Abstract
Ten chalcones were synthesized and tested as potential leishmanicidal and trypanocidal agents. All tested compounds caused concentration-dependent inhibition of the in vitro growth of Leishmania braziliensis and Trypanosoma cruzi with no significant toxic effect towards host macrophages. Our results show that the positions of the substituents seem to be critical for their antiprotozoal activities.
Among the kinetoplastid protozoa, which infect invertebrates, mammals, and plants, some species are of particular interest due to their medical importance. These include Trypanosoma cruzi (the agent of Chagas' disease), the African trypanosome responsible for sleeping sickness, and several species of Leishmania, which cause the various forms of leishmaniasis (9). The World Health Organization has identified Chagas' disease and leishmaniasis as major and increasing public health problems, particularly in Latin America (14, 15, 16, 18). In spite of the socioeconomic importance of these tropical infectious diseases, efforts directed towards the discovery of new drugs and/or vaccines against them are underdeveloped (10, 13). In addition, most of the drugs currently in use (i) were developed several decades ago, (ii) show variable efficacy, (iii) have serious side effects, (iv) are expensive, (v) can require long-term treatment, (vi) may have low activity in immunosuppressed patients, and (vii) present and/or induce resistance in parasites (9, 10, 16). Thus, the need for the development of new, effective, cheap, and safe drugs for the treatment of leishmaniasis and Chagas' disease is very important.
Chalcones, or 1,3-diaryl-2-propen-1-ones, are natural or synthetic compounds belonging to the flavonoid family. Chalcones possess a broad spectrum of biological activities, including antibacterial, anthelmintic, amoebicidal, antiulcer, antiviral, insecticidal, antiprotozoal, anticancer, cytotoxic, and immunosuppressive activities (for reviews, see references 11 and 12). The present study was designed to determine the in vitro leishmanicidal and trypanocidal activities of the 10 substitution-containing chalcones and to investigate the cytotoxic effects of these chalcones on mouse peritoneal macrophages in vitro.
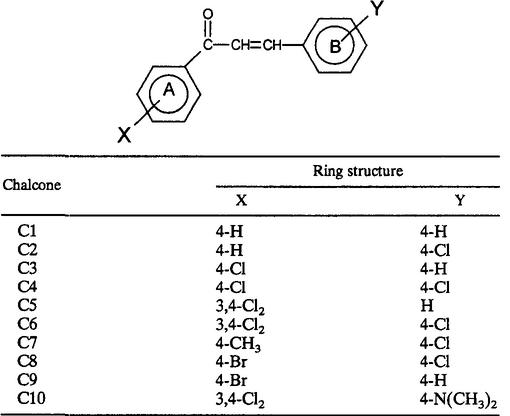
The chalcones used in the present study were synthesized in our laboratory by reaction of the appropriate aryl methyl ketone and aryl aldehyde (in a 1:1 ratio) in the presence of sodium hydroxide and ethanol. The products were then added to cooled diluted acetic acid according to the methodology previously described (8). The synthetic reaction gave substantial yields (55 to 98%) of all the chalcones, and these were characterized by 1H nuclear magnetic resonance and infrared analyses and by microanalysis. The substitution-containing chalcones were dissolved in 0.5% Tween 80 in phosphate-buffered saline to prepare a working solution with a 0.1 M concentration before being passed through 0.22-μm-pore-size Millipore filters. The structures of the chalcones are shown in Table 1.
TABLE 1.
Structures of substitution-containing chalcones
Cultures of promastigote forms of Leishmania braziliensis (strain Lb2904, kindly provided by the Evandro Chagas Institute, Belém, Brazil) and epimastigote forms of T. cruzi (strain Y) were grown at 28°C in Schneider's and TC100 media containing 5 and 10% heat-inactivated (56°C for 30 min) fetal bovine serum (FBS), respectively. For the parasite growth inhibition assays, L. braziliensis and T. cruzi were harvested on days 4 and 5 of the culture, respectively. To assess trypanocidal and leishmanicidal properties, the parasites were washed three times in phosphate-buffered saline by centrifugation at 1,000 × g for 10 min at room temperature. The concentration was adjusted to 2 × 106 parasites/ml in TC100 medium plus 10% FBS for T. cruzi or in Schneider's medium plus 5% FBS for L. braziliensis. One hundred fifty microliters of parasite suspension was added to 96-well plates and incubated at 28°C for 72 h in the presence or absence of the substitution-containing chalcones (3 to 1,000 μM), amphotericin B (10.8 to 1,082.0 nM; used as a control), or benznidazole (10 to 1,000 μM; used as a control). Three to four experiments were carried out in triplicate, and the number of surviving parasites was determined in Neubauer chambers.
The cytotoxic activity of substitution-containing chalcones to mouse peritoneal macrophages was evaluated as previously described (1, 17). To this end, cells were harvested from the peritonea of mice 2 to 3 days after injection of 2 ml of sterile thioglycolate solution (3% [wt/vol] in water). Cytotoxicity (cell viability) was assessed by an MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide] assay (17).
The following drugs were used as positive controls in the growth inhibition assays: benznidazole (Rochagan; Roche, São Paulo, Brazil) and amphotericin B (Fungizon; Bristol-Myers Squibb, São Paulo, Brazil).
The data obtained were analyzed by one-way analysis of variance followed by Dunnett's multiple-comparison test. The 50% inhibitory concentrations (IC50) were determined by linear regression analysis of data from individual experiments (GraphPad Software, San Diego, Calif.). The percentages of maximal inhibition (MI) were calculated as follows: MI = {[(number of parasites of vehicle group) − (number of parasites of drug group)]/(number of parasites of vehicle group)} × 100.
The substitution-containing chalcones clearly showed a concentration-dependent inhibitory effect on the in vitro growth of T. cruzi epimastigotes. Among the 10 tested chalcones, C1, C2, C3, C8, and C9 demonstrated distinct and potent inhibitory effects on the growth of T. cruzi, while the other chalcones inhibited parasite growth at lower but similar levels (Table 2). As observed for T. cruzi, substitution-containing chalcones strongly inhibited, in a concentration-dependent manner, the in vitro growth of L. braziliensis promastigotes. Chalcones C1 and C2 exhibited the most marked inhibitory effect on the growth of L. braziliensis, while the other chalcones (except C3, C5, and C8) inhibited the growth of the parasite to similar extents (Table 2). In a comparison of the mean IC50s, C1 and C2 were about 2- to 13-fold more potent than the other substitution-containing chalcones. However, all chalcones were less potent than the positive control drug, amphotericin B (Table 2). None of the chalcones tested over a concentration range of 10 to 300 μM showed any evidence of cytotoxicity to mouse peritoneal macrophages in vitro as assessed by MTT reduction to formazan. Cytotoxicity to macrophages was observed only at very high chalcone concentrations (≥1,000 μM; results not shown).
TABLE 2.
Trypanocidal and leishmanicidal activities of substitution-containing chalcones, benznidazole, and amphotericin B on epimastigote and promastigote forms of T. cruzi and L. braziliensis, respectively
| Chalcone or control | Trypanocidal activity
|
Leishmanicidal activity
|
||
|---|---|---|---|---|
| IC50 (μM)a | MI (%) | IC50 (μM)a | MI (%) | |
| C1 | 24.8 (11.3-54.7) | 100 | 13.7 (9.9-18.9) | 100 |
| C2 | 64.5 (45.9-90.5) | 100 | 21.9 (20.3-23.7) | 100 |
| C3 | 66.7 (47.3-94.1) | 94 ± 3 | 100.5 (76.1-132.8) | 100 |
| C4 | 100.3 (60.0-167.0) | 92 ± 5 | 98.0 (62.6-153.5) | 100 |
| C5 | 82.7 (63.3-108.0) | 88 ± 7 | 182.3 (179.6-185.0) | 100 |
| C6 | 126.4 (73.5-217.4) | 93 ± 4 | 66.7 (43.8-101.6) | 100 |
| C7 | 80.8 (53.3-122.6) | 90 ± 6 | 45.6 (38.1-54.8) | 100 |
| C8 | 65.4 (47.6-89.8) | 97 ± 3 | 129.1 (93.2-178.8) | 100 |
| C9 | 66.6 (44.5-99.8) | 100 | 61.7 (57.7-65.9) | 100 |
| C10 | 89.6 (71.2-112.8) | 90 ± 6 | 57.4 (38.0-86.5) | 100 |
| Benznidazole | 54.7 (42.8-69.8) | 100 | ||
| Amphotericin B | 0.21 (0.18-0.24) | 100 | ||
IC50 with their respective 95% confidence limits.
The results presented here show that some studied chalcones (Table 1) have a concentration-dependent inhibitory effect on the growth of L. braziliensis promastigotes and on T. cruzi epimastigotes in vitro. Although leishmanicidal and antimalarial activities have already been reported in the literature for this class of compounds (4, 5, 6; for a review, see reference 12), our results demonstrated that the synthetic chalcones reported in the present study exhibit marked in vitro leishmanicidal and trypanocidal activities. Nevertheless, the mechanisms by which the substitution-containing chalcones showed leishmanicidal and trypanocidal activities were not addressed in this work. Based on the literature, it can be predicted that chalcones could potentially inhibit the activity of fumarate reductase, succinate dehydrogenase, NADH dehydrogenase, or succinate- and NADH-cytochrome c reductases in the parasite mitochondria (2, 3, 7, 19, 20). Additional studies are in progress to address this hypothesis. Our results also show that, in vitro, leishmanicidal and trypanocidal concentrations of chalcones showed low cytotoxicity to mouse peritoneal macrophages.
In summary, we show here that some synthesized substitution-containing chalcones, especially C1, exhibited promising leishmanicidal and trypanocidal activities with no evidence of a cytotoxic effect on mouse macrophages. The positions of the substituents seem to be important for the effectiveness of the antiprotozoal activity. C1, which has no substituent groups, revealed both pronounced leishmanicidal and trypanocidal activities.
Acknowledgments
This study was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and by Programa de Apoio aos Núcleos de Excelência (PRONEX), Brazil.
We thank Moacir G. Pizzolatti for his contribution to the discussion of the manuscript.
REFERENCES
- 1.Beirith, A., T. B. Creczynski-Pasa, V. R. Bonetti, M. Konzen, I. Seifriz, M. S. Paula, C. V. Franco, and J. B. Calixto. 1999. Antinociceptive properties and nitric oxide synthase inhibitory action of new ruthenium complexes. Eur. J. Pharmacol. 369:289-297. [DOI] [PubMed] [Google Scholar]
- 2.Boveris, A. C., C. M. Hertig, and J. F. Turrens. 1986. Fumarate reductase and other mitochondrial activities in Trypanosoma cruzi. Mol. Biochem. Parasitol. 19:163-169. [DOI] [PubMed] [Google Scholar]
- 3.Chen, M., S. B. Christensen, J. Blom, E. Lemmich, L. Nadelmann, K. Fich, T. G. Theander, and A. Kharazmi. 1993. Licochalcone A, a novel antiparasitic agent with potent activity against human pathogenic protozoan species of Leishmania. Antimicrob. Agents Chemother. 37:2550-2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen, M., T. G. Theander, S. B. Christensen, L. Hviid, L. Zhai, and A. Kharazmi. 1994. Licochalcone A, a new antimalarial agent, inhibits in vitro growth of the human malaria parasite Plasmodium falciparum and protects mice from P. yoelii infection. Antimicrob. Agents Chemother. 38:1470-1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen, M., S. B. Christensen, T. G. Theander, and A. Kharazmi. 1994. Antileishmanial activity of licochalcone A in mice infected with Leishmania major and in hamsters infected with Leishmania donovani. Antimicrob. Agents Chemother. 38:1339-1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen, M., S. B. Christensen, L. Zhai, M. H. Rasmussen, T. G. Theander, S. Frekjaer, B. Steffansen, J. Davidsen, and A. Kharazmi. 1997. The novel oxygenated chalcone, 2,4-dimethoxy-4′-butoxychalcone, exhibits potent activity against human malaria parasite Plasmodium falciparum in vitro and rodent parasites Plasmodium berghei and Plasmodium yoelii in vivo. J. Infect. Dis. 176:1327-1333. [DOI] [PubMed] [Google Scholar]
- 7.Chen, M., L. Zhai, S. Brogger, S. B. Christensen, T. G. Theander, and A. Kharazmi. 2001. Inhibition of fumarate reductase in Leishmania major and L. donovani by chalcones. Antimicrob. Agents Chemother. 45:2023-2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Corrêa, R., M. A. S. Pereira, D. Buffon, L. Santos, V. Cechinel Filho, A. R. S. Santos, and R. J. Nunes. 2001. Antinociceptive properties of chalcones. Structure-activity relationships. Arch. Pharm. 334:332-334. [DOI] [PubMed] [Google Scholar]
- 9.Costa-Pinto, D., L. S. Trindade, D. McMahon-Pratt, and Y. M. Traub-Cseko. 2001. Cellular trafficking in trypanosomatids: a new target for therapies? Int. J. Parasitol. 31:537-544. [DOI] [PubMed] [Google Scholar]
- 10.Croft, S. L. 1997. The current status of antiparasite chemotherapy. Parasitology 114:83-96. [PubMed] [Google Scholar]
- 11.Dhar, D. N. 1981. The chemistry of chalcones and related compounds. Wiley-Interscience, New York, N.Y.
- 12.Dimmock, J. R., D. W. Elias, M. A. Beazely, and N. M. Kandepu. 1999. Bioactivities of chalcones. Curr. Med. Chem. 6:1125-1149. [PubMed] [Google Scholar]
- 13.Engers, H. D., R. Bergquist, and F. Moddaber. 1996. Progress on vaccines against parasites. Dev. Biol. Stand. 87:73-84. [PubMed] [Google Scholar]
- 14.Grisard, E. C., M. Steindel, J. J. Shaw, E. A. Y. Yshikawa, C. J. Carvalho-Pinto, I. Eger-Mangrich, H. K. Toma, A. J. Romanha, and D. A. Campbell. 2000. Characterization of Leishmania sp. strains from autochthonous cases of human cutaneous leishmaniasis in Santa Catarina state, southern Brazil. Acta Trop. 74:89-93. [DOI] [PubMed] [Google Scholar]
- 15.Modabber, F. 1993. Leishmaniasis, p. 77-87. In Tropical disease research progress 1991-92. UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases. World Health Organization, Geneva, Switzerland.
- 16.Opperdoes, F. R. 1994. The Trypanosomatidae: amazing organisms. J. Bioenerg. Biomembr. 26:145-146. [DOI] [PubMed] [Google Scholar]
- 17.Van De Loosdrecht, A. A., E. Nennie, G. J. Ossenkoppele, R. H. Beelen, and M. N. Langenhuijse. 1991. Cell mediated cytotoxicity against U937 cells by human monocytes and macrophages in a modified colorimetric MTT assay. A methodological study. J. Immunol. Methods 141:15-22. [DOI] [PubMed] [Google Scholar]
- 18.World Health Organization. 1991. Control of Chagas' disease. WHO Tech. Rep. Ser. 811:1-93. [PubMed] [Google Scholar]
- 19.Zhai, L., J. Blom, M. Chen, S. B. Christensen, and A. Kharazmi. 1995. The antileishmanial agent licochalcone A interferes with the function of parasite mitochondria. Antimicrob. Agents Chemother. 39:2742-2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhai, L., M. Chen, J. Blom, S. B. Christensen, T. G. Theander, and A. Kharazmi. 1999. The antileishmanial activity of novel oxygenated chalcones and their mechanism of action. J. Antimicrob. Chemother. 43:793-803. [DOI] [PubMed] [Google Scholar]