter estimates for total 1263W94 in plasma following oral administration of single 50- to 1,600-mg doses to healthy and HIV-infected subjectsa
| 1263W94 dose (mg) |
Cmax (μg/ml)
|
Median Tmax (h)
|
AUC0-∞ (μg · h/ml)
|
C8 (μg/ml), C12 (μg/ml)b
|
t1/2 (h)
|
CL/F (ml/min)
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Healthy (n = 10) | HIV infected (n = 12)c | Healthy (n = 10) | HIV infected (n = 12)c | Healthy (n = 10) | HIV infected (n = 12)c | Healthy (n = 10) | HIV infected (n = 12)c | Healthy (n = 10) | HIV infected (n = 12)c | Healthy (n = 10) | HIV infected (n = 12)c | |
| 50 | 2.66 (21) | NAd | 1.00 | NA | 10.8 (32) | NA | 0.432 (47), 0.203 (48) | NA | 3.3 (20) | NA | 83.4 (28) | NA |
| 100 | 3.32 (37) | 3.39 (29) | 2.26 | 1.50 | 16.3 (28) | 16.2 (60) | 0.811 (25), 0.343 (28) | 0.538 (76), 0.337 (91) | 3.0 (15) | 4.0 (51) | 108 (21) | 125 (37) |
| 200 | 7.45 (39) | NA | 1.26 | NA | 34.2 (49) | NA | 1.29 (56), 0.691 (80) | NA | 3.6 (30) | NA | 112 (30) | NA |
| 400 | 16.7 (34) | 17.6 (47) | 1.75 | 1.50 | 97.8 (29) | 83.2 (57) | 3.36 (39), 2.63 (49) | 3.02 (73), 1.49 (121) | 3.9 (20) | 4.1 (29) | 73.1 (27) | 105 (60) |
| 800 | 26.4 (26) | 27.0 (29) | 1.50 | 1.75 | 183 (38) | 151 (46) | 9.20 (36), 4.02 (24) | 6.47 (60), 3.07 (72) | 4.0 (25) | 4.0 (34) | 80.7 (31) | 104 (41) |
| 1,600 | 48.8 (16) | 47.8 (27) | 2.00 | 3.00 | 437 (37) | 335 (47) | 23.7 (38), 15.7 (42) | 16.1 (51), 8.72 (59) | 4.8 (32) | 4.8 (33) | 68.6 (36) | 94.8 (39) |
The values are means (percent coefficient of variation).
C8, concentration at 8 h following administration of a single dose; C12, concentration at 12 h following administration of a single dose.
n = 13 for the 400-mg dose.
NA, not applicable.
TABLE 3.
Effect of food on pharmacokinetics of 1263W94 in plasma of HIV-infected subjects
| Pharmacokinetic parameter | GLS mean
|
GLS mean ratio (90% CI)a
|
|
|---|---|---|---|
| 400-mg dose + food | 400-mg dose | 400-mg dose + food/400-mg dose | |
| Mean AUC0-∞ (μg · h/ml) | 52.1 | 72.2 | 0.72 (0.57-0.92) |
| Mean Cmax (μg/ml) | 11.5 | 16.1 | 0.72 (0.61-0.84) |
| Median Tmax (h) | 3.5b | 1.5b | 2.0 (1.0-2.5)c |
Ratio of 400-mg dose + food/400-mg dose.
Estimated by the Wilcoxon signed rank test.
Estimate for Tmax by Wilcoxon rank sum test.
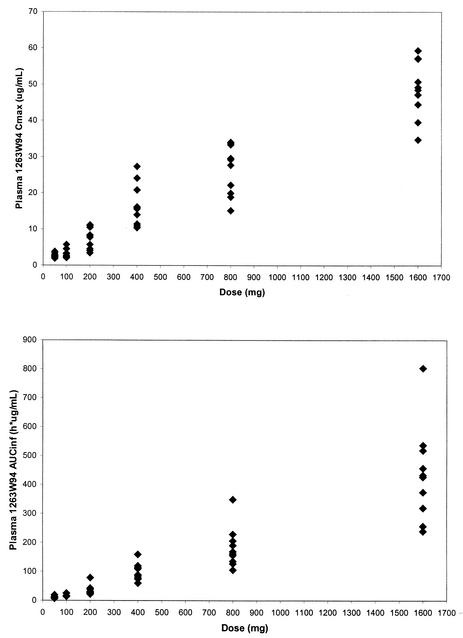
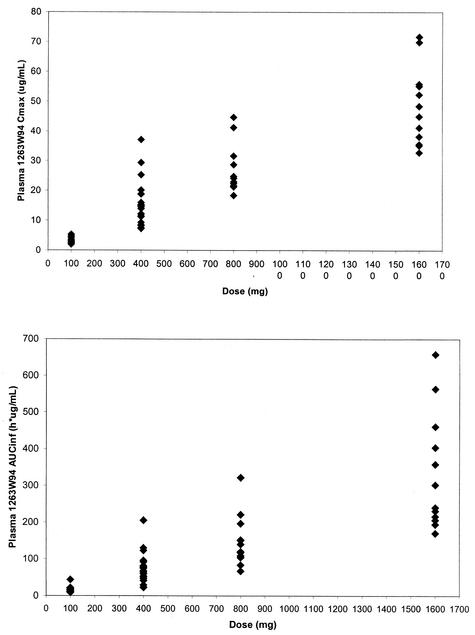
The estimates for the slope in the power model analysis for healthy subjects were 0.88 (90% CI, 0.83 to 0.94) for Cmax and 1.10 (90% CI, 1.06 to 1.14) for AUC0-∞. The estimates for HIV-infected subjects were 0.96 (90% CI, 0.88 to 1.04) for Cmax and 1.11 (90% CI, 1.03 to 1.18) for AUC0-∞. These slope estimates indicate that the Cmax of 1263W94 increases slightly less than proportionally to the dose and that the AUC0-∞ of 1263W94 increases slightly more than proportionally to the dose over the wide dose range studied (50 to 1,600 mg). The Cmax and AUC0-∞ for 1263W94 in plasma are plotted by dose in Fig. 3 for healthy subjects and in Fig. 4 for HIV-infected subjects.
FIG. 3.
Cmax and AUC0-∞ for 1263W94 versus dose in individual healthy subjects (♦).
FIG. 4.
Cmax and AUC0→∞ for 1263W94 versus dose in individual HIV-infected subjects (⧫).
1263W94 was rapidly eliminated from plasma, with an average t1/2 of 3 to 5 h in both healthy and HIV-infected subjects and across all dose levels (Table 2). Figure 2 also shows that the terminal linear portion of the semilogarithmic plots of the plasma 1263W94 concentration-versus-time curves were parallel at all dose levels for both populations.
1263W94 is extensively bound to plasma proteins in vivo. Unbound 1263W94 concentrations in plasma followed total drug concentrations; however, the unbound 1263W94 concentrations in plasma were almost 100-fold lower than the total drug concentrations at the respective dose level. Table 4 summarizes the pharmacokinetic parameter estimates for unbound 1263W94 in plasma for healthy and HIV-infected subjects. Due to the extremely low (below the limit of quantitation) concentrations of unbound 1263W94 after administration of the 200-mg dose, AUC0-∞ and t1/2 estimates were available for only two subjects. As shown by the data for the 800-mg dose, the profiles for unbound 1263W94 in plasma were similar for the two populations. On the basis of the ratios of unbound versus total drug for Cmax and AUC0-∞, the percentages of unbound 1263W94 in plasma were similar among the 200- to 1,600-mg dose levels and averaged ∼1.5%.
TABLE 4.
Pharmacokinetic parameter estimates for unbound 1263W94 in plasma following oral administration of single 50- to 1,600-mg doses to healthy or HIV-infected subjectsa
| 1263W94 dose (mg) |
Cmax (μg/ml)
|
Median Tmax (h)
|
AUC0-∞ (μg · h/ml)
|
t1/2 (h)
|
Cmax ratio (unbound/total)
|
AUC0-∞ ratio (unbound/total)
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Healthy (n = 10) | HIV infected (n = 12) | Healthy (n = 10) | HIV infected (n = 12) | Healthy (n = 10)b | HIV infected (n = 12) | Healthy (n = 10)b | HIV infected (n = 12) | Healthy (n = 10) | HIV infected (n = 12) | Healthy (n = 10)b | HIV infected (n = 12) | |
| 200 | 0.09 (43) | NDc | 2.01 | ND | 0.48 | ND | 2.9 | ND | 0.012 | ND | 0.011 | ND |
| 800 | 0.37 (22) | 0.42 (29) | 1.50 | 1.75 | 2.20 (29) | 2.28 (62) | 3.4 (20) | 3.3 (40) | 0.015 (31) | 0.016 (32) | 0.013 (30) | 0.015 (30) |
| 1,600 | ND | 0.70 (22) | ND | 2.50 | ND | 4.32 (40) | ND | 3.4 (44) | ND | 0.015 (25) | ND | 0.014 (22) |
The values are means (percent coefficient of variation).
n = 2 for AUC0-∞ and t1/2 at 200-mg dose.
ND, not done.
4469W94, the principal metabolite of 1263W94, was detectable in the plasma of all subjects within 30 min following dosing, with Cmaxs occurring at about 2 to 4 h. On the basis of molar concentrations, the median Cmax and AUC0-∞ for total 4469W94 were about 10 and 20%, respectively, of the corresponding values of the parameters for total 1263W94 in the plasma of healthy subjects across all dose levels. In HIV-infected subjects, the median molar concentration ratios (concentration of 4469W94 to concentration of 1263W94) for Cmax and AUC0-∞ were higher, about 16 and 35%, respectively. Median 4469W94 t1/2 values were approximately 4 to 8 h and were approximately 1.5 to 2 times longer than that of 1263W94. 4469W94 is also extensively bound to plasma proteins, with only 8% present as the unbound drug in plasma.
The urinary recovery data show that in both healthy and HIV-infected subjects, <2% of the oral dose was recovered as unchanged 1263W94 in urine over 24 h. The mean percentages of the dose recovered as 1263W94 and 4469W94 in urine over 24 h were about 30 to 40% across all dose levels, with almost all the dose recovered in urine being present as 4469W94. The mean renal clearances of 1263W94 were 0.6 to 1.2 ml/min in both populations, and the mean renal clearances of 4469W94 were approximately 200 to 250 ml/min in healthy subjects and 100 to 150 ml/min in HIV-infected subjects.
DISCUSSION
The phase I trials described in this report have characterized the tolerability and the plasma and urinary excretion profiles of 1263W94 and its principal metabolite, 4469W94 (the N-dealkylated metabolite), following oral administration of single escalating doses to healthy and HIV-infected subjects.
Following oral administration, 1263W94 was rapidly absorbed, with Cmaxs occurring within 1 to 3 h following dosing. At least 30 to 40% of a single oral dose of 1263W94 was absorbed, as shown by the total percentage of the dose recovered in urine collected over 24 h. Since urine was assayed only for 1263W94 and 4469W94, the proportion of the dose absorbed could be more than 40%.
1263W94 was rapidly eliminated from plasma, with a t1/2 of 3 to 5 h, which was consistent across all doses in both populations. The increases in Cmax and AUC0-∞ for 1263W94 in plasma were dose dependent. The Cmax of 1263W94 increased slightly less than proportionally to the dose and the AUC0-∞ for 1263W94 increased slightly more than proportionally to the dose over the wide dose range studied (50 to 1,600 mg).
The plasma and urinary excretion profiles of 1263W94 and 4469W94 indicate that 1263W94 is extensively metabolized. The profiles of 4469W94 in plasma suggest that the disposition of 4469W94 is not limited by its rate of formation from 1263W94, because the elimination t1/2 of 4469W94 is much longer than that of 1263W94. Preliminary experiments have shown that 4469W94 is pharmacologically inactive and is further metabolized to its glucuronide conjugate (which was not measured in this study), and thus, the percentage of the dose that is metabolized via the N-dealkylation pathway could be greater than 40%. In vitro experiments conducted by Glaxo Wellcome have shown that 4469W94 is primarily formed by a hepatic cytochrome P450 3A4 isozyme (CYP3A4), which could potentially be a target for drug interactions between 1263W94 and other concomitantly administered medications that are also metabolized by CYP3A4.
The pharmacokinetic profiles of 1263W94 appeared to be similar between the healthy and the HIV-infected subjects (Fig. 2; Table 2). However, additional work is required to support these initial findings, especially given some of the potential confounding factors in these studies. The potential confounding factors include differences in demographic variables (age and weight) and the concurrent use of other medications by the HIV-infected subjects. The HIV-infected subjects enrolled in one of these 1263W94 clinical trials were concurrently taking medications that are known to inhibit CYP3A4, such as fluconazole, ketoconazole, and clarithromycin; these medications potentially could have increased the plasma 1263W94 concentrations.
No severe safety concerns were observed in the evaluation of AEs, vital signs, ECG, and clinical laboratory tests following administration of single doses of 1263W94. The most frequently reported drug-related AEs in both populations combined were mild to moderate taste disturbance (80% of subjects) and headache (53%). On the basis of the onset and duration of the taste disturbance experiences, the event is apparently not due to the local taste of the drug product or substance upon ingestion of the 1263W94 capsules but is presumably due to secretion of 1263W94 into the salivary glands after systemic absorption. The intensity and frequency of the taste disturbance generally increased as the dose escalated above 400 mg. Although taste disturbance is not a safety concern, it may negatively affect adherence during the long-term administration of 1263W94.
The total 1263W94 concentrations in plasma were above the average in vitro 50% inhibitory concentration (IC50) for clinical HCMV isolates, 0.08 μM (0.03 μg/ml) as determined by DNA hybridization assay or 0.32 μM (0.12 μg/ml) as determined by plaque reduction assay, following the administration of single oral doses for all doses studied. However, the in vivo antiviral activity depends on many factors (e.g., the distribution of 1263W94 into target tissues and cells and the kinetics of intracellular trafficking), in addition to the concentrations in plasma. Because 1263W94 is extensively bound to plasma proteins, its antiviral activity may be more closely correlated with the unbound 1263W94 concentrations in plasma. On the basis of the data obtained from the present single-dose trials, the predicted steady-state unbound concentrations of 1263W94 in plasma following oral administration of approximately 400 mg twice a day would be maintained above the in vitro IC50 by DNA hybridization over the entire dosing interval, whereas doses of 800 mg three times a day (TID) would be required to maintain unbound concentrations above the in vitro IC50 by plaque reduction assay over the entire dosing interval. The steady-state unbound 1263W94 concentrations in plasma are based on the mean total 1263W94 concentrations in plasma observed in these studies and the assumption that the level of protein binding is 98.5% and the t1/2 is 4 h.
The results of these studies supported the design of a subsequent phase I/II clinical trial to evaluate the safety, antiviral activity, and pharmacokinetics of multiple doses of 1263W94 in HIV-infected subjects (7). In that study, doses of 100 mg TID (the lowest dose tested) demonstrated potent anti-HCMV activities in a semen model, and maximal anti-HCMV activity was observed with a dose of 200 mg TID (7). The semen model has been predictive of clinical efficacy for cidofovir. Therefore, maintenance of unbound 1263W94 concentrations in plasma above the in vitro IC50s (determined by either DNA hybridization or plaque reduction assay) over the entire dosing interval may not be necessary and requires further investigation.
Acknowledgments
Special thanks are extended to Logesvaran Yogendran and Philip Barrington and clinic staff at the Northwick Park Hospital for conducting the phase I trial with healthy subjects; to William Prince and clinic staff at the Clinical Evaluation Ltd., Academic Therapeutics, Chelsea & Westminster Hospital, for conducting the phase I trial with HIV-infected subjects; and to Michael Youle and clinic staff at the St. Stephen's Clinic, Chelsea &Westminster Healthcare NHS Trust, London, United Kingdom, for recruiting the HIV-infected subjects.
REFERENCES
- 1.Biron, K. K., R. J. Harvey, S. C. Chamberlain, S. S. Good, A. A. Smith III, M. G. Davis, C. L. Talarico, W. H. Miller, R. Ferris, R. E. Dornsife, S. C. Stanat, J. C. Drach, L. B. Townsend, and G. W. Koszalka. 2002. Potent and selective inhibition of human cytomegalovirus replication by 1263W94, a benzimidazole l-riboside with a unique mode of action. Antimicrob. Agents Chemother. 46:2365-2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chan, J., S. Chamberlain, K. Biron, M. Davis, R. Harvey, D. Selleseth, R. Dornsife, E. Dark, L. Frick, L. Townsend, J. Drach, and G. Koszalka. 2000. Synthesis and evaluation of a series of 2′-deoxy analogues of the antiviral agent 5,6-dichloro-2-isopropylamino-1-(β-l-ribofuranosyl)-1H-benzimidazole (1263W94). Nucleosides Nucleotides Nucleic Acids 19:101-123. [DOI] [PubMed] [Google Scholar]
- 3.de la Hoz, R. E., G. Stephens, and C Sherlock. 2002. Diagnosis and treatment approaches to CMV infections in adult patients. J. Clin. Virol. 25:S1-S12. [DOI] [PubMed] [Google Scholar]
- 4.Gaytant, M. A., E. A. Steegers, B. A. Semmekrot, H. M. Merkus, and J. M. Galama. 2002. Congenital cytomegalovirus infection: review of the epidemiology and outcome. Obstet. Gynecol. Surv. 57:245-256. [DOI] [PubMed] [Google Scholar]
- 5.Griffiths, P. 2002. The treatment of cytomegalovirus infection. J. Antimicrob. Chemother. 49:243-253. [DOI] [PubMed] [Google Scholar]
- 6.Koszalka, G. W., N. W. Johnson, S. S. Good, L. Boyd, S. C. Chamberlain, L. B. Townsend, J. C. Drach, and K. K. Biron. 2002. Preclinical and toxicology studies of 1263W94, a potent and selective inhibitor of human cytomegalovirus replication. Antimicrob. Agents Chemother. 46:2373-2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lalezari, J., J. Aberg, L. Wang, M. Wire, R. Miner, W. Snowden, C. Talarico, S. Shaw, M. Jacobson, and W. Drew. 2002. A phase I/II dose-escalation trial to evaluate the pharmacokinetics, anti-human cytomegalovirus activity, and safety of 1263W94 in human immunodeficiency virus-infected men with asymptomatic HCMV shedding. Antimicrob. Agents Chemother. 46:2969-2976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van der Bij, W., and R. Speich. 2001. Management of cytomegalovirus infection and disease after solid-organ transplantation Clin. Infect. Dis. 33(Suppl. 1):S32-S37. [DOI] [PubMed] [Google Scholar]