Abstract
Amphotericin B treatment was previously shown to inhibit Candida albicans reproduction and reduce the fluorescence of vitality-specific dyes without causing a corresponding increase in the fluorescence of the mortality-specific dyes bis-(1,3-dibutylbarbituric acid)trimethine oxonol and SYBR Green Ι. In the present study, we have confirmed these results and have shown that the numbers of CFU are reduced by 99.9% by treatment with 0.5 μg of amphotericin B per ml for 10 h at 35°C. This reduction was not due to fungal cell death. First, the level of reduction of the tetrazolium salt 2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-5-[(phenylamino)carbonyl]-2H-tetrazolium hydroxide increased in the presence of concentrations of amphotericin B that caused greater than 90% reductions in the numbers of CFU. Second, fungal cells treated with amphotericin B at a concentration of 0.5 μg/ml were resuscitated by further incubation at 22°C for 15 h in the continued presence of amphotericin B. Third, recovery of the ability to replicate was prevented by sequential treatment with 20 μg of miconazole per ml, which also increased the fluorescence of mortality-specific dyes to near the maximal levels achieved with 0.9 μg of amphotericin B per ml. Sequential treatment with fluconazole and flucytosine did not increase the levels of staining with the mortality-specific dyes. Itraconazole was less effective than ketoconazole, which was less effective than miconazole. The practice of equating the loss of the capacity of C. albicans to form colonies with fungal cell death may give incorrect results in assays with amphotericin B, and the results of assays with caution with other antifungal agents that are lipophilic or that possess significant postantifungal effects may need to be interpreted.
Candida is the fourth most common cause of nosocomial bloodstream infections in the United States (10), and the rate of primary bloodstream infections continues to increase (3). The most frequently isolated Candida species in these infections is Candida albicans. Candidemia is also frequently refractory to therapy, and the rate of mortality attributable to candidemia has been estimated to be 38% (36). Despite recent advances in the development of antifungal agents, amphotericin B (AMB) still remains the drug of choice for the treatment of life-threatening systemic infections (14), and the activity of AMB is the standard to which the activities of other antifungal agents are compared. AMB binds with the sterol ergosterol, resulting in the loss of membrane integrity and osmotic stability (5).
The process of cell replication deactivation is believed to involve stepwise changes in the physiological state of a cell that render an intermediate form that is incapable of initiating replicative processes but that is still capable of metabolism (21, 27). Thus, the loss of replication competency is an early event in a phase of decline that eventually leads to cell death (19, 27).
We have previously examined the effects of AMB on the vitality and mortality of C. albicans cells by measuring the intracellular ATP concentrations and levels of staining with fluorescent dyes with specific cellular affinities (22). That work delineated several physiological states produced as a consequence of the incubation of C. albicans with AMB for 10 h. These states included an alive state, a lethally injured state, and a hypothesized state in which the cells were sublethally injured because they did not stain with vitality- or mortality-specific fluorescent dyes and had a greater than 99% reduction in the numbers of CFU.
If the loss of replication competency is an early event, it was postulated that previous results could be explained by the existence of a population of cells that could no longer reproduce but that was still metabolically active (22). Since it has been shown that the CFU for only a fraction of stressed organisms can be enumerated on agar (23), this hypothesis seemed reasonable. If the cells are sublethally injured, it should be possible to demonstrate metabolic activity, recover the ability to form colonies, and convert the injury to lethality. We describe our further characterization of this interaction between C. albicans and AMB.
MATERIALS AND METHODS
Antifungal drugs.
Analytical-grade powders of the following antifungal drugs were provided by the indicated manufacturers: AMB, Bristol Myers-Squibb Co. (Princeton, N.J.); fluconazole, Pfizer, Inc. (Groton, Conn.); ketoconazole and itraconazole, Research Diagnostics, Inc. (Flanders, N.J.); flucytosine, Hoffmann-La Roche, Inc. (Nutley, N.J.); and miconazole, Sigma (St. Louis, Mo.). Stock solutions of AMB were made to 10,000 μg/ml in 100% dimethyl sulfoxide (DMSO) and frozen at −70°C. Each of the stock solutions was thawed once, and fresh dilutions were used. Stock solutions of fluconazole and flucytosine were prepared in distilled water; and those of AMB, itraconazole, ketoconazole, and miconazole were prepared in 100% DMSO (24a).
Yeast isolates.
C. albicans yeast isolate MY2417 was obtained from the National Centre for Mycology, Edmonton, Alberta, Canada. This isolate has been used repeatedly and has been well characterized in our laboratory. The isolate was stored in skim milk at −70°C and was then subcultured twice on Sabouraud dextrose agar (Difco, Sparks, Md.) before use.
Time-kill curves. (i) Initial 10-h AMB treatment.
The C. albicans strain was grown aerobically in yeast peptone dextrose broth (1% mycological peptone, 1% yeast extract, 3% glucose) on a rotary shaker at 35°C until the desired concentration of approximately 4 × 106 CFU/ml was obtained. A total of 100 ml of culture was decanted into 500-ml Erlenmeyer flasks, and the various concentrations of AMB were added. The culture flasks, which contained AMB at concentrations ranging from 0 to 4 μg/ml, were then returned to the rotary shaker for incubation at 35°C in the dark for 10 h and were then assayed directly (1 ml samples) to quantitate the following: intracellular ATP concentration, numbers of CFU per milliliter, the levels of reduction of the tetrazolium salt 2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-5-[(phenylamino)carbonyl]-2H-tetrazolium hydroxide (XTT), and the fluorescent intensity of staining with 5,(6)-carboxyfluorescein diacetate (CFDA), bis-(1,3-dibutylbarbituric acid)trimethine oxonol [DiBAC4(3)], and SYBR Green Ι.
(ii) Sequential 15-h retreatment.
Following treatment with AMB for 10 h at 35°C, a representative number of these cultures (those cultures exposed to 0, 0.5, 0.7, 0.9, and 4.0 μg of AMB per ml) were each further exposed to a battery of antifungal agents for an additional 15 h at 21°C in the dark and assayed directly as described above. The AMB present during the initial 10-h incubation was not removed prior to any additional treatment. The additional treatments that followed after 10 h included (i) retreatment with the same concentration of AMB, (ii) treatment with fluconazole at 50 μg/ml (0.16 mM), (iii) treatment with itraconazole at 50 μg/ml (0.071 mM), (iv) treatment with ketoconazole at 50 μg/ml (0.094 mM), (v) treatment with miconazole at 10 μg/ml (0.021 mM), (vi) treatment with miconazole at 20 μg/ml (0.042 mM), (vii) treatment with flucytosine at 10 μg/ml (0.31 mM), (viii) treatment with flucytosine at 100 μg/ml (0.78 mM), and (ix) a control treatment consisting of no additional treatment. The concentrations listed above were chosen to achieve the maximum inhibition of the fungi that could be expected with each respective antifungal agent.
Plate counts.
The culture samples were grown on Sabouraud dextrose agar plates at 35°C for 48 h to assess reproductive competency. The samples were plated in triplicate after appropriate serial dilution in 0.85% physiological saline.
Particle counts.
A 1-ml culture sample was centrifuged at 9,300 × g for 5 min at 25°C and resuspended in 1 ml of 0.1 M MOPS (3-morpholinepropanesulfonic acid sodium) (pH 7.0). The number of cells per milliliter in the sample was assayed with an M430 counter (Coulter Electronics, Inc., Hialeah, Fla.).
Fluorescent dyes.
The stained culture samples were aliquoted (200 μl per well) in triplicate, and the aliquots were placed into a 96-well Immuno PolySorp plate (Nunc, Nalge Nunc International, Rochester, N.Y.) and assayed for relative fluorescence intensity with an FL500 microplate fluorescence reader (Bio-Tek Instruments, Inc., Winooski, Vt.). All the fluorescent dyes were optimally evaluated by using excitation and emission wavelengths of 485 and 530 nm, respectively.
(i) CFDA staining.
One milliliter of C. albicans culture was centrifuged at 9,300 × g for 5 min and was resuspended in MOPS buffer (0.1 M MOPS [pH 7]; Sigma). The samples were washed two additional times and were resuspended the final time in MOPS buffer with citric acid (50 mM citric acid [pH 3]). Ten microliters of a 5-mg/ml stock of CFDA (Molecular Probes Inc., Eugene, Oreg.) in DMSO was added to each 1-ml sample to achieve a final concentration of 50 μg/ml. Incubation with the stain was in the dark at 35°C with shaking for 45 min.
(ii) DiBAC4(3) staining.
One milliliter of C. albicans culture was centrifuged at 9,300 × g for 5 min and was resuspended in MOPS buffer (pH 7.0). Two microliters of a 1-mg/ml stock of DiBAC4(3) (Molecular Probes Inc.) in 100% ethanol was added to each 1-ml sample to achieve a final concentration of 2 μg/ml. Incubation with the stain was in the dark at room temperature with shaking for 1 h. The samples were then washed in MOPS buffer (pH 7.0) two times, as described above.
(iii) SYBR Green Ι staining.
One milliliter of C. albicans culture was centrifuged at 9,300 × g for 5 min and was resuspended in RPMI 1640 in MOPS buffer (pH 7.0). Fifteen microliters of a 1:100 dilution of a stock of SYBR Green Ι (Molecular Probes Inc.) in MOPS buffer (pH 7.0) was added to each 1-ml sample. Incubation with the stain was in the dark on ice at 4°C for 1 h. The samples were then washed two times in MOPS buffer (pH 7.0), as described above. The samples were maintained on ice.
XTT reduction assay.
XTT reduction was measured by a modification of the assay described by Tellier et al. (33). One milliliter of C. albicans culture was centrifuged at 9,300 × g for 5 min and then resuspended in RPMI 1640 modified medium without phenol red (Sigma). The culture samples were initially incubated for 30 min at 35°C with shaking at 160 rpm. A volume of 250 μl of a mix containing XTT (Sigma) and menadione (Sigma) was added to each 1-ml sample to achieve final concentrations of 0.2 mg of XTT per ml and 1.25 μM menadione. Menadione is an electron-coupling agent that potentiates the reduction of XTT. The mix was made fresh with 8 ml of a 1-mg/ml stock of XTT in MOPS and 5 μl of a 10 mM menadione stock in acetone. The samples were incubated for 1 h at 35°C to allow color development and were then centrifuged as described above. The colorimetric change of the supernatants was measured at 470 nm. Dilutions were included as required.
ATP luciferase assay. (i) Analytical equipment and reagents.
Light emission from the bioluminescence assay was measured in a Bio-Orbit (Turku, Finland) 1258 microplate luminometer. The luminescence reaction temperature was set internally to 21°C. The ATP assay mixture (Sigma) contained luciferin and luciferase and was prepared fresh, as described by the manufacturer. Apyrase ATPase (purified grade I; Sigma) was used to eliminate extracellular ATP before the extraction of intracellular ATP. The reagents were prepared with sterile, distilled water and were monitored for contaminating ATP by the luciferase ATP assay. The assay was performed immediately and protected from the light.
(ii) Elimination of extracellular ATP.
Culture samples of 1 ml were centrifuged at 9,300 × g and resuspended in Tris-EDTA buffer (0.1 M Tris buffer [pH 7.8] containing 2 mM EDTA). A total of 50 μl of each washed sample was incubated for 15 min at 37°C with 50 μl of 0.04% apyrase ATPase.
(iii) Extraction of intracellular ATP.
After elimination of the extracellular ATP, 50 μl of the apyrase-treated sample was pipetted into 500 μl of boiling Tris-EDTA buffer. After boiling of the mixture for 90 s, the extracts were cooled in an ice bath and immediately frozen at −75°C for later analysis. This procedure inactivated the apyrase ATPase and disrupted the fungal cells.
(iv) Measurement of intracellular ATP.
The ATP assay mixture (80 μl) was added to 200 μl of each thawed sample extract (in triplicate) in a 96-well opaque plate (Corning Costar Corp., Cambridge, Mass.). The intensity of the luminescence was determined photometrically for 10,000 ms after 1 min of incubation at 560 nm. The ATP concentration present in the sample extracts was determined by comparison with a standard curve of ATP concentrations.
RESULTS AND DISCUSSION
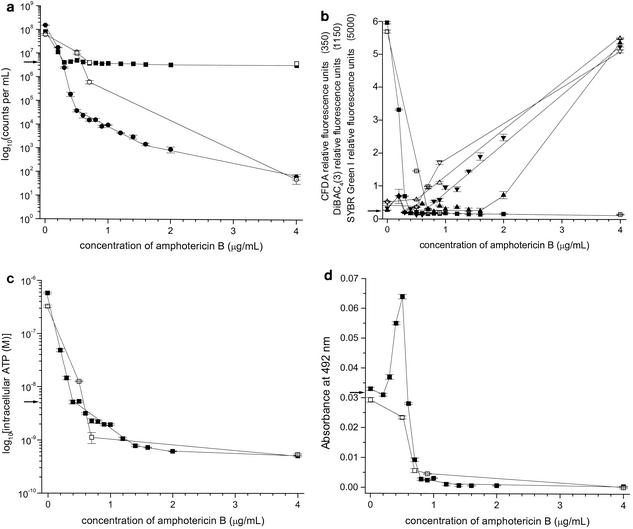
C. albicans MY2417 was exposed to AMB concentrations ranging from 0 to 4 μg/ml, and the toxic injury that resulted was characterized by measuring seven different parameters (Fig. 1a to d). The growth of C. albicans cells grown for 10 h at 37°C increased from 4 × 106 particles (as measured with a Coulter counter) and CFU per ml at the beginning of incubation to 9 × 107 particles per ml and 1.7 × 108 CFU per ml after incubation (Fig. 1a). Exposure to increasing concentrations of AMB inhibited this growth, and with AMB at 0.3 μg/ml, no net growth occurred during the 10-h period. At AMB concentrations above 0.3 μg/ml, the number of particles remained stable at the same number present at time zero, but the number of organisms capable of forming colonies declined. There were 90 and 99.9% reductions in the numbers of CFU at AMB concentrations of 0.4 and 0.5 μg/ml, respectively. With 1 μg of AMB per ml, only 9 × 103 organisms were still capable of reproduction. It has been the convention to refer to this as a fungicidal assay, with the assumption that the failure to form colonies was due to fungal cell death (24). The reason for this assumption was that, when aliquots of antifungal-treated yeast are plated onto solid medium, the diffusion of the antifungal agent into the buffer component of the medium reduces the concentration of the antifungal agent so that yeasts that were merely inhibited and not killed would resume reproduction and form colonies (1). Any cells that failed to grow are assumed to be dead (18). This assumption fails to consider antifungal agents with lengthy postantifungal effects (34); antifungal agents that do not quickly diffuse into the buffer medium, e.g., lipophilic molecules already incorporated into the cell membrane; and injured organisms that are alive but incapable of replication.
FIG. 1.
(a) Plate counts (numbers of CFU per milliliter) and particle counts per milliliter for C. albicans strain MY2417 incubated with AMB for 10 h at 35°C, followed by incubation for 15 h at 22°C. •, numbers of CFU per milliliter after incubation for 10 h at 35°C; ▪, particle counts per milliliter after incubation for 10 h at 35°C; ○, numbers of CFU per milliliter after incubation for 10 h at 35°C followed by incubation for 15 h at 22°C; □, particle counts per milliliter after incubation for 10 h at 35°C followed by incubation for 15 h at 22°C; →, numbers of CFU per milliliter and particle counts per milliliter at time zero. Error bars indicate standard errors. (b) DiBAC4(3), SYBR Green Ι, and CFDA staining of C. albicans strain MY2417 incubated with AMB for 10 h at 35°C, followed by incubation for 15 h at 22°C. ▾ and ▿, DiBAC4(3); ▴ and ▵, SYBR Green I; ▪ and □, CFDA; closed symbols, incubation for 10 h at 35°C; open symbols, incubation for 10 h at 35°C, followed by incubation for 15 h at 22°C; →, fluorescent staining values at time zero. Error bars indicate standard errors. (c) Intracellular ATP concentration for C. albicans strain MY2417 incubated with AMB for 10 h at 35°C and 10 h at 35°C, followed by incubation for 15 h at 22°C. ▪, incubation for 10 h at 35°C; □, incubation for 10 h at 35°C, followed by incubation for 15 h at 22°C; →, intracellular ATP concentration at time zero. Error bars indicate standard errors. (d) XTT reduction for C. albicans strain MY2417 incubated with AMB for 10 h at 35°C and for 10 h at 35°C, followed by incubation for 15 h at 22°C. ▪, incubation for 10 h at 35°C; □, incubation for 10 h at 35°C, followed by incubation for 15 h at 22°C; →, XTT reduction at time zero. Error bars indicate standard errors.
Both the intracellular ATP concentration (Fig. 1c) and the amount of fluorescence produced by the vitality-specific dye CFDA (Fig. 1b) decreased significantly to a plateau after treatment with approximately 0.3 μg of AMB per ml. CFDA is a nonpolar dye that is cleaved to carboxyfluorescein by nonspecific esterases in intact cells. In contrast, the fluorescence due to the mortality-specific dyes DiBAC4(3) and SYBR Green Ι remained at minimal levels until AMB concentrations above 0.6 and 1.6 μg/ml, respectively, were achieved (Fig. 1b). DiBAC4(3) is an anionic lipophilic dye that is sensitive to cytoplasmic membrane potential and that is excluded by normal cells with a negative internal charge (2). SYBR Green Ι is a nucleic acid binding dye whose fluorescence increases after intercalation into double-stranded DNA and is excluded by intact membranes (17). If the inability to form colonies in the presence of AMB at concentrations above 0.5 μg/ml was due solely to cell death, it is difficult to understand how these cells were able to maintain some degree of integrity and negative membrane potential in the presence of AMB at concentrations from 0.5 to 4 μg/ml (Fig. 1b). In summary, these findings indicate that AMB at concentrations between 0 and 0.4 μg/ml injured C. albicans cells and resulted in a 90% loss of reproduction, a complete reduction of intracellular esterase activity (CFDA), and a reduction of intracellular ATP concentrations by almost 100-fold. The results obtained with the mortality-specific dyes showed that, despite the degree of injury sustained, as described above, a negative membrane potential and cellular integrity continued to be maintained. Cell death began to occur only in the presence of AMB at concentrations higher than 1 μg/ml, as evidenced by staining with DiBAC4(3) and SYBR Green Ι. In the presence of AMB at concentrations higher than 4 μg/ml, there was no further increase in the level of mortality-specific dye fluorescence. These findings confirmed the results that we reported previously (22). We have conducted further studies on the behavior of C. albicans cells exposed to AMB at concentrations greater than 0.4 μg/ml. The observed decrease in the intracellular ATP concentration (Fig. 1c) prompted the investigation of an additional parameter that could be used to monitor the ATP production. The tetrazolium salt XTT has been used to measure the activity of the succinate-tetrazolium reductase system, which is part of the respiratory chain in mitochondria and which is involved in the production of ATP (31, 33). Figure 1d shows that the level of XTT reduction increased as C. albicans was exposed to AMB starting at a concentration of 0.2 μg/ml, with the increase in the level of XTT reduction rising to a peak at a concentration of 0.5 μg/ml and then declining to a minimum at concentrations above 0.8 μg/ml (Fig. 1d). To date, this is the only metabolic marker that we have found that indicates activity in cells exposed to this AMB concentration range. We postulate that the reduction of XTT is a marker for a state in which the yeasts are incapable of reproduction but are not yet fatally injured. It has previously been suggested that intact cells that no longer have the ability to form colonies may retain some metabolic activity and that different assays used to determine viability are measuring different fractions of the total population (19).
As the nonreplicating C. albicans cells were sublethally injured, the cells may have the capacity to recover from injury and undergo a restoration of replication (7). Consequently, we investigated conditions that would restore the capacity of C. albicans cells to replicate on agar plates. When the C. albicans cells treated with AMB for 10 h at 37°C were further incubated for 15 h at 22°C, the cells recovered the ability to replicate on agar that was lost during the initial 10-h exposure at 35°C (Fig. 1a). It is noteworthy that this resuscitation of replication occurred in the presence of AMB at the concentration present initially. At an AMB concentration of 0.7 μg/ml, the number of organisms capable of forming colonies increased to 6 × 105 from 1.5 × 104 (Fig. 1a). The rationale for using a lower incubation temperature of 22°C during the additional 15 h was based on several lines of reasoning. First, it has been empirically shown that microorganisms isolated from the environment do not form colonies on solid agar medium very well but can sometimes be induced to do so when the incubation temperature is decreased (27). Second, it was postulated that a lower incubation temperature could help prevent the outgrowth of a small population of cells and thereby aid in the identification of replication recovery for the entire culture of AMB-treated C. albicans cells, if it were to occur. The level of vitality-specific dye fluorescence and the intracellular ATP levels were not significantly affected (Fig. 1b and c). The levels of fluorescence of the mortality-specific dyes showed slight increases, indicating that some cell death occurred (Fig. 1b). With the restoration of reproduction, the cells also showed a decrease in the level of XTT reduction (Fig. 1d). The restoration of reproduction was further evidence that the C. albicans cells were injured and not dead in the presence of AMB at concentrations above 0.4 μg/ml.
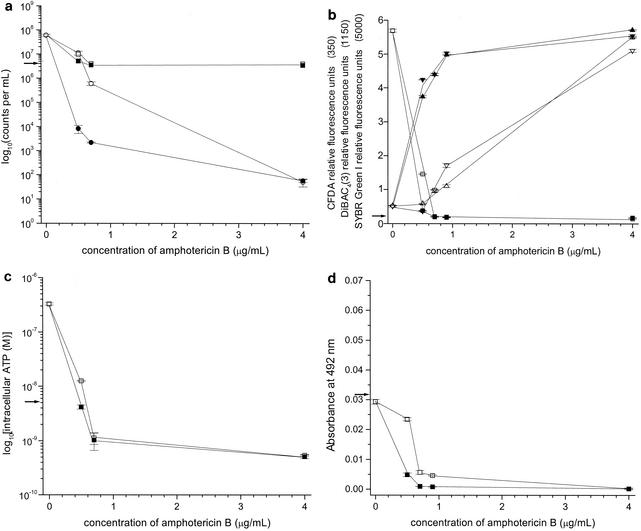
Subsequently, we investigated additional antifungal agents that could interfere with this resuscitation process and reduce the concentration of AMB required to fatally injure C. albicans cells. Miconazole was selected as a candidate because, in addition to interfering with ergosterol synthesis by inhibiting sterol C-14 demethylation, similar to other azole antifungal agents, it also has unique fungicidal activities and perturbs the lipid organization (32). Fungistatic concentrations of 0.05 μg of miconazole per ml induce minimal morphological changes at the cell periphery (13) and inhibit new hyphal outgrowth from parent yeast cells (25). Miconazole at 0.5 μg/ml has been shown to inhibit the mitochondrial ATPase (26) and to increase the cell volume and the number of peroxisomes (13). Miconazole at 5 μg/ml significantly reduces the numbers of CFU (4) and inhibits the plasma membrane ATPase (35) and the mitochondrial cytochrome oxidase (12). Miconazole at 10 μg/ml has also been shown to cause a 99% decrease in intracellular ATP concentrations. Direct membrane damage and cellular necrosis have been shown to result from exposure to miconazole at 50 μg/ml, likely due to peroxide accumulation (12, 32). For these reasons we tested C. albicans MY1417 under the resuscitation conditions in the presence or absence of 20 μg of miconazole per ml. This concentration of miconazole reduced the number of cells capable of forming colonies in the presence of 0.7 μg AMB of per ml from 6 × 105 to 2.1 × 103 (Fig. 2a). In other words, recovery of the reproductive capacity was prevented by miconazole. Furthermore, the presence of miconazole greatly decreased the concentration of AMB required to increase the fluorescence of the mortality-specific dyes DiBAC4(3) and SYBR Green Ι (Fig. 2b). The levels of DiBAC4(3) and SYBR Green Ι fluorescence became nearly maximal in the presence of 0.9 μg of AMB per ml. Miconazole is known to inhibit the synthesis of ergosterol and produce direct damage to the cytoplasmic membrane (4). The results obtained with DiBAC4(3) and SYBR Ι Green were likely a consequence of this. The level of reduction of XTT was slightly decreased (Fig. 2d), and the concentration of intracellular ATP was unchanged (Fig. 2c).
FIG. 2.
(a) Plate counts (numbers of CFU per milliliter) and particle counts per milliliter for C. albicans strain MY2417 incubated with AMB for 10 h at 35°C, followed by incubation for 15 h at 22°C or by incubation with miconazole at 20 μg/ml for 15 h at 22°C. ○, numbers of CFU per milliliter after incubation for 10 h at 35°C, followed by incubation for 15 h at 22°C; □, particle counts per milliliter after incubation for 10 h at 35°C, followed by incubation for 15 h at 22°C; •, numbers of CFU per milliliter after incubation for 10 h at 35°C, followed by incubation with miconazole at 20 μg/ml for 15 h at 22°C; ▪, particle counts per milliliter after incubation for 10 h at 35°C, followed by incubation for 15 h at 22°C with miconazole at 20 μg/ml; →, numbers of CFU per milliliter and particle counts per milliliter at time zero. Error bars indicate standard errors. (b) DiBAC4(3), SYBR Green Ι, and CFDA staining of C. albicans strain MY2417 incubated with AMB for 10 h at 35°C, followed by incubation for 15 h at 22°C or with miconazole at 20 μg/ml for 15 h at 22°C. ▾ and a▿, DiBAC4(3); ▴ and ▵, SYBR Green Ι; ▪ and □, CFDA; closed symbols, incubation for 10 h at 35°C, followed by incubation with miconazole at 20 μg/ml for 15 h at 22°C; open symbols, incubation for 10 h at 35°C, followed by incubation for 15 h at 22°C; →, fluorescent staining values at time zero. Error bars indicate standard errors. (c) Intracellular ATP concentration for C. albicans strain MY2417 incubated with AMB for 10 h at 35°C, followed by incubation for 15 h at 22°C or incubation with miconazole at 20 μg/ml for 15 h at 22°C. □, incubation for 10 h at 35°C, followed by incubation for 15 h at 22°C; ▪, incubation for 10 h at 35°C, followed by incubation with miconazole at 20 μg/ml for 15 h at 22°C; →, intracellular ATP concentration at time zero. Error bars indicate standard errors. (d) XTT reduction for C. albicans strain MY2417 incubated with AMB for 10 h at 35°C, followed by incubation for 15 h at 22°C or by incubation with miconazole at 20 μg/ml for 15 h at 22°C. □, incubation for 10 h at 35°C, followed by incubation for 15 h at 22°C; ▪, incubation for 10 h at 35°C, followed by incubation with miconazole at 20 μg/ml for 15 h at 22°C; →, XTT reduction at time zero. Error bars indicate standard errors.
A comparison of the relative abilities of miconazole, ketoconazole, fluconazole, itraconazole, and flucytosine to interfere with resuscitation at 22°C for 15 h and thereby reduce the concentration of AMB required to fatally injure C. albicans cells was conducted by measuring the DiBAC4(3) and SYBR Green Ι fluorescence of the cells initially exposed to 0.5 μg of AMB per ml for 10 h and incubated with the respective antifungal agent by using resuscitation conditions. The fluorescence intensities were compared to the maximum fluorescence intensity that was measured under the same conditions in the presence of 4 μg of AMB per ml and expressed as a percentage. Simultaneously, the number of CFU remaining after the sequential exposure to the two antifungal agents was measured. The results, shown in Table 1, indicate that 50 μg of fluconazole per ml and 10 and 100 μg of flucytosine per ml did not inhibit resuscitation or increase the level of mortality-specific staining. Of the remaining azoles tested, miconazole was the most effective at both increasing the level of mortality-specific staining and preventing resuscitation. Ketoconazole was more effective than itraconazole but was less effective than miconazole. It is possible that this difference in activity between ketoconazole and itraconazole was due to the lower concentration of itraconazole used (0.071 mM itraconazole versus 0.094 mM ketoconazole) or the decreased solubility of itraconazole compared to that of ketoconazole. The lower inhibitory effects of ketoconazole and itraconazole likely originated from their fungistatic activities. In summary, we have shown that an AMB concentration of 0.4 μg/ml is sufficient to reduce the number of CFU by 90%. This reduction was unlikely due to the death of the organisms because they (i) continued to demonstrate XTT reduction, (ii) were capable of restoring reproduction after incubation at 22°C for 15 h in the continued presence of AMB, and (iii) could be further damaged by the addition of miconazole.
TABLE 1.
Plate counts and mortality-specific staining for treatment of C. albicans MY2417 with amphotericin B for 10 h at 35°C, followed sequentially by treatment for 15 h at 22°C with the addition of a second antifungal agent
| Antifungal agent (concn [μg/ml]) | % of maximum fluorescence
|
No. of CFU/ml | |
|---|---|---|---|
| DiBAC4(3) | SYBR Green I | ||
| Growth control | 3.2 | 5.5 | 1.1 × 107 |
| Fluconazole (50) | 3.6 | 8.7 | 1.0 × 107 |
| Flucytosine (10) | 2.7 | 5.5 | 6.6 × 106 |
| Flucytosine (100) | 3.4 | 5.8 | 3.6 × 106 |
| Itraconazole (50) | 17.9 | 31.5 | 2.8 × 106 |
| Ketoconazole (50) | 36.5 | 58.5 | 8.5 × 105 |
| Miconazole (10) | 81.5 | 31.1 | 9.4 × 103 |
| Miconazole (20) | 76.1 | 63.6 | 8.5 × 103 |
Previous researchers have interpreted the failure of AMB-treated C. albicans cells to form colonies after they were plated on nutrient agar as meaning that the cells were dead. Other explanations are possible. First, AMB causes a lengthy postantifungal effect that inhibits replication for a duration of between 5 and 10 h for C. albicans, depending on the AMB concentration and the time of exposure (15, 30, 34). The postantifungal effect is a suppression of fungal growth that persists after limited exposure to an antifungal agent (34) and may have therapeutic relevance in the determination of antifungal dosing regimens (11). Lower temperatures decrease the duration of the postantifungal effect (16) and have also been shown to reduce the length of the recovery time for injured fungal cells (20). Sublethally stressed fungal cells exhibit a lag before the onset of replicative growth due to the repair of cell damage (9), and it has been suggested that the duration of the postantifungal effect induced by AMB may be related to the time required for yeast cell wall damage to be repaired (34).
Second, AMB may not have been effectively removed by dilution when the cells were plated (29). AMB is more likely to remain bound to the cell membrane of C. albicans after plating of the cells than to diffuse into the plate medium buffer. For the azoles it is well established that their postantifungal effects and lipophilic nature are correlated (29), and the nonlipophilic fluconazole is the only azole that lacks a postantifungal effect (15, 29).
If AMB concentrations previously interpreted as cidal are in fact injuring yeasts so that they cannot reproduce and this defect is reversible, then the clinical failures encountered when this antifungal agent is used are more understandable. Other clinically confounding reasons, such as differences in the conditions at the site of infection, may explain why some patients fail treatment (28). In the clinical treatment of C. albicans infections with conventional intravenous doses of AMB, peak levels in serum rise to between 0.5 and 2 μg/ml, fall rapidly, and slowly reach a plateau between 0.2 and 0.5 μg/ml (6). The risk of permanent renal impairment and the difficulties with the solubility of this agent prevent the use of doses that would achieve 4 μg of AMB per ml (8).
Our findings indicate that C. albicans sustains concentration-dependent AMB injury that can differentially compromise key cell functions. In the presence of AMB concentrations between 0 and 0.5 μg/ml, intracellular esterase activity, intracellular ATP concentrations, and levels of reproduction are greatly reduced. In the presence of intermediate AMB concentrations (between 0.5 and 4 μg/ml), cell membrane potential and integrity are affected, and on the basis of the level of XTT reduction, mitochondrial ATP pathways show increased activity. All activity ceases in the presence of AMB at concentrations above 4 μg/ml. In the presence of AMB concentrations lower than 4 μg/ml, the ability to reproduce is recoverable after 15 h at 22°C. Thus, the inability of the cell to reproduce in a fungicidal assay does not represent cell death. The discrepancy may be due to the postantifungal effect, the hydrophobicity of the drug, the inability of the yeast to undergo repair in the time available, or some combination of these. In the future it may be necessary to interpret antifungal killing more carefully by confirming with mortality-specific dyes if one of these conditions is present.
AMB has been a mainstay of therapy against systemic fungal disease since its introduction. Throughout that time, treatment failures, after seemingly adequate drug levels have been achieved, have been clinically disappointing, with serious consequences for patients. In some cases AMB may not be able to achieve concentrations for a prolonged duration sufficient to effect lethal damage. Our results indicate that the fungal cells are only sublethally injured in the presence of AMB at concentrations that are interpreted as fungicidal after cells are directly plated on solid media. Novel combination therapy with agents or combinations of agents that show significant mortality-specific dye activity at the physiologically achievable therapeutic ranges may merit further investigation. Additional experiments are required before direct comparisons can be made between the results described here and the MIC and minimum fungicidal concentration studies that use a longer (24-h) incubation with AMB.
REFERENCES
- 1.Amsterdam, D. 1996. Susceptibility testing of antimicrobials in liquid media, p. 52-111. In V. Lorian (ed.), Antibiotics in laboratory medicine, 4th ed. The Williams & Wilkins Co., Baltimore, Md.
- 2.Bashford, C. L., B. Chance, J. C. Smith, and T. Yashida. 1979. The behavior of oxonol dyes in phospholipid dispersion. Biophys. J. 25:63-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beck-Sagué, C. M., W. R. Jarvis, and the National Nosocomial Infections Surveillance System. 1993. Secular trends in the epidemiology of nosocomial fungal infections in the United States, 1980-1990. J. Infect. Dis. 167:1247-1251. [DOI] [PubMed] [Google Scholar]
- 4.Beggs, W. H. 1984. Growth phase in relation to ketoconazole and miconazole susceptibilities of Candida albicans. Antimicrob. Agents Chemother. 25:316-318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beggs, W. H. 1994. Physiochemical cell damage in relation to lethal amphotericin B action. Antimicrob. Agents Chemother. 38:363-364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bennett, J. 1990. Antifungal agents, p. 404-406. In G. L. Mandell, R. G. Douglas, and J. E. Bennett (ed.), Principles and practice of infectious diseases, 3rd ed. Churchill Livingstone, New York, N.Y.
- 7.Beuchat, L. R. 1984. Injury and repair of yeasts and moulds. Soc. Appl. Bacteriol. Symp. Ser. 12:293-306. [PubMed] [Google Scholar]
- 8.Bodey, G. P. 1996. Antifungal agents, p. 229-248. In V. T. Andriole (ed.), Current infectious disease drugs. Current Medicine, Philadelphia, Pa.
- 9.Busta, F. F. 1978. Introduction to injury and repair of microbial cells. Adv. Appl. Microbiol. 23:195-201. [DOI] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention. 1996. National nosocomial infections surveillance (NNIS) report, data summary from October 1986-April 1996. Am. J. Infect. Control 24:380-388. [PubMed] [Google Scholar]
- 11.Craig, W. A., and S. Gudmundsson. 1996. The postantibiotic effect, p. 296-329. In V. Lorian (ed.), Antibiotics in laboratory medicine, 4th ed. The Williams & Wilkins Co., Baltimore, Md.
- 12.De Nollin, S., H. Van Belle, G. Goossens, F. Thone, and M. Borgers. 1977. Cytochemical and biochemical studies of yeasts after in vitro exposure to miconazole. Antimicrob. Agents Chemother. 11:500-513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Nollin, S., and M. Borgers. 1975. The effects of miconazole on the ultrastructure of Candida albicans. Sabouraudia 13:63-73. [PubMed] [Google Scholar]
- 14.Edwards, J. E., et al. 1997. International Conference for the Development of a Consensus on the Management and Prevention of Severe Candida Infections. Clin. Infect. Dis. 25:43-59. [DOI] [PubMed] [Google Scholar]
- 15.Ernst, E. J., M. E. Klepser, and M. A. Pfaller. 2000. Postantifungal effects of echinocandin, azole, and polyene antifungal agents against Candida albicans and Cryptococcus neoformans. Antimicrob. Agents Chemother. 44:1108-1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garcia, M. T., M. T. Llorente, F. Minguez, and J. Prieto. 2000. Influence of temperature and concentration on the postantifungal effect and the effects of sub-MIC concentrations of four antifungal agents on previously treated Candida species. Chemotherapy (Basel) 46:245-252. [DOI] [PubMed] [Google Scholar]
- 17.Haugland, R. P. 1992. Handbook of fluorescent probes and research chemicals, 5th ed. Molecular Probes, Inc., Eugene, Oreg.
- 18.Jawetz, E., G. F. Brooks, J. L. Melnick, J. S. Butel, E. A. Adelberg, and L. N. Ornston. 1995. The growth, survival & death of microorganisms, p. 44-52. In Medical microbiology, 20th ed. Appleton & Lange, Norwalk, Conn.
- 19.Jones, R. P. 1987. Measures of yeast death and deactivation and their meaning, parts I and II. Process. Biochem. 22:118-134. [Google Scholar]
- 20.Katsui, N., T. Tsuchido, M. Takano, and I. Shibasaki. 1982. Viability of heat-stressed cells of micro-organisms as influenced by pre-incubation and post-incubation temperatures. J. Appl. Bacteriol. 53:103-108. [DOI] [PubMed] [Google Scholar]
- 21.Lentini, A. 1993. A review of the various methods available for monitoring the physiological status of yeast: yeast viability and vitality. Fermentation 6:321-327. [Google Scholar]
- 22.Liao, R. S., R. P. Rennie, and J. A. Talbot. 1999. Assessment of the effect of amphotericin B on the vitality of Candida albicans. Antimicrob. Agents Chemother. 43:1034-1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mason, C. A., G. Hamer, and J. D. Bryers. 1986. The death and lysis of microorganisms in environmental processes. FEMS Microbiol. Rev. 39:373-401. [Google Scholar]
- 24.McGinnis, M. R., and M. G. Rinaldi. 1996. Antifungal drugs: mechanisms of action, drug resistance, susceptibility testing, and assays of activity in biologic fluids, p. 176-211. In V. Lorian (ed.), Antibiotics in laboratory medicine, 4th ed. The Williams & Wilkins Co., Baltimore, Md.
- 24a.National Committee for Clinical Laboratory Standards. 1997. Reference method for broth dilution antifungal susceptibility testing of yeasts. M27-A. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 25.Odds, F. C., A. Cockayne, J. Hayward, and A. B. Abbott. 1985. Effects of imidazole- and triazole-derivative antifungal compounds on the growth and morphological development of Candida albicans hyphae. J. Gen. Microbiol. 131(Pt. 10):2581-2589. [DOI] [PubMed] [Google Scholar]
- 26.Portillo, F., and C. Gancedo. 1984. Mode of action of miconazole on yeasts: inhibition of the mitochondrial ATPase. Eur. J. Biochem. 143:273-276. [DOI] [PubMed] [Google Scholar]
- 27.Postgate, J. R. 1976. Death in macrobes and microbes, p. 1-18. In T. R. G. Gray and J. R. Postgate (ed.), The survival of vegetative microbes. Cambridge University Press, Cambridge, United Kingdom.
- 28.Rex, J. H., M. A. Pfaller, T. J. Walsh, V. Chaturvedi, A. Espinel-Ingroff, M. A. Ghannoum, L. L. Gosey, F. C. Odds, M. G. Rinaldi, D. J. Sheehan, and D. W. Warnock. 2001. Antifungal susceptibility testing: practical aspects and current challenges. Clin. Microbiol. Rev. 14:643-658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schiven, M., C. Scheven, K. Hahn, and A. Senf. 1995. Post-antibiotic effect and post-expositional polyene antagonism of azole antifungal agents in Candida albicans dependence on substance lipophilia. Mycoses 38:435-442. [DOI] [PubMed] [Google Scholar]
- 30.Shu, M., A. N. B. Ellepola, and L. P. Samaranayake. 2001. Effects of two different growth media on the postantifungal effect induced by polyenes on Candida species. J. Clin. Microbiol. 39:2732-2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Slater, T. F., B. Sawyer, and U. Straul. 1963. Studies on succinate-tetrazolium reductase systems. Biochim. Biophys. Acta 77:383-393. [DOI] [PubMed] [Google Scholar]
- 32.Sud, I. J., and D. S. Feingold. 1981. Heterogeneity of action mechanisms among antimycotic imidazoles. Antimicrob. Agents Chemother. 20:71-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tellier, R., M. Krajden, G. A. Grigoriew, and I. Campbell. 1992. Innovative endpoint determination system for antifungal susceptibility testing of yeasts. Antimicrob. Agents Chemother. 36:1619-1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Turnidge, J. D., S. Gudmundsson, B. Vogelman, and W. A. Craig. 1994. The postantibiotic effect of antifungal agents against common pathogenic yeasts. J. Antimicrob. Chemother. 34:83-92. [DOI] [PubMed] [Google Scholar]
- 35.Van den Bossche, H., J. M. Ruysschaert, F. Defrise-Quertain, G. Willemsens, F. Cornelissen, P. Marichal, W. Cools, and J. Van Cutsem. 1982. The interaction of miconazole and ketoconazole with lipids. Biochem. Pharmacol. 31:2609-2617. [DOI] [PubMed] [Google Scholar]
- 36.Wey, S. B., M. Mori, M. A. Pfaller, R. F. Woolson, and R. P. Wenzel. 1988. Hospital-acquired candidemia. The attributable mortality and excess length of stay. Arch. Intern. Med. 148:2642-2645. [DOI] [PubMed] [Google Scholar]