Abstract
T cell responses against an antigen are often focused on a small fraction of potentially immunogenic determinants, a phenomenon known as immunodominance. Immunodominance can be established at several stages of antigen presentation, including antigen processing, binding of peptides to MHC, and competition between T cells for dendritic cells (DCs). The mechanism of this T cell competition is unclear, but may include competition for physical access to DCs, competition for limiting soluble DC-derived factors, or a suppressive effect of one T cell population on the other(s). To model DC-specific T cell competition, normal mice were injected with one or two T cell receptor transgenic CD8 T cell populations, each with high affinity for different peptides presented by different class I MHC complexes. These mice were immunized with DCs pulsed with peptides that are recognized by the transferred T cells. Competition was detectable when both T cell populations were transferred and their target peptides were present on the same DCs. The competition resulted in fewer cells entering the response, but had no effect on the level of activation of the cells that did respond. The effect was evident very early in the response, and in fact the competing T cells needed to be present in the first 5 h of the response for competition to occur. Thus, some aspect of DCs other than peptide/MHC complexes is limiting in the earliest stages of the CD8 T cell response. These results have implications for the design of multivalent vaccines.
Keywords: immunodominance
The T cell repertoire is extremely broad and contains randomly generated T cell receptors (TCRs) that can potentially react with a wide variety of peptide epitopes derived from foreign proteins. However, in response to immunization or infection, the T cell response quickly focuses on a small number of epitopes derived from the antigen, despite the fact that the antigen may have hundreds of potential epitopes, each of which, given individually, is capable of being recognized by T cells. This phenomenon is known as immunodominance and likely serves to ensure that the resources of the immune system are mobilized economically against foreign invaders (1). It would be useful in some instances to overcome the effects of immunodominance and invoke a broader T cell response. In the case of vaccines, it would be desirable to elicit a CD8 cytotoxic T cell response against several pathogen- or tumor-derived antigens with a single vaccine. For tumor immunity, such a strategy would obviate the need for identifying antigens expressed in a particular patient’s tumor and provide a hedge against the tumor losing expression of that antigen caused by selective pressure. However, a combination of CD8 epitopes in a single vaccine has been shown in some cases to result in reduced reactivity compared with separate immunizations (2, 3). Immunodominance is therefore a vital consideration in the design of vaccines, and the relevant mechanisms must be better understood.
There are several mechanisms of immunodominance that take place at almost every level of antigen processing, presentation, and recognition by T cells. For T cell clones of the same specificity, direct competition for peptide/MHC complexes is well documented (4–9). We and others have shown that CD8 T cells of different specificities also compete with each other, (2, 6, 7, 10, 11). For example, work on the conventional B6 CD8 T cell response to BALB.B minor histocompatibility antigens identified responses against dominant epitopes that could “interfere” with the response to subdominant epitopes (10, 11). The response to these subdominant epitopes was restored either in the absence of the dominant response or if the dominant and subdominant epitopes were presented on separate antigen-presenting cells (APCs). Competition between T cells of different specificities has not been observed in all systems tested, however, and its existence is therefore controversial (4, 12, 13). This competition appears to occur at the level of the APC, because it does not occur if the antigens are presented by separate APCs. In contrast, CD4 T cells of different specificities do not appear to compete for APCs in vivo and may even act cooperatively (5, 14).
To resolve this issue, we designed a system to measure the effect of competition between CD8 T cells that recognize different peptides and class I MHC molecules. This system uses TCR transgenic T cells to facilitate analysis of the activation status and fate of responding cells in the presence or absence of competition by a variety of techniques. In this work we show that CD8 T cell competition for different peptide/MHC complexes on the same dendritic cells (DCs) is a very early event in activation, occurring before T cell DNA synthesis and division. Competition reduces the number of T cells recruited into the response and the number of proliferating cells at the peak of the response, but not the activation state of the recruited cells.
Results
T Cells Compete for DCs in Vivo.
To study the ways in which T cells compete for different MHC/peptides on the same DC in vivo, we used T cells from two transgenic mouse strains expressing TCRs specific for different MHCs and peptides: P14, specific for Db + a peptide from lymphocyte choriomeningitis virus (gp33, ref. 15) and OT-I, specific for Kb + a peptide from ovalbumin (16). P14 T cells were labeled with 5-(and 6)-carboxyfluorescein diacetate succinimidyl ester (CFSE) and transferred into C57BL/6 mice in the presence or absence of competing OT-I T cells. The next day, mice were immunized i.v. with peptide-pulsed bone marrow-derived DCs that express both Kb and Db. DCs were loaded with high concentrations of both target peptides (10 μg/ml) to ensure that antigen was not limiting, and the use of T cells that recognize different MHC proteins eliminated the possibility of direct competition for MHC molecules themselves. The effect of the few peptide-specific naïve CD8 T cells in the host C57BL/6 mice should not have been a factor because it has been previously shown that high-affinity TCR transgenic T cells compete with these cells with almost total efficiency (6). In the experiments shown here, a weak agonist version of the gp33 peptide, Y4A (17), was loaded onto the DCs to improve detection of competition; however, similar results were obtained even when using the full agonist peptide (data not shown).
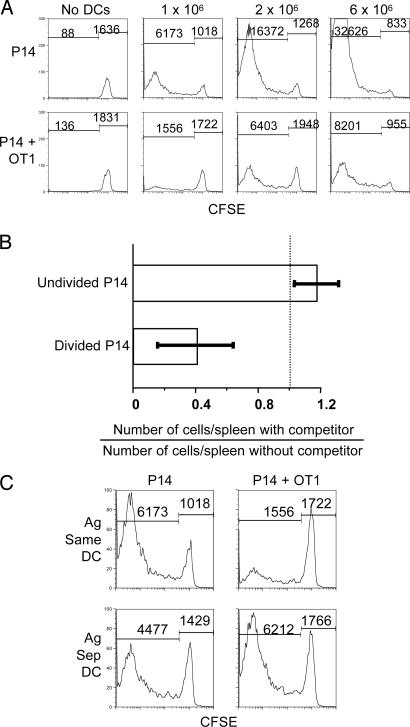
Immunization with 1 × 106 peptide-pulsed DCs in the absence of OT-I cells resulted in substantial accumulation of P14 cells that had divided several times by day 4 as shown by complete loss of their CFSE label. Coinjection of an equal number of OT-I cells reduced the number of CFSElo P14 cells by >3-fold (Fig. 1A and Fig. 5, which is published as supporting information on the PNAS web site). Higher doses of immunizing DCs resulted in larger numbers of proliferating P14 cells, but, even at the highest dose tested, competition was still readily detectable. Competition was also observed at the level of recruitment into the response, as measured by the numbers of P14 cells that did not divide at all (Fig. 1 A and B). The T cells competed for some aspect of the DCs, because simultaneous immunization with two separate DC populations, one pulsed with Y4A and the other pulsed with ovalbumin, did not result in competition (Fig. 1C). Experiments with CFSE-labeled OT-I cells, and P14 cells as the competing T cells, gave similar results, indicating that both populations show decreased recruitment in the presence of the other and that the ability to compete is not specific to one of the T cell populations (Fig. 6, which is published as supporting information on the PNAS web site).
Fig. 1.
T cells compete for antigen-bearing DCs in vivo. (A) A total of 1 × 107 P14.Thy1.1 spleen cells labeled with CFSE were transferred into C57BL/6 mice in the presence or absence of equal numbers of OT-I spleen cells. The next day, mice were immunized i.v. with various numbers of bone marrow-derived DCs pulsed with both ovalbumin and Y4A peptides. Spleen cells were analyzed 4 days after immunization. Histograms are gated on CD8, Thy1.1+, and class II MHC− cells. The number of events is noted above histogram regions. (B) The number of P14 cells per spleen that had not divided or divided at least once was determined for multiple experiments performed as in A after immunizing with 2 × 106 DCs. The number of P14 cells in the presence of OT-I cells was divided by the number in the absence of competitor. This ratio was averaged for four experiments for a total of 10 mice per group. (C) Experiment was performed as in A except that mice were immunized with 2 × 106 DCs pulsed with both peptides (same DC) or two populations of DCs pulsed separately with each peptide (separate DC, 2 × 106 each).
Competition Does Not Affect the Activation State of Responding T Cells.
We next asked whether activation marker expression on the CD8 T cells was altered by competition. CD122 (IL-2/15Rβ) and intracellular IFN-γ, markers of fully activated CD8 T cells, were measured 4 days after immunization (Fig. 2A and B). CD122 and IFN-γ expression increased in the cells that had proliferated more, as measured by dilution of CFSE, but unexpectedly, the extent of CD122 and IFN-γ expression by the P14 cells that had divided was unaffected by the presence of competing OT-I cells. This result suggests that CD8 T cell competition for DCs occurs early, rather than late, in the response. Therefore we measured the effects of competition on early events, such as initial proliferation and Bcl-XL up-regulation, 2 days after immunization. As before, the competition reduced the numbers of dividing P14 cells, but activation marker expression at any given cycle of proliferation was identical in P14 CD8 T cells in the presence or absence of competing OT-I cells (Fig. 2C and Fig. 7, which is published as supporting information on the PNAS web site). Unaffected up-regulation of Bcl-XL, in particular, indicated that the competed P14 cells received similar costimulatory signals (18). The fact that CD44 and CD122 levels were initially low on P14 (Fig. 2C) and OT-I (data not shown) cells suggests that the response is dominated by naïve CD8 T cells. Competition did not result from cytotoxic lysis of DCs by the transferred cells because the number of immunizing DCs present in the spleen after 2 days did not change in the presence or absence of antigen or either T cell population (data not shown).
Fig. 2.
Activation marker expression on and effector function of the proliferating P14 T cells are unaffected by competition. (A and B) Experiments were performed similarly to Fig. 1A, and CFSE intensity was plotted against CD122 (A) or intracellular IFN-γ (B). The mean fluorescence intensity (MFI) for CD122 expression in cells that have completely lost CFSE staining (region shown on dot plot) is noted in A. Histograms are shown for intracellular IFN-γ expression in fully divided P14 cells in B. Mice were immunized with 2 × 106 DCs. (C) CFSE-labeled P14.Thy1.1 cells and OT-I cells were transferred into mice and immunized with unpulsed or peptide-pulsed DCs as shown, and spleen cells were tested for CD44 and Bcl-XL expression at 44–48 h. MFI for CD44 and Bcl-XL are plotted for undivided cells and cells in rounds 1–4 of division. ■, P14 alone; ●, P14 + OT-I.
T Cells Proliferate at the Same Rate in the Presence or Absence of Competition.
Competition reduces the number of dividing T cells but not the activation state of these cells. This effect could be because competition reduces the number of cells that enter the cell cycle (as suggested by the results in Fig. 1), competition reduces the rate of division of cells, or competition increases the rate of cell death. The last of these explanations is unlikely, because competition is evident within 44 h and activated T cells are not thought to start dying until a later time (19). To check the first two possibilities, we determined the percentage of transferred cells that entered the cycle and the average number of cell divisions per dividing cell 2 days after immunization. Indeed, competition reduced the numbers of cells that entered the cycle (Fig. 3A Upper) and had a small effect on the average number of divisions for the cells that actually did enter the cycle (Fig. 3A Lower). To verify this result, we combined CFSE dilution with measurements of BrdU incorporation into the DNA of cycling cells. Experiments were performed as above, except that mice were injected i.p. with BrdU 4 h before death (Fig. 3B). At 18 h after injection of DCs, P14 cells had not started to synthesize DNA because they had not incorporated detectable amounts of BrdU. By 44 h, P14 cells had divided four to five times in 26 h, indicating a cell cycle time of <6 h, a remarkable rate of proliferation for mammalian cells that is nevertheless consistent with other reports (20, 21). At that time point cells were still synthesizing DNA as witnessed by incorporation of BrdU. By 92 h, BrdU incorporation was restricted to the cells that had lost all of the CFSE label, with little or no incorporation in cells at earlier cycle numbers. This result is surprising in light of the observation that fully activated CD8 T cells are programmed to divide extensively (22–24) and may reflect weaker activation of some cells because of intraclonal competition (20, 25). None of these results were affected by the presence of competitor OT-I cells. These results suggest that competition between CD8 T cells of different specificities acts at the level of recruitment, and that once recruited, cells are resistant to competition.
Fig. 3.
P14 cells that are recruited into the response proliferate to the same extent in the presence or absence of competition. (A) CFSE-labeled P14.Thy1.1 cells were transferred into mice with or without OT-I cells, and mice were immunized with 2 × 106 peptide-pulsed DCs. Spleen cells were tested for proliferation at 48 h. The proliferation platform function of FlowJo was used to determine the number of P14 cells that divided at least once and the average number of divisions for those cells. Multiple experiments were combined for a total of nine mice per group. (B) Cell transfer and immunization was performed as in A, and spleen cells were analyzed at 18, 44, and 96 h as shown. Four hours before death, mice were injected i.p. with 1 mg of BrdU in 100 μl of PBS. Cells were analyzed as in other figures except intracellular staining for BrdU incorporation was performed. MFI for BrdU staining is shown for undivided P14 cells and at rounds 1–4 of cell division for the 44-h time point. ■, P14 alone; ●, P14 + OT-I.
Competition Occurs Within the First Few Hours of the Immune Response.
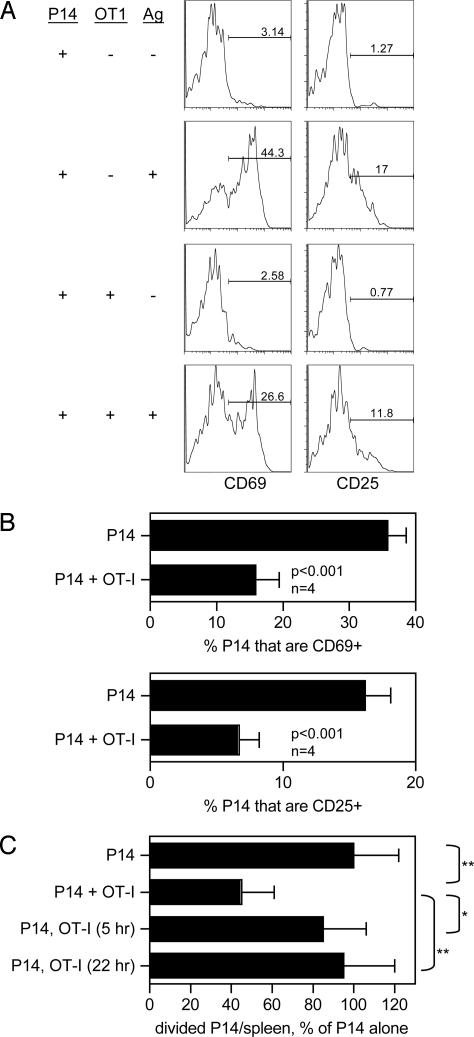
How early in the CD8 T cell response does competition take place? Cell division begins later than 18 h postimmunization (Fig. 3B), so we measured differences in the expression of early activation markers at this time point to determine whether competition is detectable before DNA synthesis. Fig. 4A shows representative data from staining of P14 cells for CD69 and CD25 (IL-2Rα) 18 h after immunization. The percentages of P14 cells expressing these markers were averaged for four mice (Fig. 4B). The results show that competitor OT-I cells significantly reduced the numbers of CD69- and CD25-bearing P14 cells. Therefore, competition occurs earlier than the onset of cell division. To address this question another way, mice were injected with competitor OT-I cells at the same time as, or after immunization, and P14 T cell division was measured at 48 h. Transfer of OT-I cells simultaneously with P14 cells resulted in competition as expected (Fig. 4C), and transfer at the time of immunization gave identical results (Fig. 8, which is published as supporting information on the PNAS web site). However, if OT-I cells were transferred as little as 5 h after immunization, competition was no longer observed. These results show that CD8 T cell competition for DCs exerts its effect in the first 5 h after immunization, and also that this form of competition is not effective after this time.
Fig. 4.
T cell competition occurs very early in the initiation of the response. (A) CFSE-labeled P14.Thy1.1 cells were injected with or without OT-I cells. Peptide-pulsed DCs and spleen cells were tested for expression of the activation markers CD69 and CD25 at 18 h. (B) Similar experiment to A except that results were averaged for mice with or without cotransfer of OT-I cells. (C) Cell transfer was performed as in other experiments except that OT-I cells were cotransferred simultaneously with P14.Thy1.1 cells or at the indicated times after immunization. n = 6–8, normalized results of two experiments. Statistical comparisons are shown. *, P < 0.01; **, P < 0.001.
Discussion
Much of the published work on immunodominance concentrates on the efficiency of processing and presentation of epitopes from the antigen. It is important to consider, however, that the dominance of a particular epitope is not necessarily linked to the abundance of the epitope on the surface of the APC (26) and that the absence of an epitope or MHC molecule can lead to the conversion of a subdominant epitope to a dominant epitope (27, 28). The results described here show that CD8 T cell immunodominance caused by competition between T cell clones for DCs can occur in the first few hours of a response. The competition occurs even though the responding T cells recognize different peptides bound to different class I MHC molecules and requires that the competing T cells react with the same DC, because presentation of the peptides on different DC populations eliminates the competition. Competition, therefore, does not result from a lack of space or some other generally available factor in the spleen, but must be caused by some resource provided by the DCs that is limiting.
Our model of CD8 T cell competition requires that the competing T cells be present in the first hours of the response. After this time the cells that have been recruited are unaffected by competition. This finding is compatible with previous work showing that T cells that recognize antigen early tend to dominate the response (29) and the fact that antigen presentation by DCs takes place within the first 24–48 h after immunization (30–32), although the remarkable speed at which this competition can take place has not been previously reported to our knowledge. The effect of competition appears to be at the stage of recruitment into the response, not the activation state of recruited cells. This conclusion is based on data taken at 48 h or less after immunization, because with experiments with longer time points (Figs. 1 and 2 A and B) do not allow measurement of individual cell generations and cell yields at longer times are affected by issues, such as cell death and migration from the spleen, other than the initial activation events. T cells that were recruited in the presence of competition displayed similar levels of activation markers at all time points tested. Furthermore, competed T cells had the same rate of proliferation as T cells in the absence of competition. These results are consistent with multiple reports that CD8 T cells require only a brief period of stimulation for full activation (22–24). The model described here uses DCs that are activated in vitro with LPS, a highly potent immunization. It is possible that in other forms of immunization, strength of stimulation, activation of CD4 T cells, and the presence of Toll-like receptor ligands might have an effect on the outcome of competition, if not on the primary response then perhaps on the generation of memory (20, 25, 33–36).
A key factor in studies of T cell competition is the ratio of T cells to DCs, as increasing the availability of DCs and antigen will eventually eliminate the effect of competition (6, 13). Our model uses transfer of a larger than physiological number of antigen-specific CD8 T cells to allow detailed measurements of the extent and quality of their response. We are also aware of a recent report demonstrating that the transfer of large numbers of antigen-specific cells affects the way in which the cells differentiate (37). However, that report addresses the generation of memory, not primary responses, and our results show that the activation profile of responding cells is not affected by the presence of a competing population. As discussed previously, immunodominance at the T cell level is present in some vaccine and infectious models and the results described here will be of use in learning whether competition is responsible for this observation and how responses may be modified.
Competition between CD8 T cells of different peptide/MHC specificities is not as effective as competition between clones of the same specificity and has not been detected in every case where this has been tested. Two such reports measured competition between clones specific for lymphocytic choriomeningitis virus-derived epitopes during acute infection (4, 12). It is possible that the extent of this infection results in a large enough pool of antigen-presenting DCs or other APCs to overwhelm the competition for these cells, but competition for a particular peptide/MHC complex presented by these cells is retained. Another report used a system similar to ours and observed only competition between clones of the same specificity (13). However, when testing competition between clones of different specificities mice were immunized with target peptides pulsed onto separate DC populations and not the same DCs. This immunization would not be expected to result in competition and is in agreement with our results.
We hypothesize that the limiting factor that results in competition is the number of productive interactions that a DC is capable of carrying out with T cells. This idea is surprising, because recent data derived from visualization of T cell/DC interactions in whole lymph nodes by in vivo multiphoton microscopy have shown that a single DC can interact with an incredible number of T cells (500–5,000 cells per h; refs. 38 and 39). This estimate implies that the surface of a DC may not be a particularly limiting resource; however, these experiments do not measure the number of T cell interactions that are productive. It is likely that the effective stimulatory capacity of a DC is not limited by surface area but by the availability of membrane proteins required for full T cell activation. We cannot rule out a soluble factor such as a cytokine as the limiting factor in competition, and there is clearly an upper limit to the expansion of T cell precursors in a given immune response that is not caused by DC availability (4, 5, 40, 41). However, in this and other published reports of competition between T cells of different specificities, the competition was alleviated or eliminated when antigen was provided on separate DCs (2, 6, 7, 10, 42). Such competition may limit the efficacy of multivalent vaccines that deliver multiple CD8 T cell epitopes simultaneously, and care must be taken in the design of such vaccines to maintain as broad of an immune response as possible.
Materials and Methods
Mice and Reagents.
C57BL/6, B6.PL-Thy1a/CyJ (Thy1.1+), and B6.SJL-Ptprca Pep3b/BoyJ (CD45.1+) mice were obtained from The Jackson Laboratory (Bar Harbor, ME). OT-I mice (specific for the ovalbumin peptide SIINFEKL, presented by H-2Kb molecules) (16) were provided by T. Potter (National Jewish Medical and Research Center), and P14 mice (specific for the lymphocytic choriomeningitis virus gp33 peptide, KAVYNFATM, or the weak agonist variant Y4A, KAVANFATM, presented by H-2Db class I molecules) (15) were from P. Ohashi (University of Toronto, Toronto, Canada). OT-I mice were crossed to B6.SJL-Ptprca Pep3b/BoyJ, and P14 mice were crossed to B6.PL-Thy1a/CyJ to generate P14.Thy1.1 and OT-I.CD45.1 mice, respectively. Kb-OVA MHC tetramers were produced in the laboratory at the National Jewish Medical and Research Center as described (43). Peptides were supplied by The Molecular Resource Center at the National Jewish Medical and Research Center. Directly conjugated mAbs were purchased from BD Pharmingen (San Diego, CA) or produced in the laboratory at The National Jewish Medical and Research Center by using Alexa 405 or Alexa 647 protein conjugation kits from Molecular Probes (Eugene, OR). CFSE was from Molecular Probes, and all other reagents were from Sigma–Aldrich (St. Louis, MO).
Cell Preparation, Adoptive Transfer, and Immunization.
Spleen and lymph node cells from TCR transgenic mice were labeled as indicated by incubation in a 1 μM solution of CFSE for 5 min at room temperature. Unincorporated dye was quenched by adding 1/10 volume of FBS. Cells were washed three times, and 1 × 107 cells were injected i.v. into the tail veins of C57BL/6 mice in 200 μl of Hanks’ balanced salt solution (HBSS). DCs were cultured from mouse bone marrow cells as described (6), then pulsed with 10 μg/ml of peptide as indicated for 2 h at 37°C, and activated by a 30-min incubation with 1 μg/ml Salmonella typhosa LPS. DCs were washed three times with HBSS and injected i.v. In some experiments, mice were injected i.p. with 100 μl of a 10 mg/ml solution of BrdU in PBS 4 h before death.
Cell Analysis.
Spleen cells from immunized mice were passed through nylon filters, and red blood cells were lysed. Cells were counted and surface-stained by using directly conjugated mAbs according to standard protocols. For intracellular cytokine staining, spleen cells were cultured for 4 h at 37°C in the presence of the appropriate peptide and 10 μg/ml brefeldin A and stained with a kit from BD Pharmingen. For BrdU staining, cells were surface-stained and then fixed in 4% paraformaldehyde and 0.1% saponin for 10 min at room temperature. Cells were treated with 10% DMSO and 0.1% saponin in PBS for 10 min on ice, fixed again as above for 5 min, and incubated with 300 μg/ml DNase in PBS for 30 min at 37°C. Staining was performed in staining buffer with 0.1% saponin and 1 μg/ml Alexa 647-conjugated anti-BrdU mAb from Molecular Probes (44). For flow cytometric analysis, 106 events were collected on a FACSCalibur (BD Biosciences, San Diego, CA) or a CyAn LX (Dakocytomation, Glostrup, Denmark), and data were analyzed by using FlowJo version 6 or later (Tree Star, Ashland, OR). Samples were gated on CD8 class II MHC− events, and transferred cells were identified by using the appropriate congenic marker or class I MHC tetramer reagent.
Statistics.
Data are presented as mean ± SD, and statistical significance was determined by using Student’s unpaired, equal variance, two-tailed t test with Microsoft Excel (Redmond, WA).
Supplementary Material
Acknowledgments
We thank Eric Clambey, Gwen Murphy, Megan MacLeod, and Tomasz Sosinowski for helpful discussions and comments on the manuscript; Tibor Vass for animal care; Bill Townend, Shirley Sobus, and Josh Loomis for flow cytometry assistance; Fran Crawford and Rachel Fruge for help with tetramer preparation; and Dirk Homann for the BrdU staining protocol. R.A.W. is a Fellow of the Leukemia and Lymphoma Society. This work was supported by U.S. Public Health Service Grants AI-17134, AI-18785, AI-52225, and P30 CA-046934.
Abbreviations
- DC
dendritic cell
- APC
antigen-presenting cell
- TCR
T cell receptor
- CFSE
5-(and 6)-carboxyfluorescein diacetate succinimidyl ester
- MFI
mean fluorescence intensity.
Footnotes
Conflict of interest statement: No conflicts declared.
References
- 1.Yewdell J. W., Bennink J. R. Annu. Rev. Immunol. 1999;17:51–88. doi: 10.1146/annurev.immunol.17.1.51. [DOI] [PubMed] [Google Scholar]
- 2.Palmowski M. J., Choi E. M., Hermans I. F., Gilbert S. C., Chen J. L., Gileadi U., Salio M., Van Pel A., Man S., Bonin E., et al. J. Immunol. 2002;168:4391–4398. doi: 10.4049/jimmunol.168.9.4391. [DOI] [PubMed] [Google Scholar]
- 3.Rosenberg S. A., Sherry R. M., Morton K. E., Yang J. C., Topalian S. L., Royal R. E., Kammula U. S., Restifo N. P., Hughes M. S., Schwarz S. L., et al. J. Immunother. 2006;29:224–231. doi: 10.1097/01.cji.0000190399.98802.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Butz E. A., Bevan M. J. Immunity. 1998;8:167–175. doi: 10.1016/s1074-7613(00)80469-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith A. L., Wikstrom M. E., Fazekas de St Groth B. Immunity. 2000;13:783–794. doi: 10.1016/s1074-7613(00)00076-5. [DOI] [PubMed] [Google Scholar]
- 6.Kedl R. M., Rees W. A., Hildeman D. A., Schaefer B., Mitchell T., Kappler J., Marrack P. J. Exp. Med. 2000;192:1105–1113. doi: 10.1084/jem.192.8.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kedl R. M., Schaefer B. C., Kappler J. W., Marrack P. Nat. Immunol. 2002;3:27–32. doi: 10.1038/ni742. [DOI] [PubMed] [Google Scholar]
- 8.Kedl R. M., Kappler J. W., Marrack P. Curr. Opin. Immunol. 2003;15:120–127. doi: 10.1016/s0952-7915(02)00009-2. [DOI] [PubMed] [Google Scholar]
- 9.Catron D. M., Rusch L. K., Hataye J., Itano A. A., Jenkins M. K. J. Exp. Med. 2006;203:1045–1054. doi: 10.1084/jem.20051954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wolpert E. Z., Grufman P., Sandberg J. K., Tegnesjo A., Karre K. J. Immunol. 1998;161:4499–4505. [PubMed] [Google Scholar]
- 11.Grufman P., Wolpert E. Z., Sandberg J. K., Karre K. Eur. J. Immunol. 1999;29:2197–2204. doi: 10.1002/(SICI)1521-4141(199907)29:07<2197::AID-IMMU2197>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 12.Probst H. C., Dumrese T., van den Broek M. F. J. Immunol. 2002;168:5387–5391. doi: 10.4049/jimmunol.168.11.5387. [DOI] [PubMed] [Google Scholar]
- 13.Kemp R. A., Powell T. J., Dwyer D. W., Dutton R. W. J. Immunol. 2004;173:2923–2927. doi: 10.4049/jimmunol.173.5.2923. [DOI] [PubMed] [Google Scholar]
- 14.Creusot R. J., Thomsen L. L., Tite J. P., Chain B. M. J. Immunol. 2003;171:240–246. doi: 10.4049/jimmunol.171.1.240. [DOI] [PubMed] [Google Scholar]
- 15.Pircher H., Burki K., Lang R., Hengartner H., Zinkernagel R. M. Nature. 1989;342:559–561. doi: 10.1038/342559a0. [DOI] [PubMed] [Google Scholar]
- 16.Hogquist K. A., Jameson S. C., Heath W. R., Howard J. L., Bevan M. J., Carbone F. R. Cell. 1994;76:17–27. doi: 10.1016/0092-8674(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 17.Sebzda E., Kundig T. M., Thomson C. T., Aoki K., Mak S. Y., Mayer J. P., Zamborelli T., Nathenson S. G., Ohashi P. S. J. Exp. Med. 1996;183:1093–1104. doi: 10.1084/jem.183.3.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boise L. H., Minn A. J., Noel P. J., June C. H., Accavitti M. A., Lindsten T., Thompson C. B. Immunity. 1995;3:87–98. doi: 10.1016/1074-7613(95)90161-2. [DOI] [PubMed] [Google Scholar]
- 19.Marrack P., Kappler J. Annu. Rev. Immunol. 2004;22:765–787. doi: 10.1146/annurev.immunol.22.012703.104554. [DOI] [PubMed] [Google Scholar]
- 20.van Stipdonk M. J., Hardenberg G., Bijker M. S., Lemmens E. E., Droin N. M., Green D. R., Schoenberger S. P. Nat. Immunol. 2003;4:361–365. doi: 10.1038/ni912. [DOI] [PubMed] [Google Scholar]
- 21.Kurts C., Kosaka H., Carbone F. R., Miller J. F., Heath W. R. J. Exp. Med. 1997;186:239–245. doi: 10.1084/jem.186.2.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Stipdonk M. J., Lemmens E. E., Schoenberger S. P. Nat. Immunol. 2001;2:423–429. doi: 10.1038/87730. [DOI] [PubMed] [Google Scholar]
- 23.Wong P., Pamer E. G. J. Immunol. 2001;166:5864–5868. doi: 10.4049/jimmunol.166.10.5864. [DOI] [PubMed] [Google Scholar]
- 24.Kaech S. M., Ahmed R. Nat. Immunol. 2001;2:415–422. doi: 10.1038/87720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gett A. V., Sallusto F., Lanzavecchia A., Geginat J. Nat. Immunol. 2003;4:355–360. doi: 10.1038/ni908. [DOI] [PubMed] [Google Scholar]
- 26.DiPaolo R. J., Unanue E. R. J. Immunol. 2002;169:1–4. doi: 10.4049/jimmunol.169.1.1. [DOI] [PubMed] [Google Scholar]
- 27.van der Most R. G., Murali-Krishna K., Lanier J. G., Wherry E. J., Puglielli M. T., Blattman J. N., Sette A., Ahmed R. Virology. 2003;315:93–102. doi: 10.1016/j.virol.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 28.Schirmbeck R., Stober D., El-Kholy S., Riedl P., Reimann J. J. Immunol. 2002;168:6253–6262. doi: 10.4049/jimmunol.168.12.6253. [DOI] [PubMed] [Google Scholar]
- 29.Bousso P., Levraud J. P., Kourilsky P., Abastado J. P. J. Exp. Med. 1999;189:1591–1600. doi: 10.1084/jem.189.10.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ingulli E., Mondino A., Khoruts A., Jenkins M. K. J. Exp. Med. 1997;185:2133–2141. doi: 10.1084/jem.185.12.2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Norbury C. C., Malide D., Gibbs J. S., Bennink J. R., Yewdell J. W. Nat. Immunol. 2002;3:265–271. doi: 10.1038/ni762. [DOI] [PubMed] [Google Scholar]
- 32.Mempel T. R., Henrickson S. E., Von Andrian U. H. Nature. 2004;427:154–159. doi: 10.1038/nature02238. [DOI] [PubMed] [Google Scholar]
- 33.Sun J. C., Bevan M. J. Science. 2003;300:339–342. doi: 10.1126/science.1083317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sun J. C., Williams M. A., Bevan M. J. Nat. Immunol. 2004;5:927–933. doi: 10.1038/ni1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Janssen E. M., Lemmens E. E., Wolfe T., Christen U., von Herrath M. G., Schoenberger S. P. Nature. 2003;421:852–856. doi: 10.1038/nature01441. [DOI] [PubMed] [Google Scholar]
- 36.Castellino F., Huang A. Y., Altan-Bonnet G., Stoll S., Scheinecker C., Germain R. N. Nature. 2006;440:890–895. doi: 10.1038/nature04651. [DOI] [PubMed] [Google Scholar]
- 37.Marzo A. L., Klonowski K. D., Le Bon A., Borrow P., Tough D. F., Lefrancois L. Nat. Immunol. 2005;6:793–799. doi: 10.1038/ni1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miller M. J., Hejazi A. S., Wei S. H., Cahalan M. D., Parker I. Proc. Natl. Acad. Sci. USA. 2004;101:998–1003. doi: 10.1073/pnas.0306407101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bousso P., Robey E. Nat. Immunol. 2003;4:579–585. doi: 10.1038/ni928. [DOI] [PubMed] [Google Scholar]
- 40.Blattman J. N., Antia R., Sourdive D. J., Wang X., Kaech S. M., Murali-Krishna K., Altman J. D., Ahmed R. J. Exp. Med. 2002;195:657–664. doi: 10.1084/jem.20001021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Laouar Y., Crispe I. N. Immunity. 2000;13:291–301. doi: 10.1016/s1074-7613(00)00029-7. [DOI] [PubMed] [Google Scholar]
- 42.Hamilton S. E., Harty J. T. J. Immunol. 2002;169:4936–4944. doi: 10.4049/jimmunol.169.9.4936. [DOI] [PubMed] [Google Scholar]
- 43.Crawford F., Kozono H., White J., Marrack P., Kappler J. Immunity. 1998;8:675–682. doi: 10.1016/s1074-7613(00)80572-5. [DOI] [PubMed] [Google Scholar]
- 44.Lenz D. C., Kurz S. K., Lemmens E., Schoenberger S. P., Sprent J., Oldstone M. B., Homann D. Proc. Natl. Acad. Sci. USA. 2004;101:9357–9362. doi: 10.1073/pnas.0400640101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.