The field of antimicrobial pharmacodynamics examines the relationship between drug pharmacokinetics and antimicrobial activity or host toxicity (9). These investigations have been valuable for defining optimal antimicrobial dosing regimens and validating in vitro susceptibility breakpoints (4, 14, 25). The concepts encompassing this discipline were defined initially with antibacterial compounds (9, 34, 35). With the advent of standardized and reproducible antifungal susceptibility testing, similar pharmacodynamic analyses have been undertaken (24). Both in vitro and in vivo models have been able to demonstrate a correlation between drug dose, the MIC for an organism, and outcome (15, 17, 18, 36, 37). These investigations have been important for describing the relative potencies of antifungal drugs against a number of important pathogens. More recent in vivo pharmacodynamic investigations have examined the relationship among drug dose, dosing interval, MIC, and treatment outcome to define the specific pharmacodynamic parameter and parameter magnitude predictive of antifungal drug activity. This minireview summarizes these in vivo antifungal pharmacodynamic investigations.
PATTERNS OF ANTIMICROBIAL ACTIVITY
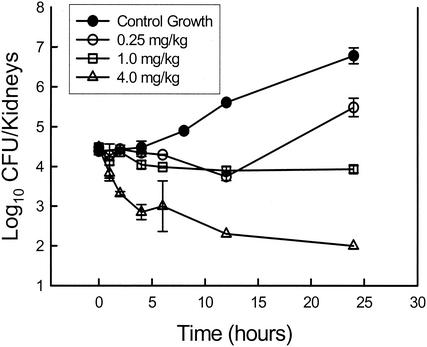
In examining pharmacodynamic relationships, three patterns of antimicrobial activity have been identified (9). The patterns of activity have been described by examining the relationship between drug concentration and antimicrobial effect over time or the time course of antimicrobial activity. Two factors have been found to be important in describing this time course of activity. The first is the impact of the drug concentration on the rate and extent of organism killing. When antimicrobial killing is enhanced by increasing drug levels, the pattern of activity is referred to as “concentration dependent.” In these studies, the polyene amphotericin B and drugs of the new echinocandin class have been shown to exhibit concentration-dependent killing (2, 5, 26). When peak serum amphotericin B levels exceeded the MIC for Candida albicans by fourfold, killing of greater than 1 log10 was observed. The highest doses examined produced a peak level in serum in relation to the MIC (peak/MIC) of 10, which resulted in nearly 2-log10 organism killing. As shown in Fig. 1, a similar peak/MIC relationship was observed with the new echinocandin derivative HMR 3270, in that killing was observed when the levels in serum exceeded the MIC by a factor of more than 3 and maximal killing was observed when the peak/MIC ratios reached a value of 10. Similarly, Petraitis et al. (26) observed a concentration-dependent reduction in the Candida burden in rabbits treated over an eightfold dose range with the echinocandin micafungin (FK463).
FIG. 1.
Relationship between echinocandin (HMR 3270) dose and burden of C. albicans in the kidneys of neutropenic mice over time. Each datum point represents the mean Candida burden in kidneys from two animals. (Modified from reference 5.)
On the other hand, when higher concentrations do not increase the rate or extent of organism death, the time course of activity is called “concentration-independent” or “time-dependent” killing. This pattern of activity has been observed with drugs of the triazole class and flucytosine (1, 3, 6). In studies with fluconazole, even a dose that produced levels exceeding the MIC by more than a factor of 200 did not result in organism killing, confirming the fungistatic character of the azole compounds.
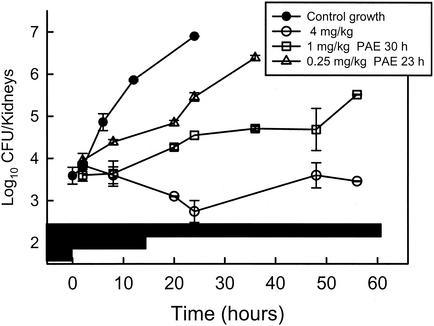
The second factor important for describing the antimicrobial activity pattern is the organism growth dynamics after drug exposure (9, 10). With some antimicrobial drug classes, organism growth continues to be suppressed after the antimicrobial is no longer present at levels defined as necessary for drug activity (the MIC). This period of growth suppression is called the postantibiotic effect (PAE). Studies with both amphotericin B and the echinocandins produced prolonged persistent growth inhibition (2, 5). For example (Fig. 2), with amphotericin B, the PAE durations exceeded 20 h and increased with dose escalation. Similarly, the PAE durations with an echinocandin lengthened with higher doses and exceeded 80 h with the highest dose studied. Prolonged in vitro PAEs have also been observed with these compounds (15, 18). However, the duration of growth suppression in the in vivo studies is much longer than that described in vitro. This phenomenon of longer in vivo PAEs than in vitro PAEs has been described for antibacterials as well (10). Several hypotheses have been offered to explain these differences, including enhanced growth of organisms in the nutrient-rich broths used for in vitro studies as well as the potential that a portion of the in vivo PAE duration could be due to sub-MIC effects.
FIG. 2.
In vivo PAE of amphotericin B against C. albicans in neutropenic mice. Each datum point represents the mean Candida burden in kidneys from two animals. The black horizontal bars represent the amount of time that each of the dose levels would produce serum drug levels exceeding the MIC for the infecting organism. (Reprinted from reference 2.)
In studies with flucytosine, only modest periods of growth suppression were observed (3). On the other hand, studies with two triazole compounds revealed prolonged in vivo PAEs durations more than four times the half-lives of these compounds in serum in the animal model used (4, 6). These findings of prolonged in vivo PAEs with triazole drugs are in contrast to the findings from in vitro investigations that have not identified PAEs (15, 18, 33). However, these in vitro studies have demonstrated a reduction in the growth rate in the presence of triazoles at concentrations below the MIC (33). It is likely that at least a portion of the in vivo PAE duration with triazoles is due to these sub-MIC effects.
Antifungal time course studies in vivo have identified three combinations of these two factors (Table 1): (i) concentration-dependent killing and prolonged PAEs with amphotericin B and the echinocandin, (ii) time-dependent killing and short or no PAEs with flucytosine, and (iii) time-dependent killing and prolonged PAEs with the triazoles.
TABLE 1.
In vivo antifungal pharmacodynamic characterizations
| Drug class | Time course of activity
|
Pharmacodynamic parameter
|
||
|---|---|---|---|---|
| Killing | PAE | Type | Magnitudea | |
| Triazole | Static | Long | AUC/MIC | 25 |
| Polyene | Cidal | Long | Peak/MIC | 4 (10) |
| Flucytosine | Static | Short | T > MIC | 25b |
| Echinocandin | Cidal | Long | Peak/MIC | 3 (10) |
| Sordarin | NAc | NA | AUC/MIC | NA |
The magnitude end point is the dose necessary to achieve 50% of the maximal effect. The data represent the ratio for AUC/MIC and the percentage of the dosing interval for T > MIC.
Dose associated with maximal efficacy.
NA, not available.
PHARMACODYNAMIC PARAMETERS
Three pharmacodynamic parameters have been shown to describe the association between antimicrobial dosing and treatment effect (9, 34). These parameters include the percentage of time that the levels of drug in serum exceed the MIC (T > MIC), the peak/MIC, and the area under the serum concentration-time curve (AUC) in relation to the MIC (AUC/MIC). Numerous studies with antibacterial drugs have demonstrated that the specific parameter predictive of activity varies for different drug classes but is usually the same for drugs within a class (9). Each of the pharmacodynamic parameters is associated with one of the time course of antimicrobial activity patterns described above (Table 1). For drugs that demonstrate time-dependent killing and short or no PAEs, drug dosing is optimized by prolonging the duration of exposure of the organism to the drug. The parameter that considers this exposure is T > MIC. Antimicrobials are often administered at lower doses but are dosed more frequently or even continuously to take advantage of this pattern of activity. For drugs exhibiting concentration-dependent killing and long PAEs, antimicrobial efficacy is optimized by the infrequent administration of large doses. The pharmacodynamic parameters that represent this type of dosing are the peak/MIC and the AUC/MIC. The final pattern of drug activity is characterized not only by concentration-independent killing but also by prolonged persistent growth suppression, which increases the importance of the concentration or the total amount of drug administered. The AUC represents the total amount of drug exposure, and the AUC/MIC is the predictive pharmacodynamic parameter.
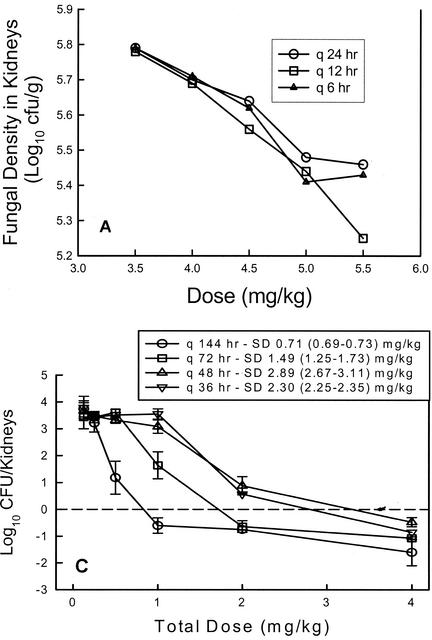
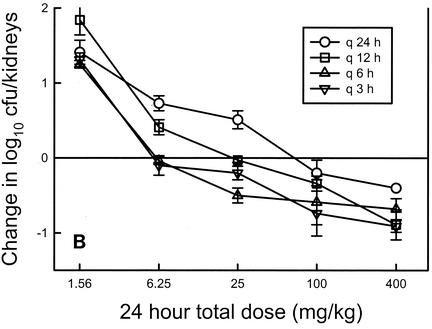
Dose fractionation studies represent another approach that has been helpful in defining which of the three pharmacodynamic parameters is best associated with drug efficacy. In traditional in vivo treatment experiments, the dose levels may vary widely but the variation in the dosing interval is most often limited (17, 26, 36, 37). In dose fractionation studies a variety of dose levels are administered by using three or more dosing intervals. In examining treatment results, if the regimens with shorter dosing intervals are more efficacious, the time-dependent parameter (T > MIC) is the more important parameter. If the large, infrequently administered dosing regimens are more active, the peak level in relation to the MIC is most predictive. Lastly, if the outcome is similar with each of the dosing intervals, the outcome is dependent on the total dose or the AUC for the dosing regimen. Louie et al. (22) (Fig. 3A) first examined the impact of fluconazole dose fractionation and demonstrated that similar outcomes were observed whether the dose was administered as a single bolus or as two or three smaller doses. These observations are consistent with the time course pattern which included time-dependent killing but prolonged persistent effects, in which it is the AUC for exposure and not the dosing interval that affects the treatment outcome. Dose fractionation studies subsequent to this confirmed the importance of the AUC/MIC parameter for both fluconazole and a new triazole, ravuconazole (1, 6). These observations suggest that the pharmacodynamic parameter associated with efficacy is similar within the azole drug class, as has been described for drugs in various antibacterial classes.
FIG. 3.
(A) Relationship between 24-h total fluconazole dose for three dosing intervals and fungal density in kidneys of mice in a candidiasis model. (Data from reference 22.) (B) Relationship between 24-h total flucytosine dose for four dosing intervals in a neutropenic candidiasis model. Each datum point represents the mean Candida burden in kidneys two animals. The solid horizontal line represents the burden of organisms in kidneys of mice at the start of therapy. (Reprinted from reference 3.) (C) Relationship between total echinocandin dose for four dosing intervals in a neutropenic candidiasis model. Each datum point represents the mean Candida burden in kidneys from two animals. The dashed horizontal line represents the burden of organisms in kidneys of mice at the start of therapy. SD, the dose necessary to produce no organism growth in the kidneys of mice relative to the burden at the start of therapy. Values in parentheses represent the 95% confidence intervals for the fungistatic dose. (Reprinted from reference 5.) In each panel, the letter q followed by a time indicates the frequency of dosing.
Dose fractionation studies have similarly been performed with flucytosine (Fig. 3B), amphotericin B, an echinocandin (Fig. 3C), and a sordarin derivative (2, 3, 5, 8). In the investigations with flucytosine, greater efficacy was observed when regimens with small, frequently administered doses were used. In regimens that used a shorter dosing interval, the amount of drug needed to achieve 50% of the maximal microbiologic effect was nearly 10-fold less than the amount needed in regimens with largely spaced dosing intervals. These studies demonstrate the importance of prolonged exposures, suggesting that T > MIC is the most important parameter. This is consistent with the time course studies which identified time-dependent killing and short PAEs.
On the other hand, in similar studies with amphotericin B and an echinocandin (HMR 3270), large doses with widely spaced dosing intervals were most efficacious. When amphotericin B was administered once every 72 h, the amount of drug needed to achieve the microbiologic endpoint of a fungistatic effect was nearly eightfold lower than that needed when the drug was provided every 12 h. In similar studies with an echinocandin, one also observes a shift in the dose-response curves to the right as the dosing interval is shortened, again suggesting that optimal killing is achieved with high peak levels. Dose fractionation experiments with a drug from the sordarin class (GM 237354) demonstrated that the outcome, whether it is measured by survival or organism number, was dependent on the total dose, not the dosing interval, similar to observations with the triazole class (8).
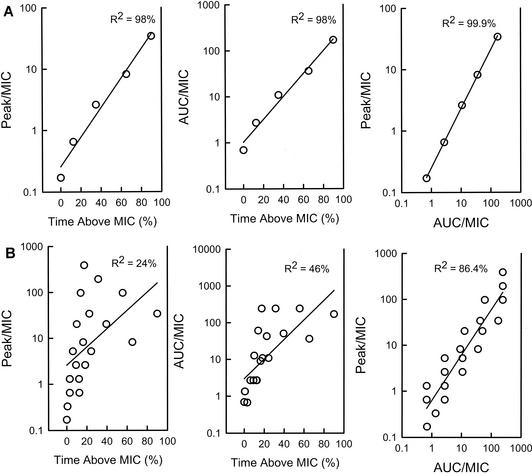
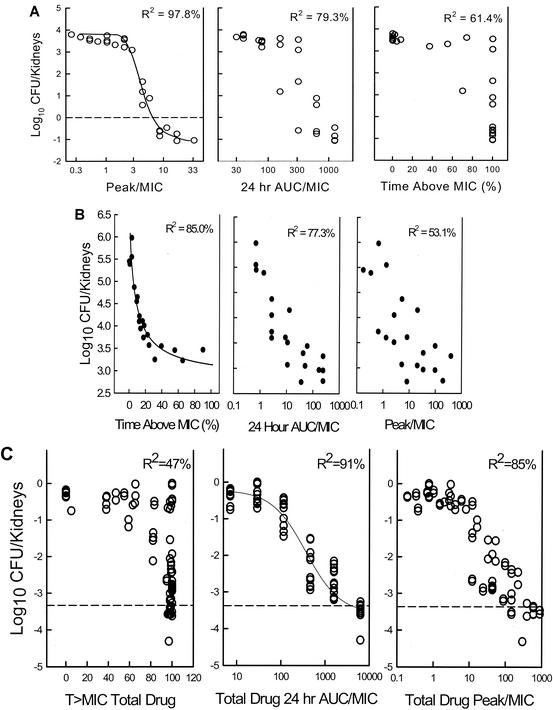
Data from these dose fractionation studies can also be used to specifically examine the relationship between outcome and each parameter by expressing each dosing regimen as a pharmacodynamic parameter value. A 1-g drug dose administered in the treatment of an infection caused by an organism for which the MIC is 1 μg/ml is expressed as (i) the percentage of time that levels in serum remain above the MIC of 1 μg/ml, (ii) the peak/MIC, and (iii) the AUC/MIC. Using nonlinear regression analysis on a sigmoid Emax (maximum-effect) model, one can then examine the correlation between the parameter and the outcome (7, 9). It is difficult for traditional dose-ranging single-dosing-interval studies to differentiate among parameters in this way due to the interrelationship among the pharmacodynamic parameters. If a higher dose of drug is administered, the peak level, the AUC, and the T > MIC each increases. As shown in Fig. 4, one can see the strong correlation among each of the parameters in studies with five dose levels but a single dosing interval of the antifungal flucytosine (4). On the other hand, one is able to reduce this correlation by also varying the dosing interval, allowing one to more easily determine which parameter is associated with treatment efficacy. This relationship has been examined by analysis of data from dose fractionation studies with each of the antifungal drug classes (Table 1) and confirmed the importance of the pharmacodynamic parameter predicted by the time course and dose fractionation studies. The relationship among the treatment data for amphotericin B was clearly strongest with the peak/MIC parameter. As shown in Fig. 5A, the relationship between echinocandin treatment and efficacy is strongest when the dosing regimen data are represented by the peak/MIC. There is more data scatter when the outcome is shown in relation to either the T > MIC or the 24-h AUC/MIC. For flucytosine (Fig. 5B), T > MIC was most closely associated with efficacy. For the triazoles (Fig. 5C) and the sordarin class, the dose fractionation study results were best correlated with the 24-h AUC/MIC.
FIG. 4.
Impact of increasing dose and a single dosing interval (A) and three dosing intervals (B) on the interrelationship among peak/MIC, AUC/MIC, and T > MIC for flucytosine. R2 is the coefficient of determination. (Reprinted from reference 7 with permission.)
FIG. 5.
(A) Relationship between three pharmacodynamic parameters and Candida organism burden in the kidneys of neutropenic animals treated with an echinocandin (A), flucytosine (B), and the triazole ravuconazole (C). Each symbol represents the mean organism burden in kidneys from two mice. The dashed lines represent the burden of Candida in the kidneys of mice at the start of therapy. R2 is the coefficient of determination. (The data in panels A, B, and C are from references 5, 3, and 6, respectively.)
PHARMACODYNAMIC PARAMETER MAGNITUDE
Studies defining the importance of a specific pharmacodynamic parameter can help determine whether dosing with a drug class is likely to be most efficacious when dosing is frequent or infrequent. These studies do not, however, tell us what drug dose is needed for efficacy. However, additional studies can be undertaken with a sufficient number of organisms with various degrees of susceptibility to the drug to help define the amount of drug or pharmacodynamic parameter magnitude necessary for treatment success. These studies have demonstrated that the magnitude of a pharmacodynamic parameter associated with efficacy is similar for drugs within the same class, provided that free drug levels are considered (9, 11, 19). Furthermore, in the extensive studies with antibacterials, this parameter magnitude has been shown to be independent of the animal species, dosing interval, site of infection, and most often, the infecting pathogen (2, 9, 12). For example, the T > MIC necessary for penicillin efficacy in a mouse is the same as that needed for efficacy in humans. This concordance among species is not surprising if one considers two factors. First, the drug target for an antimicrobial is in the organism and not in the animal and thus does not vary from species to species. Second, the expression of the drug dose as a pharmacodynamic parameter magnitude most often corrects for pharmacokinetic differences among animal species.
TRIAZOLES
Impacts of various MICs and specific resistance mechanisms.
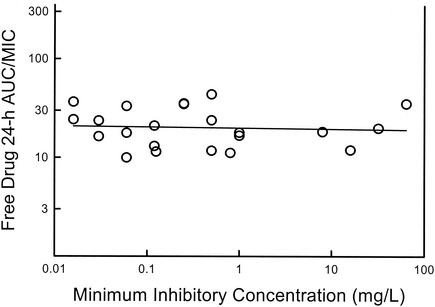
Initial fluconazole dose-ranging studies with C. albicans strains for which the MICs varied 64-fold found that a 24-h AUC/MIC magnitude near 25 produced similar microbiologic effects. As shown in Fig. 6, analysis of in vivo azole dosing data from other investigations demonstrates that efficacy is similarly predicted by a 24-h AUC/MIC near 25 (range, 12 to 44; mean ± standard deviation, 22.6 ± 10.8) (1, 6, 22, 29, 30, 34; K. Sorensen, E. Corcoran, S. Chen, et al., Abstr. 39th Intersci. Conf. Antimicrob. Agents Chemother., abstr 1271, p. 328, 1999). These studies included C. albicans strains for which MICs covered a range more than 500-fold. Among the studies represented in that analysis, other treatment variables included treatment duration (range, 24 h to 7 days), animal species (mouse and rat), treatment end point (numbers of CFU and rates of survival), and the resistance mechanism present in organisms for which MICs were higher (efflux pumps and reduced drug target affinity) (21). The concordance of the data, despite these variables, suggests that the pharmacodynamic magnitude relationship is independent of these factors, as has been reported with numerous antibacterial classes.
FIG. 6.
Relationship between the magnitude of the 24-h AUC/MIC for a free triazole and the MIC for the infecting organism in animal models of candidiasis. The study end points associated with the triazole 24-h AUC/MIC include the dose necessary to produce 50% of maximal microbiologic efficacy and the dose associated with 80% survival in infected animals.
Correlation of animal model data with clinical trial results.
Correlations of human pharmacokinetics and the outcomes of clinical trials with several antibacterial classes have suggested that the pharmacodynamic parameter magnitude which produces efficacy in animal models also predicts efficacy in humans (4, 7). The strength of this relationship has been strongest in analyses of otitis media and sinusitis, in which the study end point was microbiologic eradication. Unfortunately, analogous data from antifungal clinical trials, for which the data sets are fairly heterogeneous, are not available. In particular, it is difficult to account for important host immunity variables in these studies. In addition, the nature of these trials precludes determination of proven microbiologic eradication. With these limitations in mind, however, there are data sets that allow one to consider the relationship between drug dose, the MIC for the organism, and clinical outcome (Table 2). The largest data set is with fluconazole for the treatment of oral candidiasis (28). If one examines the data presented in the NCCLS susceptibility guideline publication (28), the relationship between treatment success and the magnitude of the 24-h fluconazole AUC/MIC is very similar to that seen in animal models (1). Clinical success rates exceeded 80% against organisms for which one would predict a 24-h AUC/MIC near 20. A failure rate of nearly 50% was reported with MICs that exceeded 64 μg/ml, with which 24-h AUC/MICs would fall far below this value. Importantly, the fluconazole AUC/MIC magnitude of nearly 25 is supportive of the susceptibility breakpoints suggested in the NCCLS publication (24). Two additional investigations allow one to examine the relationship between the fluconazole AUC/MIC and the efficacy of treatment against Candida spp. (C. J. Clancy, C. A. Kauffman, A. Morris, M. L. Nguyen, D. C. Tanner, D. R. Snydman, V. L. Yu, and M. H. Nguyen, Program Abstr. 36th Annu. Meet. Infect. Dis. Soc. Am., abstr. 98, p. 93, 1998). The importance of these additional, but smaller, studies is that the Candida infections are systemic or deep-seated. In both reports the fluconazole 24-h AUC/MICs predictive of the outcome for patients with these systemic Candida infections were similar to the values predictive of the outcome for patients with mucosal disease. A larger AUC/MIC magnitude may be required when systemic disease is complicated by certain underlying immunodeficiencies, such as neutropenia. Further analyses should address the impact of host immunity on these pharmacodynamic relationships.
TABLE 2.
Fluconazole AUC/MIC and clinical trial outcomes
| Study and MIC (μg/ml) | No. of patients | Change in 24-h AUC/MICa | % Success |
|---|---|---|---|
| Rex et al. (28), HIVb oropharyngeal candidiasis | |||
| ≤8 | 403 | 17 | 92 |
| 16-32 | 55 | 15c | 84 |
| ≥64 | 61 | 7 | 56 |
| Clancy et al., non-HIV candidemiad | |||
| ≤8 | 22 | 17 | 50 |
| 16-32 | 6 | 8-4 | 7 |
| ≥64 | 6 | 2 | 0 |
| Lee et al. (21), non-HIV candidemia and deep candidiasise | |||
| ≤8 | 24 | 30 | 79 |
| 16-32 | 6 | 15-8 | 67 |
| ≥64 | 2 | 2 | 0 |
The change in the 24-h AUC is derived from fluconazole pharmacokinetics in the literature.
HIV, human immunodeficiency virus.
A dose of 400 mg/day for an MIC of 16 μg/ml; a dose of 800 mg/day for an MIC of 32 μg/ml.
Clancy et al., Program Abstr. 36th Annu. Meet. Infect. Dis. Soc. Am. Abstr. 98, p. 93, 1998. A dose of 200 mg/day was used.
A dose of 400 mg/day was used.
Impact of protein binding and drug class.
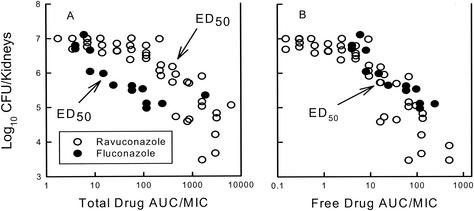
One major difference between fluconazole and the newer triazoles is the degree of protein binding (32). Fluconazole has a low degree of protein binding (10%), while several of the newer azoles having binding levels exceeding 90%. Recent investigations have begun to determine the impact of triazole protein binding on treatment outcome. In general, it is accepted that only free drug is pharmacologically active (9, 11, 19). This is related to the limited ability of protein-bound drug to diffuse across tissue and cellular membranes to reach the drug target. The impact of protein binding upon antimicrobial agents has been most clearly shown for antibacterials. These observations are perhaps most clearly demonstrated by the studies of Kunin et al. (19) with a variety of beta-lactam antibiotics with various degrees of protein binding. Several in vitro investigations have attempted to discern the impact of protein binding differences among various azole compounds (30, 31, 38). Study findings have been mixed, with some investigations suggesting that free drug levels are a better predictor of potency when compounds are being compared, while others have suggested that the relationship is poor. The methods used in those investigations have been dissimilar, varying in the type of protein and the amount of animal serum used. However, in vivo studies with one of the more highly bound azoles, ravuconazole, suggested that the outcome is linked to the degree of binding (6). If the total levels of ravuconazole are considered, studies have suggested that an inordinately larger amount of drug or a higher AUC would be needed for efficacy compared to the amount of fluconazole required. However, if one takes free drug levels into consideration, the comparative dose-response relationships are strong (Fig. 7).
FIG. 7.
Impact of protein binding on the relationship between the triazole 24-h AUC/MIC and the burden of Candida spp. in the kidneys of neutropenic mice. Solid symbols, mean Candida burden in kidneys from two mice treated with fluconazole; hollow symbols, mean Candida burden in kidneys from two mice treated with ravuconazole. Data were obtained by using total drug levels (A) and free drug levels (B). (Modified from reference 6.)
OTHER ANTIFUNGAL DRUG CLASSES
Parameter magnitude studies with other antifungal drug classes in animal models are less complete. Unfortunately, clinical trials have not lent themselves to analyses that would provide insight into this parameter magnitude question. However, several pharmaceutical companies have begun to consider pharmacodynamic analysis in the development of new compounds.
Amphotericin B.
One of the factors that has limited study of parameter magnitude predictions with amphotericin B is the small range of MICs (28). However, for strains with similar in vitro susceptibilities, studies have suggested that a net fungistatic effect is achieved when peak/MICs exceed 4 (2). Drug toxicity has limited study of peak/MICs much greater than 10, with which maximal efficacy was observed.
Flucytosine.
Pharmacodynamic parameter magnitude studies with flucytosine are even more limited. In vivo animal model data are limited to those from studies with a single strain, making magnitude predictions difficult (3). However, in the study conducted with the single strain, maximal microbiologic efficacy was observed when levels in serum exceeded the MIC for only 25% of the dosing interval. The only reason that this may be worth consideration in this review is the relationship between common flucytosine dosing in humans and the MIC for the organism. Dosing of 37.5 mg/kg of body weight every 6 h would provide levels in serum exceeding the MIC at which 90% of C. albicans isolates are inhibited for more than twice the duration of the dosing interval (13). If one considers the narrow therapeutic window of this compound, the relationship between the pharmacodynamic parameter magnitude associated with efficacy should be evaluated further (16).
Echinocandin.
A narrow MIC range has also restricted magnitude studies with the echinocandins (27). However, in a study with several C. albicans isolates and one of the new echinocandins, a peak/MIC of 3 produced a fungistatic outcome, while maximal microbiologic efficacy was observed with values near 10 (5). This is very similar to the values observed in studies with amphotericin B.
Sordarins.
Pharmacokinetic and pharmacodynamic parameter magnitude analyses with drugs from the sordarin class have not been undertaken.
SUMMARY
Application of pharmacodynamic principles to antifungal drug therapy of Candida infections has provided an understanding of the relationship between drug dosing and treatment outcome similar to that observed for antibacterial pharmacodynamics. Initial observations of the pharmacodynamics of triazoles have correlated with the results of clinical trials and have proved useful for validation of in vitro susceptibility breakpoints.
Additional antifungal pharmacodynamic areas are under active study, including the pharmacodynamics of antifungals in combination and antifungal pharmacodynamics in the treatment of infections caused by other fungal pathogens (23).
Pharmacodynamic studies have been invaluable in the design of clinical trials and in the selection of the doses of numerous antibacterial drugs in the development stage that should be used in those trials. Application of similar studies to antifungal development should be considered.
REFERENCES
- 1.Andes, D., and M. L. van Ogtrop. 1999. Characterization and quantitation of the pharmacodynamics of fluconazole in a neutropenic murine model of disseminated candidiasis model. Antimicrob. Agents Chemother. 43:2116-2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andes, D., T. Stamsted, and R. Conklin. 2001. Pharmacodynamics of amphotericin B in a neutropenic murine disseminated candidiasis model. Antimicrob. Agents Chemother. 45:922-926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andes, D., and M. van Ogtrop. 2000. In vivo characterization of the pharmacodynamics of flucytosine in a neutropenic murine disseminated candidiasis model. Antimicrob. Agents Chemother. 44:938-942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andes, D., and W. A. Craig. 1998. In vivo activities of amoxicillin and amoxicillin-clavulanate against Streptococcus pneumoniae: application to breakpoint determinations. Antimicrob. Agents Chemother. 42:2375-2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andes, D., K. Marchillo, L. Lowther, A. Bryskier, T. Stamstad, and R. Conklin. 2003.. In vivo pharmacodynamics of HMR 3270, a glucan synthase inhibitor, in a murine candidiasis model. Antimicrob. Agents Chemother. 47:1187-1192. [DOI] [PMC free article] [PubMed]
- 6.Andes, D., K. Marchillo, T. Stamstad, and R. Conklin. 2003.. In vivo pharmacodynamics of a new triazole, ravuconazole, in a murine candidiasis model. Antimicrob Agents Chemother. 47:1193-1199. [DOI] [PMC free article] [PubMed]
- 7.Andes, D., and W. A. Craig. 2002. Animal model pharmacokinetics and pharmacodynamics: a critical review. Int. J. Antimicrob. Agents 19:261-268. [DOI] [PubMed] [Google Scholar]
- 8.Aviles, P., C. Falcoz, R. San Roman, and D. Gargallo-Viola. 2000. Pharmacokinetics-pharmacodynamics of a sordarin derivative (GM 237354) in a murine model of lethal candidiasis. Antimicrob. Agents Chemother. 44:2333-2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Craig, W. A. 1998. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin. Infect. Dis. 26:1-12. [DOI] [PubMed] [Google Scholar]
- 10.Craig, W. A., and S. Gudmundsson. 1996. Postantibiotic effect, p. 296-329. In V. Lorian (ed.), Antibiotics in laboratory medicine, 4th ed. The Williams & Wilkins Co., Baltimore, Md.
- 11.Craig, W. A., and B. Suh. 1996. Protein binding and the antimicrobial effects: methods for the determination of protein binding, p. 367-402. In V. Lorian (ed.), Antibiotics in laboratory medicine, 4th ed. The Williams & Wilkins Co., Baltimore, Md.
- 12.Craig, W. A., and D. Andes. 1996. Pharmacokinetics and pharmacodynamics of antibiotics in otitis media. Pediatr. Infect. Dis. J. 15:255-259. [DOI] [PubMed] [Google Scholar]
- 13.Cutler, R. E., A. D. Blair, and M. R. Kelly. 1978. Flucytosine kinetics in subjects with normal and impaired renal function. Clin. Pharmacol. Ther. 24:333-342. [DOI] [PubMed] [Google Scholar]
- 14.Dudley, M. N., and P. G. Ambrose. 2000. Pharmacodynamics in the study of drug resistance and establishing in vitro susceptibility breakpoints: ready for prime time. Curr. Opin. Microbiol. 3:515-521. [DOI] [PubMed] [Google Scholar]
- 15.Ernst, E. J., M. E. Klepser, and M. A. Pfaller. 2000. Postantifungal effects of echinocandin, azole, and polyene antifungal agents against Candida albicans and Cryptococcus neoformans. Antimicrob. Agents Chemother. 44:1008-1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Francis, P., and T. J. Walsh. 1992. Evolving role of flucytosine in immunocompromised patients: new insights into safety, pharmacokinetics, and antifungal therapy. Clin. Infect. Dis. 15:1003-1018. [DOI] [PubMed] [Google Scholar]
- 17.Graybill, J. R., L. K. Najvar, J. D. Holmberg, A Correa, and M. F. Luther. 1995. Fluconazole treatment of Candida albicans infection in mice: does in vitro susceptibility predict in vivo response? Antimicrob. Agents Chemother. 39:2197-2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klepser, M. E., E. J. Wolfe, R. N. Jones, C. H. Nightingale, and M. A. Pfaller. 1997. Antifungal pharmacodynamic characterization of fluconazole and amphotericin B tested against Candida albicans. Antimicrob. Agents Chemother. 41:1392-1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kunin, C. M., W. A. Craig, M. Kornguth, and R. Monson. 1973. Influence of binding on the pharmacological activity of antibiotics. Ann. N. Y. Acad. Sci. 226:214-224. [DOI] [PubMed] [Google Scholar]
- 20.Lee, S. C., C. P. Fung, J. S. Huang, C. J. Tsai, K. S. Chen, N. L. Chen, L. C. See, and W. B. Shieh. 2000. Clinical correlates of antifungal macrodilution susceptibility test results for non-AIDS patients with severe Candida infections treated with fluconazole. Antimicrob. Agents Chemother. 44:2715-2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lopez-Ribot, J. L., R. K. McAtee, L. N. Lee, W. R. Kirkpatrick, T. C. White, D. Sanglard, and T. F. Patterson. 1998. Distinct patterns of gene expression associated with development of fluconazole resistance in serial Candida albicans isolates from human immunodeficiency virus-infected patients with oropharyngeal candidiasis. Antimicrob. Agents Chemother. 42:2932-2937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Louie, A., G. L. Drusano, P. Banerjee, Q. F. Liu, W. Liu, M. Kaw, H. Shayegani, H. Taber, and M. H. Miller. 1998. Pharmacodynamics of fluconazole in a murine model of systemic candidiasis. Antimicrob. Agents Chemother. 42:1105-1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Louie, A., P. Banerjee, G. L. Drusano, M. Shayegani, and M. H. Miller. 1999. Interaction between fluconazole and amphotericin B in mice with systemic infection due to fluconazole-susceptible or -resistant strains of Candida albicans. Antimicrob. Agents Chemother. 43:2841-2847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.National Committee for Clinical Laboratory Standards. 1997. Reference method for broth dilution antifungal susceptibility testing for yeast; approved standard. Document M27-A. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 25.National Committee for Clinical Laboratory Standards. 2000. Development of in vitro susceptibility testing criteria and quality control parameters; approved guidelines, 2nd ed. Document M23-A2. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 26.Petraitis, V., R. Petraitiene, A. H. Groll, K. Roussillon, M. Hemmings, C. A. Lyman, T. Sein, J. Bacher, I. Bekersky, and T. J. Walsh. 2002. Comparative antifungal activities and plasma pharmacokinetics of micafungin (FK463) against disseminated candidiasis and invasive pulmonary aspergillosis in persistently neutropenic rabbits. Antimicrob. Agents Chemother. 46:1857-1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pfaller, M. A., R. N. Jones, G. V. Doern, A. C. Fluit, J. Verhoef, H. S. Sader, S. A. Messer, A. Houston, S. Coffman, and R. J. Hollis. 1999. International surveillance of blood stream infections due to Candida species in the European SENTRY Program: species distribution and antifungal susceptibility including the investigational triazole and echinocandin agents. Diagn. Microbiol. Infect. Dis. 35:19-25. [DOI] [PubMed] [Google Scholar]
- 28.Rex, J. H., M. A. Pfaller, J. N. Galgiani, M. S. Bartlett, A. Espinel-Ingroff, M. A. Ghannoum, M. Lancaster, F. C. Odds, M. G. Rinadli, T. J. Walsh, and A. L. Barry, for the NCCLS Subcommittee on Antifungal Susceptibility Testing. 1997. Development of interpretive breakpoints for antifungal susceptibility testing: conceptual framework and analysis of in vitro and in vivo correlation data for fluconazole, itraconazole, and Candida infections. Clin. Infect. Dis. 24:235-247. [DOI] [PubMed] [Google Scholar]
- 29.Rogers, T. E., and J. N. Galgiani. 1986. Activity of fluconazole (UK 49,858) and ketoconazole against Candida albicans in vitro and in vivo. Antimicrob. Agents Chemother. 30:418-422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schafer-Korting, M., H. C. Korting, W. Rittler, and W. Obermuller. 1995. Influence of serum protein binding on the in vitro activity of antifungal agents. Infection 23:292-297. [DOI] [PubMed] [Google Scholar]
- 31.Schafer-Korting, M., H. C. Korting, F. Amman, R. Peuser, and A. Lukacs. 1991. Influence of serum protein binding on the in vitro activity of antifungal activity: results of a dynamic in vitro study. Antimicrob. Agents Chemother. 35:2053-2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sheehan, D. J., C. A. Hitchcock, and C. M. Sibley. 1999. Current and emerging azole antifungal agents. Clin. Microbiol. Rev. 12:40-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Turnidge, J. D., S. Gudmundsson, B. Vogelman, and W. A. Craig. 1994. The postantibiotic effect of antifungal agents against common pathogenic yeasts. J. Antimicrob. Chemother. 34:83-92. [DOI] [PubMed] [Google Scholar]
- 34.Van't Wout, J., H. Mattie, and R. van Furth. 1989. Comparison of the efficacies of amphotericin B, fluconazole, and itraconazole against systemic Candida albicans infection in normal and neutropenic mice. Antimicrob. Agents Chemother. 33:147-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vogelman, B., S. Gudmundsson, J. Leggett, J. Turnidge, S. Ebert, and W. A. Craig. 1988. Correlation of antimicrobial pharmacokinetic parameters with therapeutic efficacy in an animal model. J. Infect. Dis. 158:831-847. [DOI] [PubMed] [Google Scholar]
- 36.Walsh, T. J., C. E. Gonzalez, S. Piscitelli, J. D. Bacher, J. Peter, R. Torres, D. Shetti, V. Katsov, K. Kligys, and C. A. Lyman. 2000. Correlation between in vitro and in vivo antifungal activities in experimental fluconazole-resistant oropharyngeal and esophageal candidiasis. J. Clin. Microbiol. 38:2369-2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Walsh, T. J., J. W. Lee, E. Roilides, P. Francis, J. Bacher, C. A. Lyman, and P. A. Pizzo. 1992. Experimental antifungal chemotherapy in granulocytopenic animal models of disseminated candidiasis: approaches to understanding investigational antifungal compounds for patients with neoplastic diseases. Clin. Infect. Dis. 14(Supp1.):S139-S147. [DOI] [PubMed] [Google Scholar]
- 38.Zhanel, G. G., D. G. Saunders, D. J. Hoban, and J. A. Karlowsky. 2001. Influence of human serum on antifungal pharmacodynamics with Candida albicans. Antimicrob. Agents Chemother. 45:2018-2022. [DOI] [PMC free article] [PubMed] [Google Scholar]