Abstract
The penetration of 1 g of intravenous ertapenem once daily for 3 days in suction-induced skin blisters was evaluated. Ten forearm blisters were formed (n = 12) 12 h prior to the last dose. Concentrations of ertapenem in blister fluid exceeded 4 μg/ml (the MIC at which 90% of the isolates tested are eliminated) for the entire dosing interval. The area under the concentration-time curve for 0 to 24 h ratio of blister fluid to plasma was 61% (90% confidence interval, 56, 65%) suggesting good blister penetration.
Ertapenem is a new long-acting 1-β-methyl parenteral (intravenous [i.v.] and intramuscular) antibiotic in the carbapenem class of β-lactam agents. Ertapenem has a broad spectrum of activity against both gram-positive and gram-negative, aerobic and anaerobic bacteria (3) and is anticipated to be effective against a wide variety of community-acquired and aerobic-anaerobic mixed infections, such as complicated intra-abdominal infections, complicated skin and skin structure infections, and lower respiratory tract infections. Carbapenems with a hydroxyethyl side chain at C6 are resistant to β-lactamases (5). Ertapenem has demonstrated in vitro activity against strains of Escherichia coli and Klebsiella pneumoniae that express extended-spectrum β-lactamases and are resistant to broad-spectrum cephalosporins, as well as activity against resistant strains of Streptococcus pneumoniae (9) The 1-β-methyl structure confers stability against human renal dehydropeptidase-1, and thus it does not require coadministration of an inhibitor of renal dehydropeptidase (11).
This study was conducted to evaluate whether ertapenem diffuses into the interstitial fluid of the skin and whether it achieves and maintains concentrations equal to or above the expected MIC at which 90% of the isolates tested are eliminated (MIC90) for the usual skin pathogens for an adequate period of time. Numerous models have been used to evaluate the penetration of antibiotics into tissue fluid (12). In this study, the suction-induced skin blister technique was used to obtain interstitial fluid (6, 7, 10) as a model for assessing drug penetration into skin tissue. The penetration of ertapenem was studied with epidermal blisters on the arm, produced by controlled suction (4, 6, 7, 10).
Twelve healthy male and nonpregnant female volunteers who were between 19 and 45 years of age (mean age, 27 years) participated in the study. Subjects were generally healthy, as judged by medical history, physical examination, and laboratory safety tests (complete blood count, chemistry profile, and urinalysis), and had a skin type and mid-volar arm area that were judged by the investigator to be suitable for the suction-induced blistering procedure (type I or II). All subjects were nonsmokers and were within 20% of ideal body weight. Subjects were excluded if they had taken any medication within a period of 2 weeks prior to the study. All subjects provided written informed consent for the study. The protocol and informed-consent form were approved by the local ethics committee. The study was performed in accordance with the Declaration of Helsinki and the European Good Clinical Practice guidelines.
Epidermal blisters were made by application of controlled suction to the skin (4, 6, 7, 10). The suction-induced blisters were produced on the forearm by a modification of the method of Hellum and Schreiner (4, 10). All subjects had 1 blister formed on day −1 as a predose blank and 10 uniform blisters formed 12 h prior to final drug administration on day 3. Volunteers remained in a semirecumbent position throughout the blistering procedure. The area was hydrated with warm compresses for approximately 10 min prior to the start of blistering, the blister site was swabbed with 70% isopropanol, and the suction apparatus was applied. This apparatus, a Plexiglas block (Monaderm, Monaco, France) containing bores 9 mm in diameter, was placed on the volar area of the forearm, and negative pressure (controlled suction) was applied to the skin surface with an electric vacuum pump. The vacuum was increased slowly over a period of 1 min up to a maximum negative pressure of 0.3 kg/cm2 (3.104 Pa). This equilibrium was maintained for 2 to 3 h, until half-spherical blisters were formed. As soon as the blisters appeared, the vacuum was released, and the suction chamber apparatus was carefully removed without breaking the blister. Bathing was restricted from the time of blistering to completion of pharmacokinetic sampling, 36 h after blister formation (4, 10). In order to protect the blisters, they were covered with a large half-spherical protection cap. Prior to dosing, the blister area was assessed to ensure all blisters raised were still viable for sampling. If there was any sign of blood in the blister fluid, the blister was not used. Once the blisters were used, the skin area was disinfected. The course of healing of the blister site was monitored for 6 to 8 days after final dose. As the blisters healed, fusidic acid (20 mg/g) cream was applied. Generally reepithelialization of suction-induced blisters was complete within 1 week. Because there could have been temporary loss of pigment, a sunscreen was used with an SPF (sun protection factor rating) of at least 20 in order to prevent hyperpigmentation in the case of sun exposure.
Each volunteer received i.v. doses of 1 g of ertapenem infused over 30 min once daily for 3 days. Each vial contained sterile lyophilized powder with an average of 1,050 mg of ertapenem per vial. The arm for i.v. administration of drug was not used for blistering, except for a single baseline blister on day −1.
Blister fluid was sampled predose and 0.5, 1, 2, 4, 8, 12, and 24 h postdose following i.v. administration on day 3 by puncturing the blister with a minimum-dead-space syringe (Micro-fine III; Becton Dickinson). The blister fluid was stored on ice after collection until frozen at −70°C. Each blister was sampled only once, delivering approximately 0.1 to 0.25 ml of fluid. Blood was sampled for the ertapenem assay predose and 0.25, 0.5, 0.75, 1, 1.5, 2, 4, 6, 8, 12, 16, and 24 h postdose. Plasma samples were stored at −70°C until analysis.
Total ertapenem concentrations were determined in plasma and blister fluid samples by reverse-phase high-performance liquid chromatography (HPLC) with UV absorption (at 300 nm) for detection. The lower limit of quantification of ertapenem was 0.1 μg/ml for both plasma and blister fluid. The assay for the determination of ertapenem concentrations in blister fluid was based on minimal modification of the plasma ertapenem assay (8). Ertapenem in blister fluid was analyzed by UV detection after direct injection of the sample onto a high-performance liquid chromatograph with column switching. For blister or plasma samples, a standard curve was prepared in control plasma matrix. For sample analysis, quality control samples were used for blister and plasma samples, respectively. The minimum blister sample volume required for analysis was 45 μl. The concentrations for the standard curves ranged from 0.1 to 50 μg/ml. The control concentrations were 0.25, 10, and 40 μg/ml. The intraday coefficients of variation for the control concentrations were <15% for accuracy and <10% for precision. For day-to-day runs, the control concentrations were within 20% of nominal.
To assess whether the geometric mean maximum concentration (Cmax) of ertapenem in blister fluid following 3 days of 1-g i.v. daily doses infused over 30 min exceeded 4 μg/ml, a confidence interval (CI) approach was used. Cmax data for blister fluid were log transformed prior to analysis. A two-sided 90% CI (equivalent to a one-sided 95% CI) for the arithmetic mean Cmax in blister fluid on the log scale was calculated by using the between-subject standard deviation (SD). The arithmetic mean Cmax and confidence limits were then exponentiated to obtain the geometric mean Cmax and corresponding 90% CI. If the lower limit of the 90% CI was >4 μg/ml, then it was concluded that the Cmax of ertapenem in blister fluid following 3 days of 1-g i.v. daily doses of ertapenem exceeded 4 μg/ml. SAS v6.12 software was used to analyze the data.
The geometric mean and 90% CI for the geometric mean were calculated for the area under the concentration time curve from 0 to 24 h (AUC0-24) and the concentration at 24 h postdose (C24 h) in plasma and blister fluid by the approach used to determine Cmax. The geometric mean ratio and the 90% CI of the parameter in blister fluid relative to that in plasma were also calculated. In addition, the geometric mean C24 h in blister fluid was statistically compared with that in plasma. The arithmetic mean time to maximum concentration of drug in serum (Tmax) and SD for blister fluid, harmonic mean terminal half-life, and jackknife SD for plasma and mean plasma clearance were also calculated (for exploratory purposes only).
Safety data, including the incidence of adverse experiences, vital signs, and electrocardiogram, and laboratory data were monitored and tabulated throughout the study.
The day 3 plasma AUC0-24 was determined by using the linear trapezoidal rule for the increasing portion of the curve and by log-trapezoidal rule for the decreasing portion of the curve. The AUC0-24 on day 3 in blister fluid was also estimated by using the linear trapezoidal rule. The plasma clearance of ertapenem was obtained by dividing the dose of ertapenem by the corresponding ertapenem AUC0-24 in plasma. The terminal elimination rate constant, β, in plasma was estimated by fitting the terminal log-linear concentration-time points in plasma on day 3 by using linear regression of log-transformed data, and the terminal half-life was estimated as the ratio ln2/β. The commercial software EXCEL was used for making the calculations. The Cmax in blister fluid was obtained by visual inspection of the blister fluid profiles. The pharmacokinetic parameter estimates were adjusted for the actual doses of ertapenem administered on day 3 obtained by multiplying the assayed concentrations of the i.v. infusion solution by the actual volumes of infusion solution administered to each individual on day 3.
Thirteen subjects were enrolled in the study. One subject discontinued the study due to personal reasons, so the pharmacokinetic analysis was done on the 12 subjects who completed the study. (Except for two subjects, there were no 24-h plasma samples available.) Data from all 13 subjects were included in the analysis for safety. In all subjects, at least eight blisters were viable for sampling, and no blister samples were forfeited.
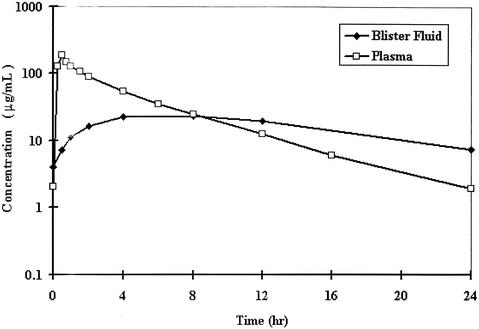
The mean plasma concentration-time curves of total ertapenem in plasma and blister fluid on the 3rd day of 1-g once-daily i.v. doses are given in Fig. 1. The highest concentration in blister fluid was reached at 8 h, at which time, the blister fluid and plasma drug concentrations were similar. Subsequently, the blister fluid concentrations declined more slowly than the plasma drug concentrations, so the concentration of ertapenem in blister fluid was higher than that in plasma.
FIG. 1.
Mean concentration profiles of ertapenem in plasma and blister fluid on the 3rd day of 1-g once-daily i.v. doses in healthy subjects.
Table 1 summarizes the pharmacokinetic parameters of ertapenem in plasma and blister fluid. The geometric mean AUC0-24 in plasma was 688.1 μg · h/ml, while that in blister fluid was 417.5 μg · h/ml (P < 0.001). The geometric mean ratio (90% CI) of AUC0-24 in blister fluid relative to that in plasma was 0.61 (90% CI, 0.56, 0.65); individual ratios ranged from 0.46 to 0.75.
TABLE 1.
Pharmacokinetics of ertapenem in plasma and blister fluid on the 3rd day of 1-g i.v. daily doses in healthy subjects
| Parameter | Result fora:
|
||||
|---|---|---|---|---|---|
| Plasma
|
Blister fluid
|
Blister/plasma GMR (90% CI) | |||
| n | GM (90% CI) | n | GM (90% CI) | ||
| Cmax (μg/ml)b | 12 | 191.9 (178.1, 206.8) | 12 | 25.0 (22.8, 27.4) | 0.13 (0.12, 0.15) |
| C24 h (μg/ml) | 10 | 1.9 (1.5, 2.4) | 12 | 7.6 (6.7, 8.6) | 3.90 (3.20, 4.76) |
| AUC0-24 (μg · h/ml) | 12 | 688.1 (640.2, 739.5) | 12 | 417.5 (385.1, 452.6) | 0.61 (0.56, 0.65) |
GM, geometric least-square mean; GMR, geometric mean ratio.
End-of-infusion concentration was used as the Cmax for plasma.
The geometric mean Cmax in plasma was 191.9 μg/ml, compared to 25.0 μg/ml in blister fluid (Table 1). The lower limit of the two-sided 90% Cl for geometric mean Cmax in blister fluid was 22.8 μg/ml, which exceeded 4 μg/ml, supporting the hypothesis that the geometric mean Cmax in blister fluid on the 3rd day of 1-g i.v. daily doses of ertapenem exceeds 4 μg/ml. At 24 h postdose, the mean concentration of antibiotic in blister fluid was statistically significantly higher (7.6 μg/ml) than that in plasma (1.9 μg/ml) (P < 0.001), with a geometric mean ratio (90% CI) in blister fluid relative to plasma of 3.90 (90% CI, 3.20, 4.76). The lower limit of the 90% CI for C24 h in blister fluid was 6.7 μg/ml, indicating that the mean concentration of ertapenem clearly exceeded 4 μg/ml at 24 h after dose administration on day 3. In addition, the C24 h values for blister fluid exceeded 4 μg/ml for all individual subjects.
The mean terminal half-life in plasma was 4.3 h, with individual values ranging from 3.7 to 5.4 h. The elimination half-life in blister fluid appeared longer, but was not calculated because of insufficient data. The mean clearance of ertapenem in plasma was 24.2 ml/min, with individual values ranging from 17.9 to 29.9 ml/min.
During the study, one subject reported a clinical adverse experience of mild headache that was considered as possibly drug related by the investigator. No clinically significant changes in biochemical or hematological parameters were found.
This was a multiple-dose study of ertapenem to evaluate the tissue penetration of ertapenem in skin blisters after a 1-g i.v. daily dose for 3 days. Numerous models have been used to evaluate the penetration of antibiotics into tissue fluid. Skin chambers, suction-induced and irritant-induced blisters, and lymphatic fluid (2, 12) have been used to obtain interstitial fluid, although there is little comparative information on these methods. However, blisters, whether induced by suction or cantharidin, have been compared. Cantharidin is an irritant, and blisters induced by this method result in some inflammatory response and injury to the blister's basement membrane, with local swelling, reddening, and turbidity of the blister fluid (10). The use of suction-induced epidermal blisters is much less invasive; the procedure does not provoke an inflammatory response or bleeding (depending on skin type) (4, 12), and the basement membrane remains intact, with minimal tissue breakdown. Although one might expect better penetration in the inflamed blister model, studies suggest no significant difference between penetration into an inflamed blister versus a noninflamed blister (e.g., suction) (4, 10). The suction-induced skin-blistering method was chosen for this study, since it is less invasive and yields very reproducible results. The technique as described was successful. All subjects had at least eight blisters raised, and blisters remained viable throughout the sampling period, with complete blister pharmacokinetic profiles obtained. Blister healing was complete within 1 week, with no complications.
Ertapenem in skin blister fluid demonstrated favorable concentrations throughout the dosing interval, with a temporal profile that resembles those of other β-lactam antibiotics (1). The mean ratio of AUC in blister fluid to AUC in plasma was high (61%), indicating that ertapenem penetrates well into suction-induced skin blisters. The concentrations of the antibiotic in suction-induced skin blister fluid on the 3rd day of 1-g once-daily i.v. dosing were above the MIC90 for generally susceptible organisms (4 μg/ml) for the entire 24-h dosing interval for each subject, and the peak concentrations were approximately sixfold higher than the MIC90. The concentrations achieved also exceeded the susceptibility breakpoints of 2 μg/ml for members of the family Enterobacteriaceae and staphylococci and 1 μg/ml for streptococci for the entire 24-h dosing interval, supporting the use of ertapenem in a once-daily regimen in the treatment of various skin infections.
Ertapenem is highly plasma protein bound (95%). The reduced AUC of total (bound and unbound) ertapenem in skin blister (61%) compared to that in plasma is consistent with lower concentrations of bound drugs in skin blister due to its lower protein concentration: the total protein content in skin blister is approximately 60% of that in serum, and the albumin content in skin blister is approximately 70% of that in serum (4, 10). Consequently, the AUC ratio (skin blister/plasma) based on unbound drug is likely to be much higher than 0.61, indicating excellent penetration of unbound drug.
In general, extravascular fluid exhibits peak concentrations of β-lactam antibiotics below those in serum (1), and peak concentrations typically occur slightly later than or simultaneously with those in serum. The peak concentration of ertapenem in plasma occurred at the end of the 0.5-h infusion, while the time of peak concentration in blister fluid occurred at ∼8 h. The delay between the peak in plasma and the peak in blister fluid indicates a slow entry and/or exit of ertapenem from the skin blisters. However, following the peak, the levels in blister fluid were higher than those in plasma, and the rate of elimination of antibiotics from extravascular fluid (e.g., skin blister) was generally slower than that from plasma (1).
The results of this study support the finding that ertapenem, at doses of 1 g i.v. once daily, penetrated blister fluid well, and the concentrations exceeded the MIC for generally susceptible organisms in complicated skin and skin structure infections.
Acknowledgments
This study was supported by Merck Research Laboratories.
We acknowledge David Sciberras for assistance in developing the blister methodology. Coordination of the study by Anne Perier of Aster is also acknowledged.
REFERENCES
- 1.Bergan, T. 1981. Pharmacokinetics of tissue penetration of antibiotics. Rev. Infect. Dis. 3:45-66. [DOI] [PubMed] [Google Scholar]
- 2.Bruun, J. N., J. E. Bredesen, P. Kierulf, and P. K. M. Lunde. 1991. Pharmacokinetic studies of antibacterial agents using the suction blister method. Scand. J. Infect. Dis. 74(Suppl.):49-53. [PubMed] [Google Scholar]
- 3.Gill, C. J., J. J. Jackson, L. S. Gerckens, B. A. Pelak, R. K. Thompson, J. G. Sundelof, H. Kropp, and H. Rosen. 1998. In vivo activity and pharmacokinetic evaluation of a novel long-acting carbapenem antibiotic, MK-826 (L-749345). Antimicrob. Agents Chemother. 42:1996-2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hellum, K. B., A. Schreiner, A. Digranes, and I. Bergman. 1978. Skin blisters produced by suction: a new model for studies of penetration of antibiotics in humans, p. 620-622. In W. Siegenthaler and R. Lüthy (ed.), Current chemotherapy. Proceedings of the 10th International Congress of Chemotherapy, vol. I. American Society for Microbiology, Washington, D.C.
- 5.Jacoby, G., P. Han, and J. Tran. 1997. Comparative in vitro activities of carbapenem L-749345 and other antimicrobials against multiresistant gram-negative clinical pathogens. Antimicrob. Agents Chemother. 41:1830-1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kiistala, U. 1968. Suction blister device for separation of viable epidermis from dermis. J. Investig. Dermatol. 50:129-137. [DOI] [PubMed] [Google Scholar]
- 7.Kiistala, U., and K. K. Mustakallio. 1964. In-vivo separation of epidermis by production of suction blisters. Lancet 1964:1444-1445. [DOI] [PubMed] [Google Scholar]
- 8.Musson, D. G., K. L. Birk, A. M. Cairns, A. K. Majumdar, and J. D. Rogers. 1998. High performance liquid chromatographic methods for the determination of a new carbapenem antibiotic, L-749,345, in human plasma and urine. J. Chromatogr. B 720:99-106. [DOI] [PubMed] [Google Scholar]
- 9.Odenholt, I., E. Löwdin, and O. Cars. 1998. In vitro pharmacodynamic studies of L-749345 in comparison with imipenem and ceftriaxone against gram-positive and gram-negative bacteria. Antimicrob. Agents Chemother. 42:2365-2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schreiner, A., K. B. Hellum, A. Digranes, and I. Bergman. 1978. Transfer of penicillin G and ampicillin into human skin blisters induced by suction. Scand. J. Infect. Dis. 14(Suppl.):233-237. [PubMed] [Google Scholar]
- 11.Sundelof, J. G., R. Hajdu, C. J. Gill, R. Thompson, H. Rosen, and H. Kropp. 1997. Pharmacokinetics of L-749,345, a long-acting carbapenem antibiotic, in primates. Antimicrob. Agents Chemother. 41:1743-1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wise, R. 1986. Methods for evaluating the penetration of β-lactam antibiotics into tissues. Rev. Infect. Dis. 8:S325-S332. [DOI] [PubMed] [Google Scholar]