Abstract
Recombinant adenoviruses have been widely used for various applications, including protein expression and gene therapy. We herein report a new and simple cloning approach to an efficient and robust construction of recombinant adenoviral genomes based on the mating-assisted genetically integrated cloning (MAGIC) strategy. The production of recombinant adenovirus serotype 5-based vectors was greatly facilitated by the use of the MAGIC procedure and the development of the Adeasy™ adenoviral vector system. The recombinant adenoviral plasmid can be generated by a direct and seamless substitution, which replaces the stuff fragment in a full-length adenoviral genome with the gene of interest in a small plasmid in Escherichia coli. Recombinant adenoviral plasmids can be rapidly constructed in vivo by using the new method, without manipulations of the large adenoviral genome. In contrast to other traditional systems, it reduces the need for multiple in vitro manipulations, such as endonuclease cleavage, ligation and transformation, thus achieving a higher efficiency with negligible background. This strategy has been proven to be suitable for constructing an adenoviral cDNA expression library. In summary, the new method is highly efficient, technically less demanding and less labor-intensive for constructing recombinant adenoviruses, which will be beneficial for functional genomic and proteomic researches in mammalian cells.
INTRODUCTION
Adenoviral vectors are a versatile tool in the investigations of gene expression and regulation as well as gene therapy. Several advantages of the use of the adenovirus have been demonstrated. These include the inability of the adenovirus to integrate into the genome of the target cells, its broad spectrum of applications in various cell types, its high expression of the gene of interest, the ability to produce high titers of recombinant viruses, and the ability to have gene transferred independent of active cell division (1–7). More recently, with the increasing application of novel RNA silencing techniques, adenoviruses have been shown to be a powerful approach to facilitating the expression of short-interfering RNA (5,8).
Over the years, many approaches have been developed for the generation of recombinant adenoviruses, which can be divided into two basic categories; direct plasmid construction of recombinant adenoviral genome (9–13) or indirect construction (14–17). The former involves the ligation of the adenoviral genome with the DNA fragments of interest, and the latter involves homologous recombination in mammalian cells or in Escherichia coli. The direct methods are handicapped by the limitation of choices of suitable restriction sites and the difficulty in the manipulation of the large adenoviral vector. On the other hand, the indirect methods utilize two plasmids coding for the homologous recombinant regions; one is a shuttle plasmid containing an expression cassette, and the other is the large plasmid containing the majority of the adenoviral genome. For example, the construction of the recombinant adenovirus through Cre-lox has been described in studies with mammalian cells and E.coli (14,16). The major advantage of indirect construction is the elimination of repeated rounds of plaque purification. Although both of these methods work, the generation of recombinant adenoviruses is still limited by several factors, including the low efficiency and difficulty in the screening of homologous recombination, the need for time-consuming plaque purification, and the frequent contamination by wild-type adenoviruses. Given that there are probably 25 000 genes present in human cells, these traditional methods do not meet the increasing reqiurements for the post-genome research on gene expression and regulation as well as the development of novel gene therapy approaches especially for the high-throughput generation of viruses required in proteomic studies (13).
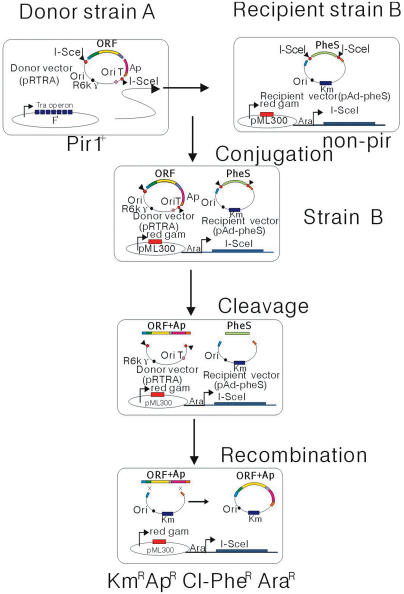
Considering the aforementioned drawbacks, we have now developed a robust and scalable system to generate recombinant adenoviruses based on a previously reported in vivo cloning system called mating-assisted genetically integrated cloning (MAGIC) (18). The newly developed MAGIC procedure utilizes bacterial mating to catalyze the transfer of a DNA fragment between a donor vector in one bacterial strain and a recipient plasmid in a separate bacterial strain (18). Then the recombination between these plasmids can be forced by inducing I-SceI to site-specific cleavage and the red and gam recombinase to homologous recombination. The donor strain contains the F factor (F′) transfer system, a low-copy plasmid containing a transfer operon (tra) and a cis-acting origin of transfer (oriT). When the oriT is ligated into the donor plasmid, the modified F′ is able to efficiently mobilize the donor plasmid, but not itself (18). A donor plasmid must also have a conditional origin of replication from R6K, oriγ, which is required for the trans-activation of factor π encoded by the gene pir1 or its relaxed copy-number control allele, pir1-116. Only the donor strain DH10β can express pir1-116, so the donor plasmid will replicate only in DH10β, but not in the recipient strain BUN21 that does not have pir1-116 (18). After the bacteria are mixed, the presence of arabinose will induce the homing endonuclease I-SceI to lyse the fragment of interest from the donor plasmid, and cut down the stuff fragment from the recipient plasmid. In addition, the plasmid pML300 contained in BUN21 will be induced to express the red recombinase gene in the presence of rhamnose. The cleavage of both the donor fragment and the recipient plasmid greatly enhances recombination events (18). The plasmid pML300 contains a temperature-sensitive mutant derivative of the pSC102 origin of replication and will not replicate when bacteria are grown at 42°C. In the present study, we cultured the bacteria at 42°C in order to eliminate the plasmid pML300. In brief, the MAGIC procedure only requires the simple mixing of bacterial strains, which would significantly save time, effort and expense. Therefore, this method may have implications in high-throughput recombinant DNA production for functional genomics studies, including the generation of recombinant adenoviruses.
Herein we report a novel approach to the generation of recombinant adenovirus based on the MAGIC procedure (18). This method utilizes site-specific and intensive recombination with random 50 bp regions of homology under red and gam recombinase, integrating the fragment of interest into the full-length adenovirus genome. It is rapid (taking only 12–14 days to generate a recombinant adenovirus) and is free of parental virus contamination. We have constructed a novel donor vector pRTRA, in which the fragment of interest is flanked by two different 50 bp homology regions, H1 and H2, which in turn are flanked with two separate I-SceI sites. The novel recipient vector pAd-pheS also contains two I-SceI-linked H1 and H2 sites (8,18). The recombination events are efficiently regulated by a series of intensive elements: an intron-encoding rare endonuclease I-SceI is induced by the sugar arabinose; and the red and gam recombinase is induced by the sugar rhamnose. Once the DNA fragment of interest in the donor vector and the stuff fragment in the recipient vector are both cut down by I-SceI, the recombination events mediated by the red and gam recombinase are stimulated. The efficiency of transfer of the DNA fragment of interest from pRTRA to pAd-pheS was shown to be very high; the positive recombination efficiency can be as high as 100%. This novel method can be used for the high-throughput creation of recombinant adenoviruses, which may be suitable for constructing an adenoviral cDNA expression library as demonstrated in the present study.
MATERIALS AND METHODS
Cell culture
The human embryonic kidney (HEK293) cells (Invitrogen) were cultured in DMEM supplemented with 10% fetal calf serum (FCS). The HEK293 cells constitutively expressed Adeasy deleted E1 gene in-trans. Infective virus particles were produced after these cells were transfected with E1-deleted adenovirus vectors. After infection with the recombinant adenoviruses, the cells were maintained in DMEM supplemented with 5% FCS.
Bacteria, plasmids and viral DNA
E.coli strains DH10β and BUN21 and the plasmid pML300 were kindly donated by Prof. Stephen J Elledge from the Harvard Medical School in Boston, (MA, USA) (18). DH10β was used for generating the recombinant donor plasmid and BUN21 was used for generating and propagating the recombinant adenoviral plasmids. The plasmid pML300 contained in BUN21 carries the red and gam recombinase gene induced by rhamnose, and is unable to replicate when the bacteria are grown at 42°C (18). The plasmid MAGIC1 and the plasmid 1202 were also provided by Prof. Stephen J Elledge (18). The adenovirus bone vector (pAdeasy) and pShuttle plasmid were obtained from Stratagene (15). We constructed the novel donor plasmid pRTRA (Figure 1), the recipient plasmid pAd-pheS (full-length adenoviral genome), the plasmid pPic-man and the plasmid pShuttle-cmv-red-sv40polA.
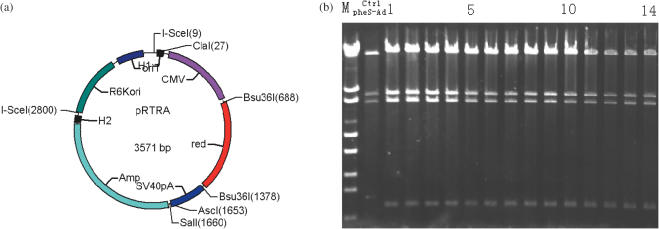
Figure 1.
(a) Restriction map of the donor plasmid pRTRA, see the text for details. (b) Construction of a recombinant adenoviral genome containing DsRed cassette. The DsRed cassette was transferred to the adenoviral vector by standard MAGIC procedure. The mixed bacteria were selected on LB plates containing 50 μg ml−1 kanamycin,100 μg ml−1ampicillin, 0.2% w/v l-arabinose and 10 mM Cl-Phe overnight. Plasmid DNA was isolated from 14 individual colonies. All plasmid DNAs were digested with SalI and the digestion products were electrophoresed on 0.7% agarose gels and stained with ethidium bromide. Expected restriction patterns for both pAd-DsRed (1–14, for each independently obtained plasmid) and pAd-pheS are shown opposite the gel.
Cloning the foreign genes gfp and man into the donor plasmid using restriction enzyme Bsu36I and T4 DNA polymerase
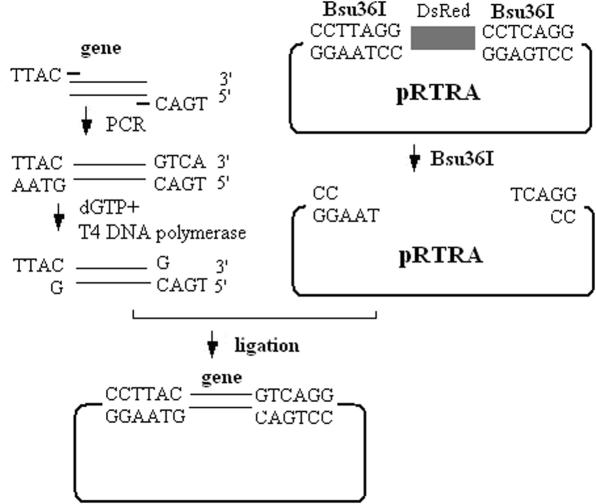
The gfp gene was amplified from pEGFP-1 (Clontech) by PCR. The forward primer was 5′-TTACGATGGTGAGCAAGGGCGAGGA-3′, and the reverse primer was 5′-TGACTTACTTGTACAGCTCGTCCATGCC-3′. The man gene was amplified from pPic-man using the primers: ZL15F, 5′-TTACTGAAGCGCATACTGTGTCGCC-3′ and ZL16R, 5′-TGACCGGATTCACTCAACGATTGG-3′. The amplified fragments were incubated with 0.5 U of T4 DNA polymerase and 4 mM dGTP (TaKaRa) at 12°C for 45 min, as described previously (19,20). For each gene, a total of 30 ng of treated fragments and 1 ng of the Bsu36I-digested (CCTTAGG and CCTGAGG) pRTRA were ligated with 5 U of T4 DNA ligase™ (TaKaRa) at 16°C for 12 h in 1 μl buffer. The gfp gene and man gene were inserted into pRTRA to form the plasmids pRTGA and pRTMA separately (Figure 2).
Figure 2.
The PCR product of a foreign gene was amplified by T4 DNA polymerase and dGTP, and then was ligated with the Bsu36I-digested pRTRA. The ligation mixture was transformed to the donor strain DH10β, and then the recombinant donor plasmid was obtained. We introduced the two different Bsu36I sites (CCTTAGG and CCTGAGG) in the pRTRA vector and the 4 nt TTAC(5′–3′) in the forward primer and the other 4 nt TGAC(5′–3′) in the reverse primer. The complete digestion of pRTRA with Bsu36I results in a linearized donor vector with overhang ends of 5′-TTA-3′ and 5′-TCA-3′, respectively. We made use of the 3′→5′ exonuclease activity and 5′→3′ polymerase activity of T4 DNA polymerase. When T4 DNA polymerase encounters the first Guanine nucleotide at the 5′ end of the DNA in the dGTP bath, the reaction will keep the balance between the exonuclease activity and polymerase activity. Therefore, the overhang ends of the gene fragments of interest will be digested to be perfectly compatible with the vector.
Modification of the donor plasmid
The fragment of chloramphenicol resistent gene was amplified from pBT (Strategene) by PCR using the forward primer [5′-TTTGTCGACATAACTTCGTATAATGTATGCTATACGAAGTTATACGGGGAGAGCCTGAGCAAAC (SalI and 34 bp loxP sites underlined)] and the reverse primer [5′-TTTGTGCACATAACTTCGTATAGCATACATTATACGAAGTTATCAGCATCACCCGACGCACTTT-3′ (ApaLI, 34 bp loxP sites underlined)]. The PCR was performed at 95°C for 5 min, followed by 25 cycles at 95°C for 45 s, 56°C for 45 s and 72°C for 1 min by using Thermo Hybrid PX2 (Thermo) and TaKaRa Extaq™ polymerase (TaKaRa). The fragment cut by ApaLI and SalI was ligated into the pRTRA vector (also cut by the same enzymes), resulting in the recombinant plasmid pRTRC.
Generating the recombinant adenoviral vector by MAGIC
The overall strategy developed is shown in Figure 3. E.coli DH10β containing the donor vector pRTRA was grown in Luria–Bertani (LB) broth containing ampicillin (100 μg/ml).The recipient strain BUN21 containing the plasmid pML300 and the recipient plasmid pAd-pheS was grown in LB broth containing spectinomycin (50 μg/ml), kanamycin (50 μg/ml) and glucose (0.2% w/v) overnight. The recipient strain was washed twice with 2 volume of LB broth the next day. The donor and recipient strains were separately diluted to 1:25, 1:50, 1:100 or 1:200 with LB broth containing 0.2% w/v rhamnose and grown at 30°C for 2 h to an A600 of 0.15∼0.25, and the donor and recipient strains were mixed to a ratio of 1:1 based on their A600 in the presence of 0.2% w/v l-arabinose. The mixture was incubated at 37°C for 2 h without shaking, and then for a further 2 h with shaking. The recombinant culture was diluted at the ratio of 1:100, plated on the selective plates containing kanamycin (50 μg/ml), ampicillin (100 μg/ml), 10 mM Cl-Phe and 0.2% w/v l-arabinose, and finally incubated at 42°C overnight (18).
Figure 3.
The procedures for generating recombinant adenoviruses through MAGIC. The donor and recipient plasmids were generated as described in the text, then transformed into the donor strain A(DH10β) and recipient strain B(BUN21). The DNA fragments of interest in the donor vector and the negative marker (pheS) in the recipient vector were both cut down by an intron-encoding rare endonuclease I-SceI, and then the recombination events intermediated by the red and gam recombinase were stimulated. The recombinant adenoviral plasmid was then generated. Restriction digestion by PacI and subsequent transfection into HEK293 cell lines yielded a high population of recombinant adenoviral particles after 7–10 day growth period.
Production of recombinant adenoviruses and proliferation
The recombinant adenoviral plasmids were amplified by incubating the colony identified in 50 ml LB broth containing kanamycin (50 μg/ml), ampicillin (100 μg/ml) and 0.2% w/v arabinose. The culture was grown overnight at 37°C. The maxiprep DNA was then prepared from the liquid culture and digested in a sufficient amount (5 μg of DNA) with PacI. Subsequently, the buffer and the excess enzyme from restriction reactions were removed by phenol extraction/ethanol precipitation. The DNA was re-suspended in 50 μl of sterile 0.1× TE buffer or dH2O, and then added to HEK293 cells in the presence of Lipofectamine 2000 (Invitrogen). At 4 h post-transfection, the cells were incubated in 5 ml DMEM containing 5% FCS. About 8 days after transfection, the cells were harvested and pelleted by low-speed centrifugation, and the viruses were liberated by three freeze/thaw cycles. The cell lysate [1 ml in 1× phosphate-buffered saline (PBS) (pH 7.4)] containing the recombinant adenoviruses was then amplified and purified. To generate higher titer viral stocks, the HEK 293 cells were infected by the cell lysate and the harvest process was repeated.
Construction of a model adenoviral cDNA expression library
The donor strain containing pRTRA and the donor strain containing pRTGA were mixed at ratios of 1:30, 1:300, 1:3000 and 1:30 000 on the basis of their A600. The mixed donor stains at different ratios were mixed with the recipient strain at a 1:1 ratio on the basis of their A600 (3,18). The subsequent experiments were based on the complete MAGIC procedure as reported previously (18). LB broth was used to wash all the colonies on the selective plates which were then incubated overnight in 100 ml LB broth containing 50 μg/ml kanamycin, 100 μg/ml ampicillin, 0.2% w/v l-arabinose and 10 mM Cl-Phe. The DNA was extracted for greater yields, treated with PacI, and the excess buffer and enzyme were removed by phenol extraction/ethanol precipitation. The DNA (5 μg) was re-suspended in 100 μl of sterile 0.1× TE buffer or dH2O, and then added to a 60 mm plate containing 1 × 106 HEK293 cells that had been incubated in DMEM with 5% FCS for 2 h. At 4 h post-transfection, the cells were incubated for 8 days in 5 ml DMEM containing 5% FCS. After three freeze-thaw cycles, the resultant lysate (1 ml) was used to infect HEK 293 cells in a 10 cm plate (70% confluent) for 1 h before being incubated with 5 ml DMEM supplemented with 5% FCS.
RESULTS
Construction of the donor vector pRTRA
As illustrated in Figure 1a, the donor vector pRTRA consists of four elements: R (R6kori), T (oriT), R [DsRed cassette [cmv-red-sv40poly(A)] and A (ampicillin resistance gene). The DsRed cassette (cmv-Bsu36I-red-Bsu36I-sv40) was obtained from pShuttle-cmv-red-sv40pol(A) by PCR. The forward primer was 5′-TTTGGTACCTTTTAGGGATAACAGGGTAATTTTATCGATCGCGGGAAAACTGAATAAGAGGA-3′(KpnI, I-SceI and ClaI sites underlined), the reverse primer was 5′-TTTGTCGACTTTGGCGCGCCCCCCACCTTATATATTCTTTCCCAC-3′(SalI and AscI sites underlined). The ampicillin resistance gene was obtained from pAdeasy by PCR. The forward primer was TTTGTCGACCCTTTGATCTTTTCTACGGGGTCTGA (SalI site underlined), and the reverse primer was TTTGAGCTCTTTTAGGGATAACAGGGTAATAAATGTGCGCGGAACCCCTAT (SacI and I-SceI sites underlined). The amplified fragments of the DsRed cassette and ampicillin resistance gene were digested with SalI, ligated and then amplified by PCR. The fragments R6kori and oriT were cut down together with ApaI and BstBI from the pMAGIC1. The cmv-red-sv40 pol(A)-amp fragment and R6kori-oriT fragment were blunted, ligated and transformed to generate the donor plasmid pRTRA.
Construction of recipient vector pAd-pheS
We amplified a fragment of kanamycin resistance gene that needs to be added with I-SceI sites and 50 bp regions of homology in the primers. The I-SceI site was internal, and the 50 bp regions of homology were external. The 50 bp regions of homology included one upstream of the CMV promoter (cmv-red-sv40polA) and the other downstream of the ampicillin gene. The forward primer was 5′-TTTCTCGAGGAATAAGAGGAAGTGAAATCTGAATAATTTTGTGTTACTCATAGCGCGTAATAGGGATAACAGGGTAATCCGCTTGGGTGGAGAGGCTATT-3′ (XhoI and I-SceI sites underlined, 50 bp between the sites), and the reverse primer was 5′-TTTGTCGACTATTTTTCTAAATACATTCAAATAGTATCCGCTCATGAGACAATAACCCTAGGGATAACAGGGTAATCAAACTGGAACAACACTCAACCC-3′ (SalI and I-SceI underlined, 50 bp between the sites). The fragment of kanamycin resistance gene cut by XhoI and SalI was then ligated into the pShuttle vector, resulting in the recombinant plasmid pShuttleK. A pheS Gly294 gene (1.3 kb fragment derived from p1202) was ligated into the pShuttleK (also double cut by the I-SceI enzyme). The recombinant plasmid pShuttle-pheS was completely digested with PmeI, and then treated with alkaline phosphatase. It was transformed into E.coli BJ5183 (containing the adenovirus bone vector pAdeasy) to produce the recombinant adenoviral plasmid (pShuttle vector plus gene of interest recombined with pAdeasy). The resultant recombinant adenoviral plasmid pAd-pheS was transformed into the recipient strain BUN21.
Modification of the donor plasmid pRTRA and excision of the resistant gene
As the size of the foreign fragment is limited to 7.5 kb in the Adeasy system, when a large fragment is required to be inserted into the recipient plasmid, the existence of the amp gene will take up the capacity of adenoviruses, limiting the size of the foreign fragments. In addition, the biosafety concerns that the amp gene may cause a spread of antibiotic resistance, hamper the application of the recombinant adenovirus in gene therapy. To avoid this problem, pRTRC, a modified form of pRTRA, was constructed. The Cm cassette was flanked by 34 nt loxP sites for subsequent excision of the antibiotic cassette by Cre recombinase. For example, 10 μg of recombinant plasmids was mixed with 20 U of Cre recombinase in 300 μl of the reaction mixture at 37°C for 3 h (data not shown). If the recombinant adenoviruses are used as tools for protein expression in laboratory research, the excision procedure may be unnecessary.
Recombination cloning efficiency of the MAGIC strategy in adenoviral vectors
On the representative plates, we observed 300–400 colonies per 1 μl mixture. Colonies selected for ampicillin were all homologous recombinants. The presence of arabinose and Cl-phe could eliminate the parental recipient plasmids (18). The pheS Gly294 gene was 1.3 kb, and the ampicillin resistance gene was 1.1 kb. We examined them further by using multiple restriction digestions. The parental recipient plasmid would be cut down to a 1.3 kb fragment (pheS gene), while the recombinant plasmid would be cut down to a 1.1 kb fragment (Amp gene). All of the screened colonies were only cut down to a 1.1 kb fragment, demonstrating that 100% of the screened clones contained only the desired recombination construct (Figure 1b), which indicates that there is no need to identify recombinants. Therefore, this method for recombinant adenoviral construction is highly efficient which warrants no need for further identification.
Expression of target genes in mammalian cells
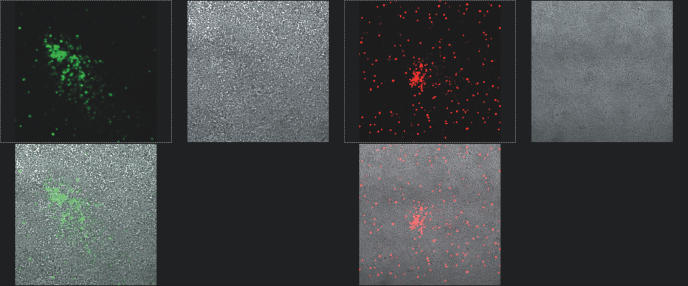
The gfp, man and DsRed genes have been cloned into the adenoviral bone vector pAd-pheS. Eight positive single clones for each gene were picked up to incubate for recombinant DNAs to be transfected into the HEK293 cells. Fluorescence expression for each sample was detected using the Leica TCS-SPII confocal system (Leica). The expression of mannanase was detected in the agar plates supplemented with 0.5% konjak mannan powder (stocked in this laboratory), and 0.02% trypan blue (Sigma). The 10 μl supernatant of HEK293 cells transfected with the recombinant adenoviral plasmids was blotted on the plate at 37°C for 10 h to assess the expression of mannanase. The supernatant of HEK293 cells transfected with the parental plasmids and the wild-type adenoviruses were used as controls (data not shown). All of the screened clones constructed through MAGIC contained the correct construct (Figure 4).
Figure 4.
PacI-digested pAdeasy-GFP and pAdeasy-DsRed were transfected separately into 293 cells. After 8 days, the recombinant adenoviruses were harvested and then used to infect HEK293 cells. Adenovirus-producing foci and the fluorescence expression were detected using Leica TCS-SPII confocal system (Leica) at 3–4 days post-infection.
Construction of an adenoviral cDNA expression library in a model experiment
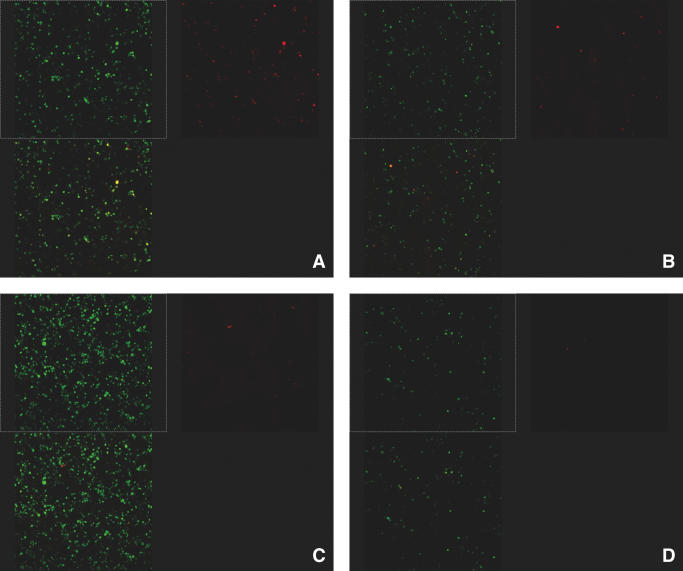
In a small-scale pilot experiment, we were able to demonstrate the potential complexity generated in the AdLibrary as constructed by MAGIC (Figure 5). Two donor bacteria, which were identical except for the transgene expressed (GFP and DsRed) and formed a donor library, were mixed and converted to recipient bacteria simultaneously. A dilution series was made, and the two donor bacteria were mixed at four ratios; 1:30, 1:300, 1:3000 and 1:30 000 (3). The bacteria-based donor library containing a test DsRed at an abundance of about 0.003% could be successfully converted to the recipient strain to form a randomly desired DNA expression adenoviral genome library (3). We could observe the red fluorescence protein expression in HEK293 cells at all ratios (Table 1), even at the dilution of 1:30 000, which suggests that a donor cDNA library can be efficiently converted into an adenoviral library with a fair coverage of cDNAs represented in the donor bacteria-based library. In HEK293 cells, the expression of red fluorescence protein was detectable up to the ratio of 1:30 000, suggesting that this library can represent almost all the mRNA species present in the original cells and the complexity of the library required could be achieved (3,13). A donor bacteria-based cDNA expression library must be initially constructed by the conventional method and then mated with the recipient strain containing the recipient plasmid, which leads to a successful construction of a bacteria-based cDNA adenoviral expression library. The results indicate that the simple method for construction of recombinant adenoviruses through MAGIC is also easily adapted to the challenge of generating libraries (Figure 5). Therefore, the method that constructs adenoviral cDNA expression libraries effectively may offer a practical way to facilitate the identification of genes based on specific biological functions for a variety of purposes.
Figure 5.
Experimental design of dilution experiments to demonstrate MAGIC can be used to construct an adenoviral cDNA expression library. The donor strain containing the plasmid pRTGT was mixed with the donor strain containing the pRTRA at various ratios, and then the mixture was combined with the recipient strain containing the recipient plasmid pAd-pheS. The mixed adenoviral plasmids containing pAd-GFP and pAd-DsRed were extracted and transfected into the HEK293 cells. After 2–3 days, the green and red fluorescence expression was detected. The ratios of donor strain containing pRTRA mixed with donor strain containing pRTGA were 1:30, 1:300, 1:3000 and 1:30 000, each marked with A, B, C and D, respectively. The quantities of the cells which can be observed with red fluorescence expression are consistent with the ratios.
Table 1.
pAd-DsRed representation shows the ratio of pAd-DsRed to pAd-GFP, comprising the Ad5 genome used to convert to adenoviruses
| pAd-DsRed representation | DsRed-positive cells |
|---|---|
| 1/30 | >2000 |
| 1/300 | 1000 |
| 1/3000 | 200 |
| 1/30 000 | 15 |
DsRed-positive cells show the number of Red-positive per plate for each dilution point.
DISCUSSION
Following the completion of the Human Genome Project and the significant progress made in identifying disease-causing genes, an increasing need arises for the development of high-throughput adenovirus production for various researches and development projects. Traditional methods of constructing recombinant adenoviruses cannot meet these ever-growing needs. More recently, the site-specific recombination of an expression cassette into the adenovirus genome to generate recombinant adenoviruses in E.coli has become popular. This method is rapid since it eliminates the numerous manipulations of sub cloning in vitro and multiple rounds of plaque purification.
In this study, we described a novel, simple and efficient method for the construction of recombinant adenovirus. As illustrated in Figure 3, the novel strategy developed involves three major steps. First, the gene of interest is cloned into a donor vector pRTRA. Second, the donor and the recipient bacteria are mixed for mating, and the recombinants are selected by using kanamycin, ampicillin and Cl-Phe. Multiple restriction digestion analysis is performed for further identification. Third, the recombinant adenoviral plasmid is cleaved with PacI to expose its inverted terminal repeats and then transfected into a packaging cell line (e.g. HEK 293 cells). The positive selection by antibiotics, the negative selection by Cl-Phe and the ability to bypass further identification steps make the screening of recombinants simple and efficient.
To our knowledge, the method described herein is the first successful example to apply MAGIC to the construction of recombinant adenoviruses. For adenoviral vector construction, this method has several advantages over other conventional methods. First, a small size plasmid (3.5 kb) is used, obviating the manipulation of the large adenoviral genome. Second, the recombination events depend on a λ red and gam recombinase-mediated cassette exchange in E.coli, eliminating multiple repeated rounds of plaque purification. Third, the efficiency of the recombination can reach 100% since the red and gam recombination system is highly efficient and the recombinants are selected by both arabinose and Cl-Phe (18,21). This efficiency was further demonstrated in constructing an adenoviral cDNA expression library. Fourth, the system has potential for the modification of any adenoviral sequences (22). Therefore, the approach described here is a highly efficient, less time-consuming and labor-intensive method for constructing recombinant adenoviruses. In addition, we speculate that the method can also be useful in the construction of other viruses.
Many standard recombinant adenoviral vector systems have been established, including Adeasy™ and adenoviral vector system based on Cre-lox (15,17). Compared with the original system (the Adeasy™ adenoviral vector system) (15), we have improved the design and the implementation of vector systems in three ways. First, in the Adeasy system, the recombinant adenoviral plasmid cannot be effectively amplified in the recombinant strain BJ5183, so it is necessary to transform the recombinant plasmid to a recA, endA strain, (such as DH10B, XL-Gold). In contrast, for the MAGIC system, the desired recombinant adenoviral plasmid can be achieved in the recipient strain with greater yields. Second, in the Adeasy system, the pShuttle plasmid carries a foreign gene, rendering the treatment with PmeI and alkaline phosphatase necessary, which is not needed in the MAGIC procedure. Third, the MAGIC method is much more efficient than the Adeasy system, and it can save 2–3 days in constructing recombinant Adenoviruses. The high efficiency is attributable to that the combination via the Red system is 500–1000 times more efficient than the BJ5183 platform (15), that inducing I-SceI also results in a 20-fold enrichment for recombinants, and that an additional 25-fold enrichment can be achieved by pheS. More recently, a new method called recombinase-mediated cassette exchange based on Cre-lox recombination in mammalian cells has been reported, which needs several repeated rounds of vector purification and suffers from frequent contamination of the replication-competent adenoviruses (16). Although the rest of this method is rapid and simple, the laborious manipulation of vector purification will remarkably limit the applications of this elegant system.
In conclusion, we have described a robust approach for the construction of recombinant adenoviruses based on MAGIC and the Adeasy™ adenoviral vector system, and it yields the desired recombinant adenoviral genome 100% of the time with high yields. The resulting plasmid containing the recombinant adenoviral genome is truly clonal, obviating the need for screening and several repeated rounds of plaque purification. Using this improved system based on MAGIC, a set of hundreds or even thousands of recombinant adenoviral plasmids could be obtained by mating with a large set of donor bacteria. Therefore, the method is compatible with high-throughput applications including the construction of cDNA expression library. Unlike conventional in vitro methods that use restriction enzymes or site-specific recombinases, recombinant adenoviral DNA assembled by MAGIC achieves a seamless transfer of genetic elements through homologous recombination in vivo without the need for DNA preparation and in vitro manipulation. Moreover, the highly efficient λ Red system will be of great use for the knockout or modification of other adenoviral sequences (21,22). This robust, scalable and highly efficient method of constructing recombinant adenoviruses will be of great use in genomics and proteomics researches.
Acknowledgments
The authors thank Dr Zhan Yang for contributing helpful ideas and Prof Stephen J Elledge for donating plasmids and E.coli strains. This work was supported by grants from the following organizations: the China National Human Liver Proteomics Project (2004BA711A19), the Nature Science Foundation of Hubei Province (2003ABA114), and the Young-Tech Chenguang Program of Wuhan (20035002016). Funding to pay the Open Access publication charges for this article was provided by HuBei University.
Conflict of interest statement. None declared.
REFERENCES
- 1.Sharp P.A., Moore C., Haverty J.L. The infectivity of adenovirus 5 DNA-protein complex. Virology. 1976;75:442–456. doi: 10.1016/0042-6822(76)90042-8. [DOI] [PubMed] [Google Scholar]
- 2.Berkner K.L. Development of adenovirus vectors for the expression of heterologous genes. Biotechniques. 1988;6:616–629. [PubMed] [Google Scholar]
- 3.Hatanaka K., Ohnami S., Yoshida K., Miura Y., Aoyagi K., Sasaki H., Asaka M., Terada M., Yoshida T., Aoki K. A simple and efficient method for constructing an adenoviral cDNA expression library. Mol. Ther. 2003;8:158–166. doi: 10.1016/s1525-0016(03)00138-2. [DOI] [PubMed] [Google Scholar]
- 4.Benihoud K., Yeh P., Perricaudet M. Adenovirus vectors for gene delivery. Curr. Opin. Biotechnol. 1999;10:440–447. doi: 10.1016/s0958-1669(99)00007-5. [DOI] [PubMed] [Google Scholar]
- 5.Arts G.-J., Langemeijer E., Tissingh R., Ma L., Pavliska H., Dokic K., Dooijes R., Mešić E., Clasen R., Michiels F., et al. Adenoviral vectors expressing siRNAs for discovery and validation of gene function. Genome Res. 2003;13:2325–2332. doi: 10.1101/gr.1332603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kovesdi I., Brough D.E., Bruder J.T., Wickham T.J. Adenoviral vectors for gene transfer. Curr. Opin. Biotechnol. 1997;8:583–589. doi: 10.1016/s0958-1669(97)80033-x. [DOI] [PubMed] [Google Scholar]
- 7.Mullan B., Dugue C., Moutard V., Raoux D., Tremp G., Denefle P., Perricaudet M., Robert J.J. Robust functional gene validation by adenoviral vectors: one-step Escherichia coli-derived recombinant adenoviral genome construction. Gene Ther. 2004;11:1599–1605. doi: 10.1038/sj.gt.3302333. [DOI] [PubMed] [Google Scholar]
- 8.Paddison P.J., Silva J.M., Conklin D.S., Schlabach M., Li M., Aruleba S., Balija V., O'Shaughnessy A., Gnoj L., Scobie K., et al. A resource for large-scale RNA-interference-based screens in mammals. Nature. 2004;428:427–431. doi: 10.1038/nature02370. [DOI] [PubMed] [Google Scholar]
- 9.Anderson R.D., Haskell R.E., Xia H., Roessler B.J., Davidson B.L. A simple method for the rapid generation of recombinant adenovirus vectors. Gene Ther. 2000;7:1034–1038. doi: 10.1038/sj.gt.3301197. [DOI] [PubMed] [Google Scholar]
- 10.Miyake S., Makimura M., Kanegae Y., Harada S., Sato Y., Takamori K., Tokuda C., Saito I. Efficient generation of recombinant adenoviruses using adenovirus DNA-terminal protein complex and a cosmid bearing the full-length virus genome. Proc. Natl Acad. Sci. USA. 1996;93:1320–1324. doi: 10.1073/pnas.93.3.1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Munz P.L., Young C.S. End-joining of DNA fragments in adenovirus transfection of human cells. Virology. 1991;183:160–169. doi: 10.1016/0042-6822(91)90129-y. [DOI] [PubMed] [Google Scholar]
- 12.Gao G., Zhou X., Alvira M.R., Tran P., Marsh J., Lynd K., Xiao W., Wilson J.M. High-throughput creation of recombinant adenovirus vectors by direct cloning, green-white selection and I-SceI-mediated rescue of circular adenovirus plasmids in 293 cells. Gene Ther. 2003;10:1926–1930. doi: 10.1038/sj.gt.3302088. [DOI] [PubMed] [Google Scholar]
- 13.McVey D., Zuber M., Brough D.E., Kovesdi I. Adenovirus vector library: an approach to the discovery of gene and protein function. J. Gen Virol. 2003;84:3417–3422. doi: 10.1099/vir.0.19446-0. [DOI] [PubMed] [Google Scholar]
- 14.Hardy S., Kitamura M., Harris-Stansil T., Dai Y., Phipps M.L. Construction of adenovirus vectors through Cre-lox recombination. J. Virol. 1997;71:1842–1849. doi: 10.1128/jvi.71.3.1842-1849.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.He T.C., Zhou S., da Costa L.T., Yu J., Kinzler K.W., Vogelstein B. A simplified system for generating recombinant adenoviruses. Proc. Natl Acad. Sci. USA. 1998;95:2509–2514. doi: 10.1073/pnas.95.5.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nakano M., Odaka K., Takahashi Y., Ishimura M., Saito I., Kanegae Y. Production of viral vectors using recombinase-mediated cassette exchange. Nucleic Acids Res. 2005;33:e76. doi: 10.1093/nar/gni074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chartier C., Degryse E., Gantzer M., Dieterle A., Pavirani A., Mehtali M. Efficient generation of recombinant adenovirus vectors by homologous recombination in Escherichia coli. J. Virol. 1996;70:4805–4810. doi: 10.1128/jvi.70.7.4805-4810.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li M.Z., Elledge S.J. MAGIC, an in vivo genetic method for the rapid construction of recombinant DNA molecules. Nature Genet. 2005;37:311–319. doi: 10.1038/ng1505. [DOI] [PubMed] [Google Scholar]
- 19.Lehman I.R. T4 DNA polymerase. Meth. Enzymol. 1974;29:46–53. [PubMed] [Google Scholar]
- 20.Wartell R.M., Reznikoff W.S. Cloning DNA restriction endonuclease fragments with protruding single-stranded ends. Gene. 1980;9:307–319. doi: 10.1016/0378-1119(90)90329-p. [DOI] [PubMed] [Google Scholar]
- 21.Zhang P., Li M.Z., Elledge S.J. Towards genetic genome projects: genomic library screening and gene-targeting vector construction in a single step. Nature Genet. 2002;30:31–39. doi: 10.1038/ng797. [DOI] [PubMed] [Google Scholar]
- 22.Campos S.K., Barry M.A. Rapid construction of capsid-modified adenoviral vectors through bacteriophage lambda Red recombination. Hum. Gene Ther. 2004;15:1125–1130. doi: 10.1089/hum.2004.15.1125. [DOI] [PubMed] [Google Scholar]