Abstract
Previous in vitro studies have shown that the neurotransmitter glutamate is important in brain development. Paradoxically, loss-of-function mouse models of glutamatergic signaling that are generated by genetic deletion of glutamate receptors or glutamate release show normal brain assembly. We examined the direct consequences on brain development of extracellular glutamate buildup due to the depletion of the glutamate transporters GLAST and GLT1. GLAST/GLT1 double knockout mice show multiple brain defects, including cortical, hippocampal, and olfactory bulb disorganization with perinatal mortality. Here, we report abnormal formation of the neocortex in GLAST/GLT1 mutants. Several essential aspects of neuronal development, such as stem cell proliferation, radial migration, neuronal differentiation, and survival of SP neurons, were impaired. These results provide direct in vivo evidence that GLAST and GLT1 are necessary for brain development through regulation of extracellular glutamate concentration and show that an important mechanism is likely to be maintenance of glutamate-mediated synaptic transmission.
Keywords: axon/dendrite development, cortex, radial fiber
Neuronal activity is important in the process of refining neural connections in the developing brain (1). Activity, however, may also act to influence earlier developmental events, such as proliferation, migration, differentiation, and survival (2, 3). As key mediators of neuronal activity, neurotransmitters released by neuronal activity are thought to have important signaling roles in shaping the early development of the CNS (3). Previous observations suggest that the major excitatory neurotransmitter, glutamate, provides important communication signals in the developing brain. Indeed, glutamate has been shown to modulate cell proliferation, radial migration, neuronal survival linked to apoptosis, and neuronal differentiation (4–7). In contrast to these observations, most studies in which glutamatergic activity was blocked, whether at the level of ligand or receptor, have demonstrated little, if any, developmental defects (8–14). Thus, according to in vivo experiments using loss-of-function models, early glutamate signaling appears to be dispensable. However, because compensatory mechanisms, coupled with a redundancy in glutamate receptor mechanisms, could reduce the severity of a brain phenotype in loss-of-function models, glutamate may still play a role at an early stage of brain development. To investigate this issue, we generated a genetically manipulated animal in which glutamate receptors are overstimulated by genetic deletion of glutamate transporters. Glutamate transporters are essential for the maintenance of low extracellular levels of glutamate (15). The glutamate transporters GLAST, GLT1, and EAAC1 are expressed in the embryonic mouse CNS (16). Previous studies demonstrated that mice lacking GLAST, GLT1, or EAAC1 have seemingly normal brain development (17–19). These results raised the possibility that glutamate transporter subtypes can functionally substitute for one another in CNS development. In the present study, we inactivated two members of the glutamate transporter family to elucidate roles of the glutamate system in CNS development.
Results
GLAST−/−/GLT1−/− Mutants Have Multiple Brain Defects.
Normal development of the CNS was observed in mutant mice lacking GLAST/EAAC1 or EAAC1/GLT1 (data not shown). By contrast, mice lacking GLAST/GLT1 died in utero [around embryonic days (E17–18)] and exhibited abnormal brain development after E15 (Fig. 1; see also Figs. 8 and 9, which are published as supporting information on the PNAS web site). The lateral ventricles in GLAST−/−/GLT1−/− mice were dilated relative to those in control mice, and alterations in the structure of the pallial–subpallial boundary were observed in the mutant mice (Fig. 1 A–D).The E16 neocortex is laminated, with the following layers: marginal zone, cortical plate (CP), subplate (SP), intermediate zone, and ventricular zone (VZ) (Fig. 1E). In the GLAST−/−/GLT1−/− mutants, the CP border on the intermediate zone was obscured, and the SP could not be identified (Fig. 1F). In addition, cell number in the VZ was decreased in mutant brains (23,558.3 ± 111.6 cells per mm2 for WT; 20,216.6 ± 119.7 cells per mm2 for the double mutant; n = 4; P < 0.0001; Fig. 1 E and F). Moreover, there were distortions in the organization of the hippocampus and the olfactory bulb of GLAST−/−/GLT1−/− mice (Fig. 8). In the hippocampus, pyramidal neurons were less densely packed. In the olfactory bulb of GLAST−/−/GLT1−/− mice, the mitral cell layer was absent. The present study focused on the cortical malformation of GLAST−/−/GLT1−/− mice.
Fig. 1.
Morphological abnormalities in brains of GLAST−/−/GLT1−/− mutants. Coronal sections of whole brain (A–D) and neocortex (E and F) at E16 from WT (A, C, and E) and GLAST−/−/GLT1−/− double mutant (DM) (B, D, and F) embryos stained with hematoxylin. Images in A and B are located anterior to those in C and D. Boxed regions in C and D are enlarged in E and F, respectively. The corpus callosum did not cross the midline, and a Probst bundle (asterisk in B) was formed. Arrowhead in C indicates the anterior commissure, which was absent in GLAST−/−/GLT1−/− mutants. Delamination of the pallial–subpallial junction can be seen in the region between arrows in B and D. MZ, marginal zone; IZ, intermediate zone. (Scale bars: A, 500 μm; E, 100 μm.)
Expression of GLT1 and GLAST in the Embryonic Brain.
GLAST is an astrocytic glutamate transporter in the adult CNS (20). Previous immunocytochemical studies have shown that GLT1 is strongly expressed, primarily by astrocytes, in the adult brain (20). However, we have recently demonstrated that GLT1 is also expressed by some neurons in the hippocampus (21). Because the two transporters show dynamic changes in expression during CNS development (16), we examined cellular elements expressing the two transporters in the embryonic cortex of C57BL mice by immunohistochemistry with subtype-specific antibodies. At E16, GLT1 was expressed in the globus pallidus, perirhinal cortex, lateral hypothalamus, hippocampus, and fimbria and in the axonal pathways interconnecting the neocortex, basal ganglia, and thalamus (Fig. 2 A and C). In the cerebral cortex, GLT1 immunoreactivity was seen in the SP and along fiber bundles in the intermediate zone (Fig. 2C; see also Fig. 10A, which is published as supporting information on the PNAS web site). To investigate further the cellular localization of GLT1 in the cortex, we performed double immunostaining of GLT1 with GAP-43 (growth-associated protein 43), a neuronal marker. GLT1 immunoreactivity was double-labeled by GAP-43, suggesting that GLT1 was expressed in neurons at E16 (Fig. 10). In contrast, GLAST protein was expressed in radial glial cells in the VZ of telencephalon and diencephalon at E16 (Fig. 2 B and D). GLAST immunoreactivity was also found in a palisade of radial glial fibers originating in the VZ near the lateral ganglionic eminence–pallium angle and coursing to the pial surface (Fig. 2B). GLT1 and GLAST are thus localized during development on neurons and radial glial cells, respectively, suggesting that the two glutamate transporters might play cooperative and complementary roles in neural development (22). Although GLT1 accounts for ≈94% of the total glutamate uptake activity in the adult forebrain (17), both GLAST and GLT1 are major glutamate transporters in the embryonic brain. Therefore, brain development is disturbed in GLAST/GLT1 double knockout mice but not in GLAST or GLT1 knockout mice.
Fig. 2.
GLT1 and GLAST immunoreactivity in the mouse forebrain at E16. (A and C) GLT1 was expressed in the globus pallidus, perirhinal cortex, lateral hypothalamus, hippocampus, and fimbria and the axonal pathways interconnecting the neocortex, basal ganglia, and thalamus. (B and D) In contrast, strong GLAST immunoreactivity was seen in radial glial cells and the radial glial fascicle. Boxed regions in A and B are enlarged in C and D, respectively. Arrows in B indicate the radial glial fascicle. AC, anterior commissure; LOT, lateral olfactory tract. (Scale bars: 100 μm.)
Cell Birth and Death in the GLAST−/−/GLT1−/− Cortex.
A decrease in cell number in the VZ of mutant brains could be caused by reduced cell proliferation or accelerated cell death. To assess the proliferation profile of mutant neuronal progenitors, embryos were pulse-labeled in utero with BrdU. At E14, the percentage of BrdU-positive cells in the VZ was similar in WT and mutant mice (25.0 ± 1.7% for WT; 26.0 ± 2.3% for double mutant; n = 6; Fig. 3 A and B). At E16, however, the percentage of BrdU-positive cells in the VZ was decreased in mutants (18.3 ± 0.3% for WT; 16.2 ± 0.4% for double mutant; n = 4; P < 0.01; Fig. 3 C and D). Proliferation of neuronal progenitor cells was also examined by immunohistochemistry for proliferating cell nuclear antigen (PCNA), a marker of proliferating cells. The pattern of PCNA immunostaining replicated the BrdU results (Fig. 3 E–H).This finding is consistent with the results of a previous in vitro study that showed that application of glutamate decreased the number of embryonic cortical cells that incorporate BrdU (5). To determine whether cell death was increased in the cortex of double mutants, we used the TUNEL method to stain apoptotic cells. Apoptosis was not increased in the neocortex of mutants at E14 or E16 (data not shown). These results suggest that both GLT1 and GLAST regulate neurogenesis by controlling the extracellular glutamate concentration at E16.
Fig. 3.
Reduced cell proliferation and abnormal neural migration in the GLAST−/−/GLT1−/− mutant neocortex. Immunoreactivities of BrdU and PCNA are visualized as green fluorescence. (A–J and M and N) All nuclei were counterstained with propidium iodide (PI) (red). Thus, the BrdU- and PCNA-positive cells appear yellow because they are colabeled for PI and BrdU or PCNA. However, some BrdU- and PCNA-positive cells appear green, because green fluorescence is intensified for easy identification of the BrdU- and PCNA-positive cells. There were a comparable number of BrdU-positive cells in WT (A) and mutant (B) E14 cortices. However, at E16, the number of BrdU-positive cells was reduced in mutant (D) compared with WT (C) cortex. (E–H) PCNA expression at E14 (E and F) and E16 (G and H). BrdU was injected at E12 (I and J) or E14 (M and N), followed by examination of the distribution of BrdU-positive cells at E16. (I–P) To quantify migration, we counted heavily labeled cells (first generation at time of BrdU injection) at E16. The cortices were divided into eight equal areas (numbered 1–8). The percentage of BrdU-positive cells (percentage of total) in each area was determined, and results were plotted as histograms of WT (K and O) and GLAST−/−/GLT1−/− double mutants (DM) (L and P). (Scale bars: 100 μm.)
Migration of CP Neurons Is Impaired in the GLAST−/−/GLT1−/− Cortex.
The disturbed laminar organization of the GLAST−/−/GLT1−/− mutant cortex suggested that cortical cell migrations were abnormal. To investigate neuronal migration in vivo, we injected pregnant mice at E12 or E14 with BrdU and examined the labeling patterns at E16. The mutant E12 neurons were spread in a broader gradient compared with WT (Fig. 3 I–L), and the mutant E14 neurons failed to migrate to the CP and remained in the VZ (Fig. 3 M–P). Thus, radial migration is impaired in GLAST−/−/GLT1−/− mutants. Correct neuronal migration requires both the radial glial fibers, which guide postmitotic neurons during their migration, and Cajal–Retzius neurons, which secrete the Reelin protein and thus have a critical role in radial migration. The alignment and density of radial glial fibers stained with anti-nestin antibody were comparable in WT and mutant E14 cortices (Fig. 4 A and B), but the disruption of radial fibers was apparent in mutant E16 cortices (Fig. 4 C and D). SEM analysis also revealed that, at E16, the radial glial fibers in the cerebral wall were disrupted in GLAST−/−/GLT1−/− mutants (Fig. 4 E and F). Furthermore, radial glial cell arrangement was severely disorganized in the VZ of GLAST−/−/GLT1−/− mutants (Fig. 4 G and H). These cells had lost radial morphology but had become round in shape. By contrast, neither the number of Cajal–Retzius neurons nor their immunolabeling intensity for Reelin was changed in the GLAST−/−/GLT1−/− cortex (Fig. 4 I and J). These data suggest that a disrupted radial glial fiber system contributes to the abnormal radial migration of GLAST−/−/GLT1−/− mutants.
Fig. 4.
Altered radial glial systems and normal Reelin expression in GLAST−/−/GLT1−/− mutants. (A–D) Nestin staining of E14 and E16 cortices. The pattern and distribution of radial glial fibers is comparable in WT (A) and double mutant (DM) (B) mice at E14. At E16, however, disruption of radial fibers was apparent in mutant cortices (D) compared with WT (C). (E–H) SEM analysis was performed with E16 cortices. (G) In WT mice, radial glial cells are aligned radially in the VZ. (E) Their radial fibers make bundles and run perpendicular to the pial surface. (F) In GLAST−/−/GLT1−/− mutants, the alignment and density of radial glial fibers are destroyed. (H) In addition, cells in the VZ lost radial morphology. (I and J) In contrast, Reelin expression (green) was comparable in WT (I) and mutant (J) mice at E16. Counterstaining of nuclei was performed with propidium iodide (red). MZ, marginal zone. (Scale bars: A, C, and I, 100 μm; E and G, 5 μm.)
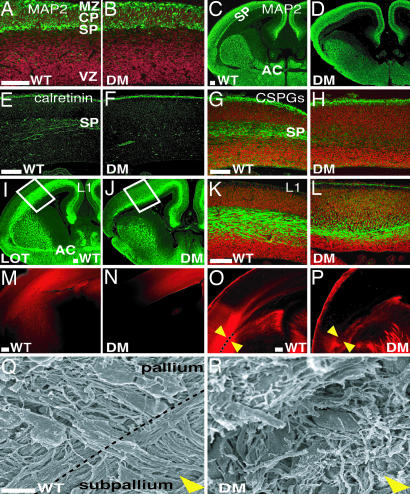
Lack of SP Neurons and Defective Cortical Connections in the GLAST−/−/GLT1−/− Mutants.
In the double mutants, the SP is difficult to discern (Fig. 1F). Because SP neurons are vulnerable to excitotoxic cell death (23), it is possible that GLAST−/−/GLT1−/− mutants may exhibit SP defects. To investigate possible SP defects in double mutants, we studied microtubule-associated protein 2 (MAP2)-positive SP neurons and expression of SP-specific markers, calretinin and chondroitin sulfate proteoglycans (CSPGs). In double mutants, no MAP2-positive SP neurons were detected at E14 or E16 (Fig. 5A–D). Furthermore, calretinin and CSPGs were scarcely present in the SP at E16 (Fig. 5 E–H). These results suggest a lack of mature SP neurons in double mutants. SP neurons have been implicated in the development of cortical afferent and efferent connections, including the corticothalamic (CT) and thalamocortical (TC) pathways (23–26). To study these pathways, we used L1 immunostaining and 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine (DiI) tracing. In WT brains, L1-positive fascicles of CT axons pass through the striatum (Fig. 5I). WT L1-positive TC axons leave the diencephalons for the internal capsules and subsequently enter the cortex at E16 (Fig. 5 I and K). In double mutants, L1-poisitive TC axons scarcely innervated the cortex (Fig. 5 J and L). DiI injection in the cortex at E16 revealed that CT axons did not exit the telencephalon in GLAST−/−/GLT1−/− mutants (Fig. 5 M and N). DiI injection in the thalamus at E16 confirmed that TC axons did not enter the GLAST−/−/GLT1−/− cortex (Fig. 5 O and P). SEM analysis of GLAST−/−/GLT1−/− double mutants revealed that, at E16, the radial glial fascicle at the pallial–subpallial boundary was absent and that CT and TC axons could not cross the pallial–subpallial boundary (Fig. 5 Q and R). The corpus callosum did not cross the midline but formed a Probst bundle (Fig. 1B). Also, the anterior commissure was absent in mutants (Fig. 1D). These results indicate that the TC, CT, and callosal projections are severely affected in GLAST−/−/GLT1−/− mutants.
Fig. 5.
Loss of SP neurons and impaired cortical connections in GLAST−/−/GLT1−/− mutants. (A–D) MAP2 staining of E14 (A and B) and E16 (C and D) cortices reveals that MAP2-positive SP neurons are difficult to discern in GLAST−/−/GLT1−/− mutants (B and D) at E14 and E16 compared with WT (A and C). (E–H) Immunostaining (green) against calretinin (E and F) and chondroitin sulfate proteoglycans (CSPGs) (G and H) on coronal sections of E16 WT (E and G) and GLAST−/−/GLT1−/− mutant (F and H) cortices shows that SP neurons are mostly missing in mutants. (I–L) Immunostaining (green) against L1 on coronal sections of E16 WT (I and K) and GLAST−/−/GLT1−/− mutant (J and L) cortices reveals defective cortical connections. Boxes in I and J are enlarged in K and L, respectively. All nuclei were stained with propidium iodide (red) in A, B, G, H, K, and L. (M–P) DiI labeling (red) from cortex (M and N) and from thalamus (O and P) at E16 also confirms that TC and CT projections are severely affected in GLAST−/−/GLT1−/− mutants. (M and O) WT. (N and P) GLAST−/−/GLT1−/− mutants. (Q and R) SEM analysis was performed for the region indicated by arrowheads in O and P of E16 WT (Q) and GLAST−/−/GLT1−/− mutant (R) brain. In GLAST−/−/GLT1−/− mutants, the radial glial fascicle at the pallium–subpallium junction is absent, and TC and CT axons cannot cross the junction. DM, double mutant; MZ, marginal zone; AC, anterior commissure; LOT, lateral olfactory tract. (Scale bars: A, C, E, G, I, K, M, and O, 100 μm; Q, 5 μm.)
Maturation of CP Neurons Is Impaired in the GLAST−/−/GLT1−/− Mutants.
At E16, pyramidal-like retrogradely labeled cells in the CP after a DiI injection in thalamus were observed in WT mice (Fig. 6A). In contrast, the morphology and neurite outgrowth of the retrogradely labeled cells in the CP were affected in mutant mice (Fig. 6B). To determine the onset of these changes, we examined E14 GLAST−/−/GLT1−/− mutant cortex. Although hematoxylin staining did not show abnormal morphology of CP neurons in the GLAST−/−/GLT1−/− mutants at E14 (Fig. 6 C and D), SEM analysis of GLAST−/−/GLT1−/− mutants showed that, at E14, the radial distribution of the CP neurons and their neurites was not conspicuous; these cells had lost their pyramidal-like morphology and had become round in shape (Fig. 6 E and F). In contrast, the alignment and density of radial glial cells in the VZ were comparable in WT and mutant E14 cortices (Fig. 6 G and H). These results indicate that maturation of CP neurons is impaired in the GLAST−/−/GLT1−/− mutants from E14 onward, whereas abnormal maturation of radial glial cells in the VZ was apparent at E16 (Fig. 4 G and H).
Fig. 6.
Impaired maturation of CP neurons in GLAST−/−/GLT1−/− mutants. (A) At E16, pyramidal-like retrogradely labeled cells are observed in the CP of WT mice after DiI injection in the thalamus. (B) In mutants, the morphology and neurite extension of the retrogradely labeled cells were affected. (C and D) Coronal sections of neocortex at E14 from WT mice (C) and GLAST−/−/GLT1−/− mutants (D) stained with hematoxylin. (E–H) SEM analysis was performed with E14 cortices. Although the hematoxylin staining could not reveal impaired maturation of CP neurons in mutants at E14, SEM analysis revealed that the cellular morphology was affected in GLAST−/−/GLT1−/− mutants (F) compared with WT (E). In contrast, the cellular morphology in the VZ was comparable in WT (G) and mutant (H) E14 cortices. DM, double mutants. (Scale bars: A and C, 100 μm; E and G, 5 μm.)
Partial Rescue of the GLAST−/−/GLT1−/− Brain Phenotypes by Glutamate Receptor Antagonists.
Because we previously showed that basal levels of extracellular glutamate in the hippocampus of GLT1 mutant mice were significantly higher than those of WT mice (27), it is reasonable to expect that genetic deletion of both GLT1 and GLAST would bring about an increase in extracellular glutamate levels, resulting in cortical malformation by excess activation of glutamate receptors. To assess this hypothesis, we first examined whether expression of glutamate receptors is affected in the GLAST−/−/GLT1−/− mutant cortex. The relative expression of the glutamate receptors GluR1, GluR2, and GluR4 and NMDA receptor 1 was unchanged in GLAST−/−/GLT1−/− mice compared with WT animals (n = 3 for each) (Fig. 7A). Next, we examined whether the GLAST−/−/GLT1−/− brain phenotype could be reversed by pharmacological administration of glutamate receptor antagonists. Injections of both the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor antagonist 2,3-dihydroxy-6-nitro-7-sulfamoylbenzo[f]quinoxaline (NBQX) and the NMDA receptor antagonist CGS-19755 in pregnant mice between E8 and E16 resulted in a partial rescue of the abnormal stratification of the mutant cerebral cortex (Fig. 7 B–D) and hippocampus (Fig. 7 E and G). In GLAST−/−/GLT1−/− mutants treated with glutamate receptor antagonists, the cerebral cortex showed some restoration of laminar structure, although it remained less organized than in WT mice (Fig. 7 B–D). The hippocampus of GLAST−/−/GLT1−/− mice is characterized by loose packing of pyramidal neurons (Fig. 7F). In contrast, GLAST−/−/GLT1−/− mice treated with glutamate receptor antagonists had hippocampal formations that seemed almost indistinguishable from WT hippocampus (Fig. 7 E and G). The excess activation of the NMDA receptors in GLAST−/−/GLT1−/− mutant brains was also suggested by the examination of expression levels of NMDA receptor-regulated gene 1 (NARG1). A previous study demonstrated that NARG1 is down-regulated by NMDA receptor activation (28). We found that NARG1 mRNA expression was down-regulated in GLAST−/−/GLT1−/− mutant brains by in situ hybridization (Fig. 7 H and I) and real-time quantitative PCR (Fig. 11, which is published as supporting information on the PNAS web site). Injection of the NMDA receptor antagonist CGS-19755 alone could not rescue the GLAST−/−/GLT1−/− brain phenotypes. Moreover, both the AMPA receptor antagonist and the NMDA receptor antagonist only partially rescued the cortical malformation of mutant mice, suggesting that, in addition to excess activation of both AMPA and NMDA receptors, overactivation of other glutamate receptors, including metabotropic glutamate receptors, may contribute to the multiple severe defects in GLAST−/−/GLT1−/− mutants.
Fig. 7.
Partial rescue of the GLAST−/−/GLT1−/− mutant brain phenotype by injection of glutamate receptor antagonists. (A) The relative expression of the glutamate receptors GluR1, GluR2, and GluR4 and NMDA receptor 1 was unchanged in GLAST−/−/GLT1−/− mice compared with WT animals. (B–G) Coronal sections of cortex (B–D) and sagittal sections of hippocampus (E–G) of E16 WT (B and E), GLAST−/−/GLT1−/− (C and F), and GLAST−/−/GLT1−/− mice treated from E8 to E16 with 2,3-dihydroxy-6-nitro-7-sulfamoylbenzo[f]quinoxaline (NBQX) and CGS-19755 (D and G) stained with hematoxylin. Injections of both NBQX and CGS-19755 resulted in a partial rescue of the abnormal stratification of the mutant cerebral cortex and hippocampus. Arrowheads in E–G indicate the pyramidal cell layer in the hippocampus. (H and I) In situ hybridization using NARG1 riboprobe on coronal sections of E16 WT (H) and GLAST−/−/GLT1−/− mutants (I). The expression of NARG1 was down-regulated in mutants. DM, double mutants; IZ, intermediate zone. (Scale bar: 100 μm.)
Oxidative Glutamate Toxicity Does Not Contribute to the Cortical Malformation of GLAST−/−/GLT1−/− Mice.
Excessive extracellular glutamate leads to cell injury by means of both glutamate receptor-mediated and glutamate receptor-independent mechanisms (29). Glutamate receptor-independent toxicity is caused by oxidative glutamate toxicity. In oxidative glutamate toxicity, high levels of glutamate block the cystine/glutamate exchange system Xc−, resulting in glutathione depletion and cell injury (30). To determine whether oxidative glutamate toxicity is involved in the cortical malformation of GLAST−/−/GLT1−/− mice, we measured the total cortical glutathione levels. Total glutathione levels were slightly increased in the cortex of GLAST−/−/GLT1−/− mice at E16 compared with WT levels (Fig. 12, which is published as supporting information on the PNAS web site), demonstrating that oxidative glutamate toxicity does not play a significant role in the cortical malformation of GLAST−/−/GLT1−/− mice.
Discussion
A large body of in vitro evidence indicates that the neurotransmitter glutamate acts to influence earlier developmental events, such as proliferation, migration, and differentiation (4–7). However, nearly all of the genetic experiments to date, in which glutamatergic signaling was blocked, have shown little, if any, developmental defects (8–14). Our work represents a unique analysis of the direct consequences on brain development of extracellular glutamate buildup due to the depletion of glutamate transporters. In contrast to loss-of-function studies, in vivo excess activation of glutamate receptors can modulate brain maturation, such as stem cell proliferation, radial migration, survival of SP neurons, and neuronal differentiation, including neurite elongation and path finding. This discrepancy may be due to compensation by other neurotransmitters such as GABA, acetylcholine (Ach), and glycine, all of which can depolarize embryonic cortical neurons as does glutamate (31). GABA is also one of the most abundant neurotransmitters detected during mammalian brain development, and its involvement in shaping brain development has also been suggested by recent in vitro investigations (5, 32, 33). However, mice lacking the two primary GABA biosynthetic enzymes, GAD65 and GAD67, show no discernible defects of neural development despite having only 0.02% of the normal GABA content (34). This discrepancy might also be due to compensation by other neurotransmitters in vivo. Glutamate, GABA, Ach, and glycine can all depolarize embryonic cortical neurons, so pathways involving more than one of these transmitters could potentially show mitigated severity of defects in single-neurotransmitter loss-of-function mutations. In the future, it will be important to analyze the direct consequences of overactivation of individual neurotransmitter receptors. Such studies could reveal functional roles of early appearing transmitter signaling during development.
The prevailing view of CNS development is that neural activity is, for the most part, important only in the refinement of axonal projections and synaptic connections, whereas early development of the nervous system is likely to be genetically programmed. Two recent studies have challenged this view by providing evidence that neural activity is required for spinal motor neurons to make accurate early path-finding decisions (35) and for embryonic spinal cord neurons to determine which types of neurotransmitters to produce (36). Combined with these studies, the present study suggests that neural activity is likely to be important in shaping early brain development and that glutamate, as a key mediator of neural activity, may play an important role in shaping the early CNS development. For these influences to be physiologically relevant, however, glutamate must be released at an early developmental stage and diffuse to stimulate glutamate receptors. Several observations support this hypothesis: (i) functional glutamate receptors are expressed by neuronal precursors and neurons of several brain areas at a very early stage (3, 37), (ii) exocytosis of glutamate occurs from growing axons and cones before synapse formation (38), (iii) paracrine nonvesicular release of glutamate exists before synapse formation and modulates neuronal migration (39, 40), and (iv) an efficient glutamate transport system is operative at early developmental stages (39). Depending on the neural activity and the location and properties of glutamate receptors and transporters, it is possible that excess activation of glutamate receptors can occur and modulate brain development. Therefore, normal brain development requires tight control of extracellular glutamate by the glutamate transporters GLAST and GLT1. This hypothesis was also confirmed by the severe developmental defects that were observed in the regions of the brain where both GLAST and GLT1 are expressed, such as the inner half of the cortex, the pallium–subpallium boundary, and the SP neurons (Fig. 13, which is published as supporting information on the PNAS web site). GLAST−/−/GLT1−/− double mutants have enabled us to clarify how glutamatergic signaling regulates the molecular pathways that control brain development.
Previous in vitro studies demonstrated that glutamate is involved in modulating the radial migration of cortical projection neurons. Blockade of NMDA receptors decreases cell migration, whereas enhancement of NMDA receptor activity or inhibition of extracellular glutamate uptake increases the rate of cell movement (4, 7). Interestingly, radial migration is impaired in GLAST−/−/GLT1−/− double mutants, in which excess activation of the NMDA receptor may occur. This impairment could be attributed to the fact that excess activation of glutamate receptors in GLAST−/−/GLT1−/− double mutants leads to disruption of the radial glial fiber system. Recent studies have indicated that glutamate released from corticofugal axons could lead to NMDA and AMPA/kainate receptor activation in tangentially migrating cells and thereby modulate their response to guidance cues (41, 42). Furthermore, GLT1 is expressed in corticofugal axons. Future experiments investigating the tangential migration of interneurons in GLAST−/−/GLT1−/− double mutants may clarify the functional significance of glutamate for tangential migration as well as radial migration.
It has been shown that SP cells are necessary for the development of many efferent and afferent cortical connections (23–26). We found that SP neurons were deficient in the neocortex of GLAST−/−/GLT1−/− mutants from E14 onward. Consistent with the defect in SP neurons, TC and CT projections were lacking in mutant mice.
Abnormal development of the brain during fetal life is now thought to contribute to the etiology of many neurological disorders that manifest throughout life (43). Cerebral hypoxia-ischemia is considered to be a major cause of perinatal brain injury. A dysfunction of glutamate transporters and the resulting excess glutamate are important pathophysiological mechanisms in brain injury after hypoxia-ischemia. Therefore, GLAST−/−/GLT1−/− mutants may be useful for characterizing lesions formed in response to hypoxia-ischemia and for developing neuroprotective strategies to reduce the burden of altered brain growth and poor functional and behavioral outcomes (44).
Materials and Methods
Mice.
The GLT1, GLAST, and EAAC1 mutant mice are described in refs. 17–19. To generate all combinations of double mutants, double-heterozygous mice (GLT1+/−/GLAST+/−, GLAST+/−/EAAC1+/−, and EAAC1+/−/GLT1+/−) were crossed. All mice were on a C57BL/6J background. The day of vaginal plug detection was designated as E0.5.
Histological Analysis, BrdU Labeling, TUNEL Assay, Western Blot Analysis, Real-Time PCR, and Glutathione Assay.
All detailed information specific to the experiments described here can be found in Supporting Materials and Methods, which is published as supporting information on the PNAS web site.
Effect of AMPA and NMDA Receptor Antagonism on Brain Abnormalities of Mutant Mice.
This experiment was performed as described in ref. 45. Between E8 and E16, pregnant mice received i.p. injections of the AMPA and NMDA receptor antagonists. Detailed procedures are described in Supporting Materials and Methods.
Supplementary Material
Acknowledgments
We thank R. A. Corriveau (Wayne State University, Detroit, MI) for his gift of the NARG1 probe and H. Kamiguchi (RIKEN Brain Science Institute) for his gift of the L1 antibody. This work was supported by research grants from RIKEN Brain Science Institute; a grant-in-aid for Scientific Research from the Japan Society for the Promotion of Sciences; and a grant-in-aid for Scientific Research on Priority Areas from the Ministry of Education, Culture, Sports, and Technology of Japan (to K. Tanaka).
Abbreviations
- En
embryonic day n
- PCNA
proliferating cell nuclear antigen
- MAP2
microtubule-associated protein 2
- CT
corticothalamic
- TC
thalamocortical
- DiI
1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- VZ
ventricular zone
- CP
cortical plate
- SP
subplate.
Footnotes
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Cohen-Cory S. Science. 2002;298:770–776. doi: 10.1126/science.1075510. [DOI] [PubMed] [Google Scholar]
- 2.Ben-Ari Y. Trends Neurosci. 2001;24:353–360. doi: 10.1016/s0166-2236(00)01813-0. [DOI] [PubMed] [Google Scholar]
- 3.Nguyen L., Rigo J. M., Rocher V., Belachew S., Malgrange B., Rogister B., Leprince P., Moonen G. Cell Tissue Res. 2001;305:187–202. doi: 10.1007/s004410000343. [DOI] [PubMed] [Google Scholar]
- 4.Komuro H., Rakic P. Science. 1993;260:95–97. doi: 10.1126/science.8096653. [DOI] [PubMed] [Google Scholar]
- 5.LoTurco J. J., Owens D. F., Heath M. J., Davis M. B., Kriegstein A. R. Neuron. 1995;15:1287–1298. doi: 10.1016/0896-6273(95)90008-x. [DOI] [PubMed] [Google Scholar]
- 6.Ikonomidou C., Bosch F., Miksa M., Bittigau P., Vockler J., Dikranian K., Tenkova T. I., Stefovska V., Turski L., Olney J. W. Science. 1999;283:70–74. doi: 10.1126/science.283.5398.70. [DOI] [PubMed] [Google Scholar]
- 7.Behar T. N., Scott C. A., Greene C. L., Wen X., Smith S. V., Maric D., Liu Q. Y., Colton C. A., Barker J. L. J. Neurosci. 1999;19:4449–4461. doi: 10.1523/JNEUROSCI.19-11-04449.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kutsuwada T., Sakimura K., Manabe T., Takayama C., Katakura N., Kushiya E., Natsume R., Watanabe M., Inoue Y., Yagi T., et al. Neuron. 1996;16:333–344. doi: 10.1016/s0896-6273(00)80051-3. [DOI] [PubMed] [Google Scholar]
- 9.Messersmith E. K., Feller M. B., Zhang H., Shatz C. J. Mol. Cell. Neurosci. 1997;9:347–357. doi: 10.1006/mcne.1997.0646. [DOI] [PubMed] [Google Scholar]
- 10.Zamanillo D., Sprengel R., Hvalby O., Jensen V., Burnashev N., Rozov A., Kaiser K. M., Koster H. J., Borchardt T., Worley P., et al. Science. 1999;284:1805–1811. doi: 10.1126/science.284.5421.1805. [DOI] [PubMed] [Google Scholar]
- 11.Meng Y., Zhang Y., Jia Z. Neuron. 2003;39:163–176. doi: 10.1016/s0896-6273(03)00368-4. [DOI] [PubMed] [Google Scholar]
- 12.Verhage M., Maia A. S., Plomp J. J., Brussaard A. B., Heeroma J. H., Vermeer H., Toonen R. F., Hammer R. E., van den Berg T. K., Missler M., et al. Science. 2000;287:864–869. doi: 10.1126/science.287.5454.864. [DOI] [PubMed] [Google Scholar]
- 13.Wojcik S. M., Rhee J. S., Herzog E., Sigler A., Jahn R., Takamori S., Brose N., Rosenmund C. Proc. Natl. Acad. Sci. USA. 2004;101:7158–7163. doi: 10.1073/pnas.0401764101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fremeau R. T., Jr., Kam K., Qureshi T., Johnson J., Copenhagen D. R., Storm-Mathisen J., Chaudhry F. A., Nicoll R. A., Edwards R. H. Science. 2004;304:1815–1819. doi: 10.1126/science.1097468. [DOI] [PubMed] [Google Scholar]
- 15.Tanaka K. Neurosci. Res. 2000;37:15–19. doi: 10.1016/s0168-0102(00)00104-8. [DOI] [PubMed] [Google Scholar]
- 16.Shibata T., Watanabe M., Tanaka K., Wada K., Inoue Y. NeuroReport. 1996;7:705–709. doi: 10.1097/00001756-199602290-00006. [DOI] [PubMed] [Google Scholar]
- 17.Tanaka K., Watase K., Manabe T., Yamada K., Watanabe M., Takahashi K., Iwama H., Nishikawa T., Ichihara N., Kikuchi T., et al. Science. 1997;276:1699–1702. doi: 10.1126/science.276.5319.1699. [DOI] [PubMed] [Google Scholar]
- 18.Peghini P., Janzen J., Stoffel W. EMBO J. 1997;16:3822–3832. doi: 10.1093/emboj/16.13.3822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Watase K., Hashimoto K., Kano M., Yamada K., Watanabe M., Inoue Y., Okuyama S., Sakagawa T., Ogawa S., Kawashima N., et al. Eur. J. Neurosci. 1998;10:976–988. doi: 10.1046/j.1460-9568.1998.00108.x. [DOI] [PubMed] [Google Scholar]
- 20.Danbolt N. C. Prog. Neurobiol. 2001;65:1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- 21.Chen W., Mahadomrongkul V., Berger U. A., Bassan M., DeSilva T., Tanaka K., Irwin N., Aoki C., Rosenberg P. A. J. Neurosci. 2004;24:1136–1148. doi: 10.1523/JNEUROSCI.1586-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yamada K., Watanabe M., Shibata T., Nagashima M., Tanaka K., Inoue Y. J. Neurosci. 1998;18:5706–5713. doi: 10.1523/JNEUROSCI.18-15-05706.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McConnell S. K., Ghosh A., Shatz C. J. Neuroscience. 1994;14:1892–1907. doi: 10.1523/JNEUROSCI.14-04-01892.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hevner R. F., Shi L., Justice N., Hsueh Y., Sheng M., Smiga S., Bulfone A., Goffinet A. M., Campagnoni A. T., Rubenstein J. L. Neuron. 2001;29:353–366. doi: 10.1016/s0896-6273(01)00211-2. [DOI] [PubMed] [Google Scholar]
- 25.Ghosh A., Antonini A., McConnell S. K., Shatz C. J. Nature. 1990;347:179–181. doi: 10.1038/347179a0. [DOI] [PubMed] [Google Scholar]
- 26.De Carlos J. A., O’Leary D. D. J. Neurosci. 1992;12:1194–1211. doi: 10.1523/JNEUROSCI.12-04-01194.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mitani A., Tanaka K. J. Neurosci. 2003;23:7176–7182. doi: 10.1523/JNEUROSCI.23-18-07176.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sugiura N., Patel R. G., Corriveau R. A. J. Biol. Chem. 2001;276:14257–14263. doi: 10.1074/jbc.M100011200. [DOI] [PubMed] [Google Scholar]
- 29.Schubert D., Piasecki D. J. Neurosci. 2001;21:7455–7462. doi: 10.1523/JNEUROSCI.21-19-07455.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rimaniol A. C., Mialocq P., Clayette P., Dormont D., Gras G. Am. J. Physiol. 2001;281:C1964–C1970. doi: 10.1152/ajpcell.2001.281.6.C1964. [DOI] [PubMed] [Google Scholar]
- 31.Ben-Ari Y. Nat. Rev. Neurosci. 2002;3:728–739. doi: 10.1038/nrn920. [DOI] [PubMed] [Google Scholar]
- 32.Behar T. N., Li Y. X., Tran H. T., Ma W., Dunlap V., Scott C., Barker J. L. J. Neurosci. 1996;16:1808–1818. doi: 10.1523/JNEUROSCI.16-05-01808.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Obata K. Dev. Neurosci. 1997;19:117–119. doi: 10.1159/000111195. [DOI] [PubMed] [Google Scholar]
- 34.Ji F., Kanbara N., Obata K. Neurosci. Res. 1999;33:187–194. doi: 10.1016/s0168-0102(99)00011-5. [DOI] [PubMed] [Google Scholar]
- 35.Hanson M. G., Landmesser L. T. Neuron. 2004;43:687–701. doi: 10.1016/j.neuron.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 36.Borodinsky L. N., Root C. M., Cronin J. A., Sann S. B., Gu X., Spitzer N. C. Nature. 2004;429:523–530. doi: 10.1038/nature02518. [DOI] [PubMed] [Google Scholar]
- 37.Lujan R., Shigemoto R., Lopez-Bendito G. Neuroscience. 2005;130:567–580. doi: 10.1016/j.neuroscience.2004.09.042. [DOI] [PubMed] [Google Scholar]
- 38.Soeda H., Tatsumi H., Katayama Y. Neuroscience. 1997;77:1187–1189. doi: 10.1016/s0306-4522(96)00465-4. [DOI] [PubMed] [Google Scholar]
- 39.Demarque M., Represa A., Becq H., Khalilov I., Ben-Ari Y., Anirksztejn L. Neuron. 2002;36:1051–1061. doi: 10.1016/s0896-6273(02)01053-x. [DOI] [PubMed] [Google Scholar]
- 40.Manent J. B., Demarque M., Jorquera I., Pellegrino C., Ben-Ari Y., Aniksztejn L., Represa A. J. Neurosci. 2005;25:4755–4765. doi: 10.1523/JNEUROSCI.0553-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Poluch S., Drian M. J., Durand M., Astier C., Benyamin Y., Konig N. J. Neurosci. Res. 2001;63:35–44. doi: 10.1002/1097-4547(20010101)63:1<35::AID-JNR5>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 42.Soria J. M., Valdeolmillos M. Cereb. Cortex. 2002;12:831–839. doi: 10.1093/cercor/12.8.831. [DOI] [PubMed] [Google Scholar]
- 43.Rees S., Inder T. Early Hum. Dev. 2005;81:753–761. doi: 10.1016/j.earlhumdev.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 44.Tanaka K. Trends Mol. Med. 2005;11:259–262. doi: 10.1016/j.molmed.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 45.Simonian S. X., Herbison A. E. J. Neurosci. 2001;21:934–943. doi: 10.1523/JNEUROSCI.21-03-00934.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.