Abstract
Here we report the antiretroviral activity of the experimental nucleoside reverse transcriptase inhibitor (NRTI) compound stampidine in cats chronically infected with feline immunodeficiency virus (FIV). Notably, a single oral bolus dose of 50 or 100 mg of stampidine per kg resulted in a transient ≥1-log decrease in the FIV load of circulating peripheral blood mononuclear cells in five of six FIV-infected cats and no side effects. A 4-week stampidine treatment course with twice-daily administration of hard gelatin capsules containing 25 to 100 mg of stampidine per kg was also very well tolerated by cats at cumulative dose levels as high as 8.4 g/kg and exhibited a dose-dependent antiretroviral effect. One of three cats treated at the 25-mg/kg dose level, three of three cats treated at the 50-mg/kg dose level, and three of three cats treated at the 100-mg/kg dose level (but none of three control cats treated with placebo pills) showed a therapeutic response, as evidenced by a ≥1-log reduction in the FIV load in peripheral blood mononuclear cells within 2 weeks. The previously documented in vitro and in vivo antiretroviral activity of stampidine against primary clinical human immunodeficiency virus type 1 isolates with genotypic and/or phenotypic NRTI resistance, together with its favorable animal toxicity profile, pharmacokinetics, and in vivo antiretroviral activity in FIV-infected cats, warrants further development of this promising new NRTI compound.
Stavudine (STV) is a pyrimidine nucleoside analogue used in the treatment of human immunodeficiency virus (HIV) infection. It inhibits viral reverse transcriptase (RT), as do zidovudine (ZDV), didanosine, zalcitabine, and lamivudine, which make up the family of nucleoside RT inhibitors (NRTIs). The 5′ triphosphates of these NRTI, which are generated intracellularly by the action of nucleoside and nucleotide kinases, are potent inhibitors of HIV type 1 (HIV-1) RT (13). The rate-limiting step in the generation of the bioactive STV metabolite STV triphosphate is conversion of STV to its monophosphate derivative (3, 13, 23). To overcome the dependence of STV on intracellular nucleoside kinase activation, we prepared stampidine (STAMP) HI-113, STV-5′-(p-bromophenyl methoxyalaninyl phosphate), a novel aryl phosphate derivative of STV (18, 23, 25). In preliminary studies, we found that STAMP is substantially more potent than STV at inhibiting HIV-1 replication in thymidine kinase-deficient T cells (23). STAMP was a much more potent anti-HIV agent than STV, and it was active against phenotypically and/or genotypically NRTI-resistant HIV strains for which the 50% inhibitory concentrations (IC50s) are in the low nanomolar-to-subnanomolar range (21). Similarly, STAMP inhibited the replication of laboratory HIV-1 strains and primary clinical HIV-1 isolates with non-NRTI (NNRTI) (1) binding site mutations and NNRTI-resistant phenotypes for which the IC50s are in the low nanomolar-to-subnanomolar range (21). STAMP was very well tolerated by rodent species, with no detectable acute or subacute toxicity at single intraperitoneal or oral bolus dose levels as high as 500 mg/kg (19). Notably, daily administration of STAMP intraperitoneally or orally for up to 8 consecutive weeks was associated with no detectable toxicity at cumulative dose levels as high as 6.4 g/kg (19, 22). STAMP exhibited dose-dependent and potent in vivo anti-HIV activity in Hu-PBL-SCID mice against a genotypically and phenotypically NRTI-resistant clinical HIV-1 isolate at nontoxic dose levels (22). The pharmacokinetics and metabolism of STAMP have been studied in mice, dogs, and cats. Notably, concentrations of STAMP in plasma >4 logs higher than its IC50 for HIV can be achieved in mice, dogs, and cats at nontoxic dose levels (4, 5). The marked potency of STAMP against primary clinical HIV-1 isolates with genotypic and/or phenotypic NRTI or NNRTI resistance, together with its anti-HIV activity in Hu-PBL-SCID mice and favorable toxicity profile in rodents, prompted us to further evaluate the clinical potential of STAMP as a new anti-HIV agent. The goal of the present preclinical study was to evaluate the toxicity and antiretroviral activity of STAMP in chronically feline immunodeficiency virus (FIV)-infected cats.
MATERIALS AND METHODS
FIV isolates.
Six cats used in single-dose toxicology and efficacy studies were chronically infected with FIV isolates Bangston (FIVBang, subtype B; cat identification [ID] no. CV5), Petaluma (FIVPet, subtype A; cat ID no. 93J and NJ2), Shizuoka (FIVShiz, subtype D; cat ID no. CT1), or Glasgow-8 (FIVUK-8, subtype A; cat ID no. CU3 and C3D). FeT-J is an interleukin-2-independent feline T-cell line highly susceptible to FIV infection. The Bang/FeT-J cell line is chronically infected with FIVBang. In 4-week toxicity studies with chronically infected cats, we used eight FIVBang-infected cats that had previously been inoculated intravaginally with Bang/FeT-J cells (5 × 103 to 5 × 106 cells/0.4 ml) >6 months prior to initiation of the study.
In vitro assay of anti-FIV activity of STAMP.
Phenotypic FIV drug susceptibility studies were performed by measuring the levels of RT activity in the culture supernatants of T-cell-enriched peripheral blood mononuclear cells (PBMC) from specific-pathogen-free (SPF) cats after exogenous infection with FIV as previously described (12, 26). T-cell-enriched PBMC were derived from SPF cats by stimulating Ficoll-Hypaque-separated PBMC with concanavalin A for 3 days and culturing them for an additional 2 weeks in interleukin-2 (100 U/ml)-supplemented RPMI culture medium in 24-well tissue culture plates as previously described (26). T-cell-enriched PBMC, at 106/ml, were treated with ZDV (control) or STAMP at the time of FIV inoculation (100 50% tissue culture infective doses). Culture supernatants were harvested at 3-day intervals, on days 9, 12, and 15, and the cells were concurrently resuspended in fresh culture medium containing the indicated concentrations of ZDV or STAMP. Viral replication was monitored by measuring the RT activity levels of the culture supernatants (12). Drug toxicity in these cultures was also monitored by viability and absolute cell count analysis by the trypan blue exclusion method. All cultures were set up in triplicate. Controls included uninfected PBMC (negative control), FIV-infected but untreated PBMC (positive control), and FIV-infected and ZDV-treated PBMC. The results are presented as percentages of the mean control RT titer obtained by using the following formula: percent control replication = 100 × [(mean RT titer of drug-treated culture − mean RT titer of negative control culture)/(mean RT titer of untreated positive control culture − mean RT titer of negative control culture)].
Animals.
SPF male or female domestic cats (body weight, 2.9 to 6.2 kg) were obtained from Liberty Research, Inc. (Waverley, N.Y.); Cedar River Laboratories (Mason City, Iowa); or Harlan Sprague-Dawley (Indianapolis, Ind.). All husbandry and experimental contact made with the cats maintained SPF conditions (12-h light-12-h dark photoperiod, 18 to 29°C, 30 to 70% relative humidity). The cats were housed in galvanized gang cages with Plexiglas in a well-ventilated room with no air recirculation. We used FIV-infected cats to simulate the clinical setting in which STAMP will be used. At the time of the present study, all cats were off antiretroviral therapy for 4 weeks and had viral culture evidence of FIV infection in their PBMC. The animal rooms were cleaned daily, and cat litters were changed daily. Cats were provided with dry and wet Purina cat chow and tap water ad libitum. Cats were acclimated to the study room conditions for at least 5 days prior to initiation of the experiment. Animal studies were approved by the Parker Hughes Institute Animal Care and Use Committee, and all animal care procedures conformed to the National Research Council's Guide for the Care and Use of Laboratory Animals (7a). Cats were treated at the University of Florida under a contract service agreement between the Parker Hughes Institute and the University of Florida. Therefore, cat studies were also approved by the University of Florida Animal Care and Use Committee.
Toxicity studies of STAMP in chronically FIV-infected cats.
Cats were allowed free access to dry and wet Purina cat chow and tap water throughout the feline toxicity experiments and monitored at least twice daily for morbidity and mortality. Physical examinations were performed at least twice weekly to evaluate the oral cavity and determine the rectal body temperature, heart rate, and respiratory rate. Body weights were determined three times a week. Clinical laboratory testing was done two or three times a month, at weeks −2, −1, 0 (day of first treatment), +2, +4, +6, and +8. Eleven chronically FIV-infected cats were treated orally with STAMP capsules (25 to 100 mg/kg/dose) or placebo capsules twice daily for 28 consecutive days as detailed in Results. Eight chronically FIV-infected cats, including seven cats treated orally with STAMP capsules twice daily (25, 50, or 100 mg/kg/dose) for 28 consecutive days and one untreated control cat, were electively sacrificed at the end of the 8-week observation period of the oral STAMP safety and efficacy study. Six additional cats treated with single bolus doses of STAMP in a single-dose toxicity and efficacy study were electively sacrificed on day 30 after administration of STAMP. Thirty-one tissue types (adrenal gland, brain, cervix, esophagus, gall bladder, heart, large intestine, small intestine, kidney, liver, lung, lymph node, mammary gland, ovary, oviduct, pancreas, parathyroid gland, peripheral nerve, salivary gland, skeletal muscle, skin, spinal cord, spleen, stomach, thymus, thyroid, tongue, trachea, urinary bladder, uterus, and vagina) were collected, fixed in 10% neutral buffered formalin, dehydrated, and embedded in paraffin by routine methods (17). Glass slides with affixed 4- to 5-μm tissue sections were prepared and stained with hematoxylin and eosin. Histopathologic examinations were performed by a veterinary pathologist (B.W.).
STAMP efficacy in chronically FIV-infected cats.
We studied the antiretroviral activity of STAMP in chronically FIV-infected cats. At the indicated times before and after administration of STAMP, the FIV load in PBMC was measured by quantitative virus isolation as previously described (2, 15). In brief, the PBMC from STAMP-treated and placebo-treated cats were cocultured in 25-cm2 tissue culture flasks (total volume, 5 ml) for 3 weeks with 5 × 106 T-cell-enriched PBMC from SPF cats in a total volume of 5 ml at a ratio of 1:1, 1:10, 1:100, 1:1,000, 1:10,000, 1:100,000, or 1:1,000,000. Culture supernatants were harvested and cells were resuspended in fresh culture medium every 3 days. FIV replication was monitored by measuring the levels of RT activity in the culture supernatants at the termination of the cultures (2, 9). RT values were considered positive if they were ≥10,000 cpm/ml. The FIV load in PBMC is presented as the highest dilution (log x) of infected PBMC from the FIV-infected cats needed to cause detectable FIV infection in uninfected PBMC from healthy SPF cats (VI-RT values) by the above-described coculture technique (16), whereby the undiluted infected PBMC concentration is set at 106 cells/ml. Thus, the FIV load can range from 1, which is equivalent to FIV detection at an undiluted cell concentration of 106/ml, to 6, which is equivalent to FIV detection at a 1:1,000,000 dilution. The FIV load (VI-RT value) was arbitrarily assigned a value of zero when no FIV was detected even at 106 cells/ml. Student t tests (unequal variances) were performed to evaluate the statistical significance of the differences between the viral load (VI-RT) values determined prior to treatment versus those determined 2, 4, and 6 weeks after treatment with STAMP at one of the three dose levels (25, 50, or 100 mg/kg/dose) or with a placebo. To increase the statistical power of the t test, analyses were also performed with the pooled viral load values for all three dose levels. When the standard error for the comparisons was zero, a nonparametric test (Wilcoxon rank sum or Mann-Whitney test) was also performed to determine the effect of STAMP. P values of <0.05 were deemed significant.
Statistical methods for comparison for toxicity-related laboratory parameters.
Values for the laboratory parameters were pooled for vehicle controls and STAMP treatments, and for each parameter, differences between means were evaluated for statistical significance by using Student's t test (vehicle versus STAMP treatment, unequal variances, two tailed). The calculations were performed in Excel spreadsheets. To determine significant effects, the P values were adjusted by using the Bonferroni method to control for random variation.
RESULTS
In vitro anti-FIV activity of STAMP.
The in vitro anti-FIV activity of STAMP was examined in two independent experiments, each performed in triplicate. T-cell-enriched PBMC from SPF cats were exogenously infected with FIV and then cultured for 9 days at 37°C in the presence or absence of STAMP. FIV replication was monitored by measuring RT activity in the culture supernatants. Controls included uninfected PBMC (negative control), FIV-infected but untreated PBMC (positive control), and FIV-infected and ZDV-treated PBMC. The results are presented as mean RT activity (in counts per minute) ± the standard deviation and percent inhibition of FIV replication as described in Materials and Methods. As shown in Table 1, STAMP exhibited significant anti-FIV activity and inhibited FIV replication in feline PBMC by >90% at concentrations of ≥1 μM. Thus, FIV, for which the 90% inhibitory concentrations (IC90s) are micromolar, appears to be less sensitive to STAMP than is HIV, for which the IC90s are nanomolar (20, 21). Nevertheless, we have previously reported that micromolar concentrations of STAMP can be achieved in FIV-infected cats after oral administration of a single 50- or 100-mg/kg bolus dose of STAMP (5). Hence, these results demonstrated that FIV-infected domestic cats represent an appropriate animal model for further evaluation of the therapeutic potential of STAMP. Therefore, we next set out to evaluate the toxicity and efficacy of STAMP in chronically FIV-infected domestic cats.
TABLE 1.
In vitro anti-FIV activity of STAMPa
| Treatment | RT activity
|
|
|---|---|---|
| Mean cpm ± SD | % Inhibition | |
| Expt 1 | ||
| None (positive control) | 23,830 ± 2,682 | NAb |
| None (negative control) | 1,505 ± 263 | NA |
| STAMP, 0.1 μM | 13,422 ± 1,338 | 46.6 |
| STAMP, 1.0 μM | 1,274 ± 74 | >99.9 |
| STAMP, 10.0 μM | 1,257 ± 154 | >99.9 |
| ZDV, 10.0 μM | 1,482 ± 228 | >99.9 |
| Expt 2 | ||
| None (positive control) | 38,197 ± 8,546 | NA |
| None (negative control) | 2,812 ± 784 | NA |
| STAMP, 0.1 μM | 10,215 ± 2,274 | 79.1 |
| STAMP, 1.0 μM | 4,452 ± 342 | 95.4 |
| STAMP, 10.0 μM | 4,122 ± 100 | 96.3 |
| ZDV, 10.0 μM | 4,192 ± 1,254 | 96.1 |
The in vitro anti-FIV activity of STAMP was examined in two independent experiments, each done in triplicate. T-cell-enriched PBMC from SPF cats were exogenously infected with FIV and than cultured for 9 days at 37°C in the presence or absence of STAMP. FIV replication was monitored by measurement of RT activity in the culture supernatants. Controls included uninfected PBMC (negative control). FIV-infected but untreated PBMC (positive control), and FIV-infected and ZDV-treated PBMC.
NA, not applicable.
Toxicity and efficacy of an oral bolus dose of STAMP in chronically FIV-infected cats.
We first examined the toxicity profile and anti-FIV activity of STAMP administered in hard gelatin capsules as a single 50-mg/kg (n = 3) or 100-mg/kg (n = 3) oral bolus dose on an empty stomach to six chronically FIV-infected cats. All six cats tolerated the STAMP treatments without any immediate adverse reactions. Cats remained healthy, with stable vital signs (no fever, no significant change in respiratory rate or heart rate), no weight loss, and no evidence of morbidity throughout the 31-day observation period. Blood tests done at 1 week and 2 weeks after treatment did not suggest any significant systemic toxicity. In particular, STAMP did not cause (i) anemia, thrombocytopenia, neutropenia, or lymphopenia suggestive of hematologic toxicity; (ii) elevations of blood urea nitrogen (BUN) or creatinine or electrolyte disturbances suggestive of renal toxicity or metabolic abnormalities; (iii) elevations of alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase, or bilirubin suggestive of hepatotoxicity; (iv) decreases in serum albumin or total protein suggestive of protein-losing renal or gut toxicity; or (v) hyperglycemia, hypoglycemia, or hypercholesterolemia suggestive of abnormalities in carbohydrate or fat metabolism. Two of the cats (cat 93J from the 50-mg/kg dose group and cat C3D from the 100-mg/kg dose group) were electively euthanized on day 14 for histopathologic examination of their organs. No toxic lesions were found in any of the 31 organs examined.
The single-dose STAMP treatment exhibited potent antiretroviral activity, as evidenced by a transient decrease in the FIV load (VI-RT value) of circulating PBMC at 1 week in five of six cats (Table 2). In addition, we observed an increase in the absolute numbers of CD4+ T cells at 1 to 2 weeks after administration of STAMP (Table 2).
TABLE 2.
Antiretroviral activity of a single oral bolus dose of STAMP in chronically FIV-infected cats
| FIV strain, cat ID no.: (STAMP dose [mg/kg]), and laboratory parameter | Pretreatment | Posttreatment
|
|
|---|---|---|---|
| Wk 1 | Wk 2 | ||
| FIVShiz, CT1 (50) | |||
| VI-RTPBMC | 2 | 1 | 1 |
| CD4 (103/μl) | 0.5 | 1.4 | 1.1 |
| CD4/CD8 ratio | 1.2 | 1.8 | 7.9 |
| FIVVK-8, CV3 (50) | |||
| VI-RTPBMC | >5 | 3 | 4 |
| CD4 (103/μl) | 0.4 | 1.0 | 1.0 |
| CD4/CD8 ratio | 1.0 | 3 | 4 |
| FIVPet, 93J (50) | |||
| VI-RTPBMC | 4 | 2 | 3 |
| CD4 (103/μl) | 0.1 | 1.8 | 0.8 |
| CD4/CD8 ratio | 1.3 | 1.8 | 2.8 |
| FIVBang, CV5 (100) | |||
| VI-RTPBMC | 3 | 4 | 3 |
| CD4 (103/μl) | 0.2 | 0.7 | 0.7 |
| CD4/CD8 ratio | 1.1 | 1.6 | 1.7 |
| FIVUK-8, C3D (100) | |||
| VI-RTPBMC | 4 | 2 | 4 |
| CD4 (103/μl) | 0.1 | 0.2 | 0.3 |
| CD4/CD8 ratio | 0.7 | 1.2 | 1.7 |
| FIVPet, NJ2 (100) | |||
| VI-RTPBMC | 4 | 2 | 4 |
| CD4 (103/μl) | 0.2 | 0.5 | 0.5 |
| CD4/CD8 ratio | 0.7 | 0.8 | 0.9 |
| Group (n = 6) | |||
| VI-RTPBMC | 3.7 ± 0.4a | 2.3 ± 0.4b | 3.0 ± 0.6 |
| CD4 (103/μl) | 0.3 ± 0.1 | 0.9 ± 0.2b | 0.7 ± 0.1c |
| CD4/CD8 ratio | 1.0 ± 0.1 | 1.4 ± 0.2 | 1.7 ± 0.3b |
Group values are means ± standard deviation.
P < 0.005 (t test).
P < 0.001 (t test).
Toxicity of a 4-week STAMP treatment course in chronically FIV-infected cats.
We next examined the safety of a 4-week STAMP treatment course in six chronically FIV-infected cats at cumulative dose levels of 1.4 g/kg (n = 3) and 2.8 g/kg (n = 3). Specifically, three cats were treated with STAMP in hard gelatin capsules at 25 mg/kg/dose twice daily for 28 consecutive days (cumulative dose = 1.4 g/kg) and three other cats were treated with STAMP at 50 mg/kg/dose twice daily for 28 consecutive days (cumulative dose = 2.8 g/kg). Six control cats were treated with placebo pills (identically prepared hard gelatin capsules with no active drug). The treatments were well tolerated and caused no significant toxicity. All six STAMP-treated cats and two of the six placebo-treated cats showed some evidence of nausea during 1 to 5 days of the 28-day treatment. At the beginning of the second treatment week, between days 10 and 14, one cat treated at the 25-mg/kg dose level and two cats treated at the 50-mg/kg dose level showed a transient decrease in appetite. Of the six STAMP-treated cats, only one that was treated at the 25-mg/kg dose level lost weight (loss = 8.3% of baseline weight). No weight loss was observed in placebo-treated cats. Blood tests done at 2, 4, 6, and 8 weeks after initiation of therapy showed that STAMP did not cause (i) anemia, thrombocytopenia, neutropenia, or lymphopenia suggestive of hematologic toxicity; (ii) elevations of BUN or creatinine or electrolyte disturbances suggestive of renal toxicity or metabolic abnormalities; (iii) elevations of ALT, AST, alkaline phosphatase, or bilirubin suggestive of hepatotoxicity; (iv) decreases in serum albumin or total protein levels suggestive of protein-losing renal or gut toxicity; or (v) hyperglycemia, hypoglycemia, or hypercholesterolemia suggestive of abnormalities in carbohydrate or fat metabolism. One of the cats treated at the 25-mg/kg dose level had a slightly elevated baseline ALT level (106 U/liter), and its ALT level fluctuated between 88 and 168 U/liter during and after treatment. The AST, alkaline phosphatase, and bilirubin levels in this cat remained normal throughout the study. Another cat treated at the 25-mg/kg level had an isolated slightly elevated ALT level at 2 weeks without any other liver function abnormalities. None of the cats treated at the 50-mg/kg dose level and none of the placebo-treated cats had abnormal liver function test results. Two of the cats treated at the 25-mg/kg dose level, one of the cats treated at the 50-mg/kg dose level, and one of the placebo-treated cats were electively euthanized, and 31 organs were subjected to histopathologic examination. No STAMP-related toxic lesions were found in any of the tissues examined. Thus, 4 weeks of treatment with STAMP at 50 or 100 mg/kg/day is well tolerated by chronically FIV-infected cats.
Four chronically FIV-infected cats were treated with STAMP at 100 mg/kg/dose in hard gelatin capsules twice daily (200 mg/kg/day) for 28 consecutive days (cumulative dose = 5.6 g). Three of these cats had been treated 2 months earlier with either 1.4 g/kg (n = 1) or 2.8 g/kg (n = 2), so that their total cumulative doses were 7.0 g/kg (n = 1) and 8.4 g/kg (n = 2), respectively. In these cats, STAMP caused nausea and vomiting for 2 to 3 days during the first week of treatment. Cat no. 009 vomited only on days 1 and 2; cats no. 035 and 065 vomited on days 1, 7, and 8; and cat no. C3B vomited on days 1 and 4. All cats exhibited a decreased appetite for at least 3 weeks during the 4-week treatment course and transiently lost weight (cat no. 009, 28.6%; cat no. 035, 1.9%; cat no. 065, 18.0%; cat no. C3B, 16.7% [mean ± standard error = 16.3% ± 5.5%]; Table 3) because of malnutrition and/or dehydration during the first 1 to 3 weeks of treatment. Diarrhea was observed in three of the four cats and only on 1 to 3 days of the 4-week treatment period. Otherwise, the treatments were well tolerated and caused no significant toxicity. Blood tests done at 2, 4, 8, and 10 weeks after initiation of therapy showed that STAMP did not cause (i) anemia, neutropenia, lymphopenia, or thrombocytopenia suggestive of hematologic toxicity; (ii) elevations of BUN or creatinine or electrolyte disturbances suggestive of renal toxicity; or (iii) hyperglycemia, hypoglycemia, or hypercholesterolemia suggestive of abnormalities in carbohydrate or fat metabolism. However, all four cats showed an elevation of serum ALT level lasting 1 to 3 weeks without a concomitant elevation in total bilirubin or alkaline phosphatase levels. Selected laboratory data are depicted in Table 3. The average ALT level was 1.8-fold higher than the upper end of the normal range (normal range, 8.3 to 100.0 U/liter; mean ± standard error at 2 weeks = 183.5 ± 77.1 U/liter; P = 0.06). Only one of the four cats had an elevated serum AST level as well. These laboratory results are consistent with hepatotoxicity. Furthermore, serum albumin and total protein levels showed statistically significant decreases at weeks 2 (P = 0.0001 for albumin levels and P = 0.001 for total protein levels) and 4 (P = 0.007 for albumin levels and P = 0.0.002 for total protein levels) consistent with the impaired nutritional status of the cats even though the serum albumin levels were still within the normal range (normal range for albumin, 1.7 to 3.7 g/dl; normal range for total protein, 5.7 to 8.0 g/dl) (Table 3). Three cats (cat ID no. 035 and 065, cumulative dose = 8.4 g/kg; cat ID no. C3B, cumulative dose = 7.0 g/kg) were electively euthanized, and 31 organs were subjected to histopathologic examination. No STAMP-related toxic lesions were found in any of the tissues examined. These results indicate that that STAMP causes transient, mild-to-moderate gastrointestinal and liver toxicity at the 200-mg/kg daily dose level. Thus, 4-weeks of treatment of chronically FIV-infected cats with STAMP is not associated with severe systemic toxicity even at the 200-mg/kg/day dose level and a cumulative dose level as high as 8.4 g/kg.
TABLE 3.
Effects of a 4-week treatment course with orally administered STAMP capsules on health, blood chemistry profiles, and peripheral blood counts of chronically FIV-infected cats
| Parameter | Placebo (n = 6)
|
STAMP at 25 mg/kg (n = 3)
|
STAMP at 50 mg/kg (n = 3)
|
STAMP at 100 mg/kg (n = 4)
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pretreatment | Wk 2 | Wk 4 | Wk 8 | Pretreatment | Wk 2 | Wk 4 | Wk 8 | Pretreatment | Wk 2 | Wk 4 | Wk 8 | Pretreatment | Wk 2 | Wk 4 | Wk 8 | |
| Morbidity | 0/6 | 0/6 | 0/6 | 0/6 | 0/3 | 1/3 | 0/3 | 0/3 | 0/3 | 2/3 | 0/3 | 0/3 | 0/4 | 4/4 | 4/4 | 0/4 |
| Wt loss | NAa | 0/6 | 0/6 | 0/6 | NA | 1/3 | 0/3 | 0/3 | NA | 0/3 | 0/3 | 0/3 | NA | 4/4b | 0/4 | 0/4 |
| Hematology | ||||||||||||||||
| Hemoglobin (g/dl) | 12.7 ± 04 | 12.3 ± 0.6 | 10.7 ± 0.8 | 10.3 ± 0.6 | 12.5 ± 1.0 | 12.6 ± 0.9 | 11.8 ± 2.2 | 11.1 ± 1.0 | 13.2 ± 1.3 | 12.6 ± 1.5 | 13.0 ± 1.5 | 10.6 ± 0.3 | 13.1 ± 0.5 | 15.2 ± 1.0 | 12.0 ± 0.9 | 13.0 ± 0.9 |
| White blood cells (103/μl) | 14.6 ± 1.4 | 13.5 ± 1.6 | 15.9 ± 3.8 | 11.7 ± 2.3 | 12.5 ± 1.6 | 15.7 ± 2.4 | 15.5 ± 2.4 | 7.8 ± 0.4 | 17.3 ± 2.9 | 22.0 ± 6.7 | 23.6 ± 5.5 | 16.0 ± 2.6 | 13.3 ± 2.1 | 18.8 ± 4.4 | 27.5 ± 10.2 | 13.7 ± 3.2 |
| Platelets (103/μl) | 183.8 ± 48.8 | 213.8 ± 36.5 | 190.8 ± 26.5 | 208.5 ± 29.8 | 205.3 ± 22.6 | 170.0 ± 17.6 | 243.0 ± 41.3 | 207.3 ± 16.9 | 175.0 ± 24.9 | 166.7 ± 25.9 | 253.3 ± 57.8 | 278.3 ± 56.3 | 201.3 ± 26.7 | 261.8 ± 36.4 | 223.8 ± 36.0 | 221.8 ± 27.3 |
| Liver function | ||||||||||||||||
| ALT (U/liter) | 73.3 ± 11.2 | 51.8 ± 4.2 | 52.3 ± 18.8 | 54.2 ± 18.5 | 67.3 ± 19.3 | 88.0 ± 17.3 | 83.7 ± 42.9 | 60.7 ± 31.8 | 59.3 ± 5.2 | 57.3 ± 6.6 | 46.0 ± 8.1 | 45.3 ± 4.1 | 61.8 ± 16.8 | 183.5 ± 77.1c | 94.3 ± 47.4 | 53.5 ± 5.5 |
| Total bilirubin (mg/dl) | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.1 ± 0.0 | 0.2 ± 0.0 | 0.1 ± 0.0 | 0.0 ± 0.0 |
| Alkaline phosphatase (U/liter) | 37.7 ± 4.7 | 32.7 ± 5.7 | 29.5 ± 6.4 | 23.3 ± 3.0 | 46.7 ± 7.4 | 36.7 ± 4.3 | 36.0 ± 3.2 | 33.0 ± 3.2 | 40.7 ± 4.4 | 43.7 ± 3.9 | 39.0 ± 9.1 | 39.7 ± 4.2 | 32.5 ± 2.8 | 20.8 ± 4.3 | 60.5 ± 24.8 | 42.8 ± 5.7 |
| Albumin (g/dl) | 2.8 ± 0.1 | 3.2 ± 0.1 | 3.0 ± 0.1 | 2.9 ± 0.0 | 3.1 ± 0.1 | 2.9 ± 0.1 | 2.8 ± 0.1 | 3.1 ± 0.1 | 2.9 ± 0.1 | 2.8 ± 0.2 | 2.7 ± 0.2 | 3.2 ± 0.1 | 3.0 ± 0.1 | 2.4 ± 0.1c | 2.2 ± 0.2c | 3.1 ± 0.1 |
| Renal function/ metabolism | ||||||||||||||||
| BUN (mg/dl) | 23.2 ± 1.2 | 22.5 ± 1.8 | 20.8 ± 1.3 | 22.3 ± 1.5 | 23.0 ± 1.0 | 17.7 ± 0.3 | 20.3 ± 0.9 | 22.0 ± 1.5 | 23.0 ± 3.0 | 19.3 ± 1.3 | 27.0 ± 1.5 | 28.0 ± 2.1 | 21.0 ± 1.9 | 24.5 ± 2.1 | 18.3 ± 0.8 | 21.8 ± 1.0 |
| Creatinine (mg/dl) | 1.6 ± 0.1 | 1.4 ± 0.1 | 1.5 ± 0.0 | 1.5 ± 0.1 | 1.6 ± 0.1 | 1.3 ± 0.2 | 1.1 ± 0.1 | 1.4 ± 0.1 | 1.5 ± 0.1 | 1.2 ± 0.1 | 1.1 ± 0.2 | 1.3 ± 0.1 | 1.3 ± 0.1 | 1.5 ± 0.2 | 1.3 ± 0.1 | 1.3 ± 0.1 |
NA, not applicable.
Weight loss averaged 16.3% ± 5.5%. (standard deviation.
The only sign of sickness at the 25- and 50-mg/kg dose levels was decreased appetite. At the 100-mg/kg dose level, cats developed decreased appetite, weight loss, diarrhea, and vomitting, as detailed in the text.
Efficacy of a 4-week STAMP treatment course in chronically FIV-infected cats.
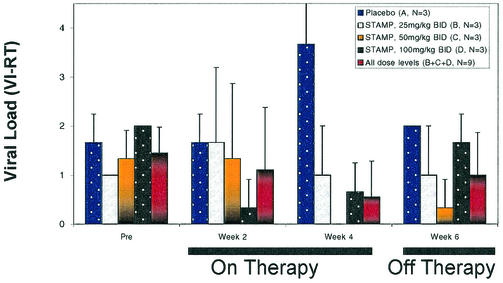
We examined the efficacy of a 4-week STAMP treatment course at daily dose levels ranging from 50 to 200 mg/kg in chronically FIV-infected cats. Specifically, three cats were treated with STAMP at 25 mg/kg/dose in hard gelatin capsules twice daily for 28 consecutive days (group B; cumulative dose = 1.4 g/kg), three cats were treated with STAMP at 50 mg/kg/dose twice daily for 28 consecutive days (group C; cumulative dose = 2.8 g/kg), and three cats were treated with STAMP at 100 mg/kg/dose twice daily for 28 consecutive days (group D; cumulative dose = 5.6 g/kg). Group D was composed of cats that were previously treated with STAMP at a lower dose level and that experienced either a failure or a relapse after an initial transient response to the lower dose. Three control cats (group A) were treated with placebo pills (identically prepared hard gelatin capsules with no active drug). One of three cats treated at the 25-mg/kg dose level, three of three cats treated at the 50-mg/kg dose level, and three of three cats treated at the 100-mg/kg dose level (but none of three control cats treated with placebo pills) showed a therapeutic response, as evidenced by a >1-log reduction in the FIV load in PBMC within 2 weeks (Fig. 1 and Table 4). The average pretreatment, week 2, and week 4 VI-RT values for the FIV burden of placebo-receiving cats were 1.7 ± 0.3, 1.7 ± 0.3, and 3.7 ± 0.9 logs, respectively, consistent with progressive infection while on treatment with placebo. In contrast, the average pretreatment, week 2, and week 4 VI-RT values for the FIV burden of cats receiving STAMP at 50 mg/kg twice daily were 1.3 ± 0.3, 1.3 ± 0.9, and 0.0 ± 0.0 logs, respectively, and the average pretreatment, week 2, and week 4 VI-RT values for the FIV burden of cats receiving STAMP at 100 mg/kg twice daily were 2.0 ± 0.0, 0.3 ± 0.3, and 0.7 ± 0.3 logs, respectively, consistent with a ≥1-log reduction in the virus burden while on treatment with STAMP at 100 to 200 mg/kg/day.
FIG. 1.
In vivo anti-FIV activity of STAMP in chronically FIV-infected cats. Chronically FIV-infected cats were treated twice daily for 28 consecutive days with STAMP administered orally in hard gelatin capsules. The viral loads of PBMC (bars represent mean viral loads ± the standard deviations) were measured by serial-dilution RT assays as described in Materials and Methods for placebo and STAMP treatment for n replicates.
TABLE 4.
In vivo anti-FIV activity of STAMP in chronically FIV-infected cats
| Treatment and cat no. or group | FIV load (VI-RT) in PBMC
|
|||
|---|---|---|---|---|
| Pretreatment | On therapy
|
Off therapy, wk 6 | ||
| Wk 2 | Wk 4 | |||
| Placebo | ||||
| 1 | 2 | 2 | 5 | 2 |
| 2 | 2 | 2 | 4 | 2 |
| 3 | 1 | 1 | 2 | 2 |
| Group A (n = 3) | 1.7 ± 0.6 | 1.7 ± 0.6 | 3.7 ± 1.5 | 2.0 ± 0.0 |
| STAMP: 25 mg/kg BIDg | ||||
| 4 | 1 | 3 | 2 | 2 |
| 5 | 1 | 0 | 0 | 0 |
| 6 | 1 | 2 | 1 | 1 |
| Group B (n = 3) | 1.0 ± 0.0 | 1.7 ± 1.5 | 1.0 ± 1.0 | 1.0 ± 1.0 |
| STAMP: 50 mg/kg BID | ||||
| 7 STAMP, 50mg/kg BID | 1 | 0 | 0 | 0 |
| 8 | 1 | 1 | 0 | 0 |
| 9 | 2 | 3 | 0 | 1 |
| Group C (n = 3) | 1.3 ± 0.6 | 1.3 ± 1.5 | 0.0 ± 0.0a,b,c,e | 0.3 ± 0.6b,c,f |
| STAMP: 100 mg/kg BID | ||||
| 4 STAMP, 100mg/kg BID | 2 | 0 | 1 | 2 |
| 8 | 2 | 0 | 0 | 2 |
| 9 | 2 | 1 | 1 | 1 |
| Group D (n = 3) | 2.0 ± 0.0 | 0.3 ± 0.6a,d,e | 0.7 ± 0.6a,d,e | 1.7 ± 0.6 |
| All doses (n = 9), groups B, C, D | 1.4 ± 0.5 | 1.1 ± 1.3 | 0.6 ± 0.7c,f | 1.0 ± 0.9f |
P < 0.05 versus pretreatment (nonparametric Wilcoxon rank sum test).
P < 0.05 versus placebo (nonparametric Wilcoxon rank sum test).
P < 0.05 versus pretreatment (t test).
P < 0.01 versus pretreatment (t test).
P < 0.05 versus placebo (t test).
P < 0.01 versus placebo (t test).
BID, twice a day.
DISCUSSION
A large proportion of all HIV-1-infected patients receiving contemporary antiretroviral therapies experience virologic failure initially because of RT mutations associated with NRTI resistance (1, 6-8, 10, 11, 13, 14, 24). Thus, for an increasing percentage of both treatment-experienced and treatment-naive HIV-1-infected patients, available NRTIs offer limited therapeutic options. There is an urgent need for new NRTIs that are active against HIV-1 strains that have developed resistance to the available drugs within the NRTI class.
Our recent studies established the novel NRTI compound STAMP as an anti-HIV agent with potent activity against genotypically and phenotypically NRTI-resistant primary clinical HIV-1 isolates with B and non-B envelope subtypes (21). In a side-by-side comparison against 10 ZDV-sensitive primary clinical HIV-1 isolates, 9 of which had a non-B envelope subtype, STAMP was 100-fold more potent than STV and twice as effective as ZDV (21). STAMP was also active against phenotypically and/or genotypically NRTI-resistant HIV strains and inhibited the replication of 20 ZDV-resistant primary clinical HIV-1 isolates for which the IC50s were in the low nanomolar-to-subnanomolar range (21).
We recently investigated the in vivo pharmacokinetics and metabolism of this promising new anti-HIV agent in mice, rats, cats, and dogs (4, 5, 19). STAMP was found to form two active metabolites, namely, alaninyl-STV-monophosphate (ala-STV-MP) and STV, with favorable pharmacokinetics after systemic administration (4, 5, 19). Notably, plasma concentrations of STAMP and ala-STV-MP >4 logs higher than the corresponding anti-HIV IC50s could be achieved in mice, dogs, and cats at nontoxic dose levels (4, 5). We have further evaluated the clinical potential of STAMP as a new anti-HIV agent by examining its acute, subacute, and chronic toxicity profile in mice and rats (19) and by testing its antiviral activity in a surrogate Hu-PBL-SCID mouse model of human AIDS (22). STAMP was very well tolerated by BALB/c and CD-1 mice, with no detectable acute or subacute toxicity at single intraperitoneal or oral bolus dose levels as high as 500 mg/kg. Notably, daily administration of STAMP intraperitoneally or orally for up to 8 consecutive weeks was not associated with any detectable toxicity at cumulative dose levels as high as 6.4 g/kg. STAMP was very well tolerated by Lewis rats as well, with no toxicity at cumulative dose levels of >1 g/kg (19). Therapeutic micromolar concentrations of STAMP and its active metabolites ala-STV-MP and STV in plasma were rapidly achieved and maintained several hours after intraperitoneal administration of nontoxic 25- to 50-mg/kg bolus doses of STAMP. STAMP exhibited dose-dependent and potent in vivo anti-HIV activity in Hu-PBL-SCID mice against a genotypically and phenotypically NRTI-resistant clinical HIV-1 isolate (BR/92/019; D67N, L214F, T215D, K219Q) at nontoxic dose levels (22).
We have previously reported that the para-bromine group in the phenyl moiety of STAMP contributes to its ability to undergo rapid hydrolysis, yielding the key active metabolite ala-STV-MP in a thymidine kinase-independent fashion (23). Hence, the potency of STAMP against genotypically and phenotypically NRTI-resistant HIV-1 isolates may be due to the rapid kinetics of the generation of its active triphosphate metabolite, yielding much higher inhibitor concentrations at the catalytic site sufficient to overcome the binding restrictions imposed by the NRTI resistance-associated RT mutations. It has also been proposed that aryl phosphate derivatives of STV enter target cells more easily than STV (23), which could also contribute to higher inhibitor concentrations at the catalytic site. It is also possible that the presence of an alaninyl side chain may promote the binding and/or incorporation of the triphosphate metabolite of STAMP. The latter possibility and elucidation of the intracellular metabolic pathways of STAMP will be the subject of our future investigations aimed at elucidating the molecular mechanisms contributing to the remarkable potency of STAMP against ZDV-resistant HIV-1 isolates, which is unprecedented for an NRTI compound.
In the present study, we evaluated the toxicity and antiretroviral activity of STAMP in chronically FIV-infected cats. In accordance with its safety profile in rodent species, a 4-week STAMP treatment course with twice-daily administration of hard gelatin capsules containing STAMP at 25 to 100 mg/kg (50 to 200 mg/kg/day) was very well tolerated by cats at cumulative dose levels as high as 8.4 g/kg. Except for the sporadic occurrence of nausea and vomiting after its administration and elevation of serum ALT levels in some of the cats, STAMP therapy was not associated with any clinical or laboratory evidence of toxicity. No STAMP-related toxic lesions were found in any of the organs from STAMP-treated cats. Notably, a single oral bolus dose of STAMP at the 50- or 100-mg/kg dose level resulted in a transient ≥1-log decrease in the FIV load of circulating PBMC in five of six FIV-infected cats and no side effects. A 4-week course of treatment with STAMP administered in gelatin capsules twice daily showed a dose-dependent antiretroviral effect in chronically FIV-infected cats. One of three cats treated at the 25-mg/kg dose level, three of three cats treated at the 50-mg/kg dose level, and three of three cats treated at the 100-mg/kg dose level (but none of three control cats treated with placebo pills) showed a therapeutic response, as evidenced by a ≥1-log reduction in the FIV load in PBMC within 2 weeks. In contrast, the ZDV-lamivudine two-NRTI combination was found to be ineffective in reducing the FIV burden of PBMC in the same model of chronically FIV-infected cats even at dose levels as high as 150 mg/kg/day for each drug, which were associated with substantial side effects (2).
On the basis of its potency, favorable pharmacokinetics, and safety profile, we postulate that STAMP may provide the basis for effective salvage therapies for patients harboring highly drug-resistant strains of HIV-1. The documented in vitro potency of STAMP against primary clinical HIV-1 isolates with genotypic and/or phenotypic NRTI or NNRTI resistance and a non-B envelope subtype, together with its in vivo antiretroviral activity in HIV-infected Hu-PBL SCID mice and FIV-infected cats, warrants further development of this promising new NRTI compound for possible clinical use in both treatment-naive and treatment-experienced HIV-1-infected persons.
Acknowledgments
F.M.U. holds a Hughes Chair in Immunology at the Parker Hughes Institute. We thank R. Pu and J. K. Yamamoto (University of Florida) for services under the FIV contract research agreement between the Parker Hughes Institute and the University of Florida.
We thank Gregory C. Mitcheltree, Hao Chen, Thao Tran, Beth Lisowski, and Heather Wendorf (Parker Hughes Institute) for technical assistance.
REFERENCES
- 1.Albrecht, M. A., R. J. Bosch, S. M. Hammer, S. H. Liou, H. Kessler, M. F. Para, J. Eron, H. Valdez, M. Delinger, and D. A. Katzenstein. 2001. Nelfinavir, efavirenz, or both after the failure of nucleoside treatment of HIV infection. N. Engl. J. Med. 345:398-407. [DOI] [PubMed] [Google Scholar]
- 2.Arai, M., D. D. Earl, and J. K. Yamamoto. 2002. Is AZT/3TC therapy effective against FIV infection or immunopathogenesis? Vet. Immunol. Immunopathol. 85(3-4):189-204. [DOI] [PubMed] [Google Scholar]
- 3.Balzarini, J., H. Egberink, K. Hartmann, D. Cahard, T. Vahlenkamp, H. Thormar, E. Declercq, and C. Mcguian. 1996. Antiretrovirus specificity and intracellular metabolism of 2′,3′-didehydro-2′3′-dideoxythymidine (stavudine) and its 5′-monophosphate triester prodrug So324. Mol. Pharmacol. 50:1207-1213. [PubMed] [Google Scholar]
- 4.Chen, C.-L., T. K. Venkatachalam, Z.-H. Zhu, and F. M. Uckun. 2001. In vivo pharmacokinetics and metabolism of anti-human immunodeficiency virus agent d4T-5′-[p-bromophenyl methoxyalaninyl phosphate] (Sampidine) in mice. Drug Metab. Dispos. 29:1035-1041. [PubMed] [Google Scholar]
- 5.Chen, C.-L., G. Yu, T. K. Venkatachalam, and F. M. Uckun. 2002. Metabolism of stavudine-5′-[p-bromophenyl methoxyalaninyl phosphate], stampidine, in mice, dogs, and cats. Drug Metab. Dispos. 30(12):1523-1531. [DOI] [PubMed] [Google Scholar]
- 6.Freedberg, K. A., E. Losina, M. C. Weinstein, A. D. Paltiel, C. J. Cohen, G. R. Seage, D. E. Graven, H. Zhang, A. D. Kimmel, and S. J. Goldie. 2001. The cost effectiveness of combination antiretroviral therapy for HIV disease. N. Engl. J. Med. 344:824-831. [DOI] [PubMed] [Google Scholar]
- 7.Lerma, J. G., and W. Heneine. 2001. Resistance of human immunodeficiency virus type 1 to reverse transcriptase and protease inhibitors: genotypic and phenotypic testing. J. Clin. Virol. 21:197-212. [DOI] [PubMed] [Google Scholar]
- 7a.National Research Council. 1996. Guide for the care and use of laboratory animals. National Academy Press, Washington, D.C.
- 8.O'Brien, W. A. 2000. Resistance against reverse transcriptase inhibitors. Clin. Infect. Dis. 30(Suppl. 2):S185-S192. [DOI] [PubMed] [Google Scholar]
- 9.Okada, S., R. Pu, E. Young, W. V. Stoffs, and J. K. Yamamoto. 1994. Superinfection of cats with feline immunodeficiency virus subtypes A and B. AIDS Res. Hum. Retrovir. 10:1739-1746. [DOI] [PubMed] [Google Scholar]
- 10.Pillay, D., S. Taylor, and D. D. Richman. 2000. Incidence and impact of resistance against approved antiretroviral drugs. Rev. Med. Virol. 10:231-253. [DOI] [PubMed] [Google Scholar]
- 11.Rey, D., M. P. Schmitt, M. Partisani, G. Hess-Kempf, V. Krantz, E. de Mautort, C. Bernard-Henry, M. Priester, C. Cheneau, and J. M. Lang. 2001. Efavirenz as a substitute for protease inhibitors in HIV-1-infected patients with undetectable plasma viral load on HAART: a median follow-up of 64 weeks. J. Acquir. Immun. Defic. Syndr. 27:459-462. [DOI] [PubMed] [Google Scholar]
- 12.Rey, M. A., B. Spire, D. Dormont, F. Barre-Sinoussi, L. Montagnier, and J. C. Chermann. 1984. Characterization of the RNA dependent DNA polymerase of a new human T-lymphotropic retrovirus. Biochem. Biophys. Res. Commun. 121:126-133. [DOI] [PubMed] [Google Scholar]
- 13.Richman, D. D. 2001. HIV chemotherapy. Nature 410:995-1001. [DOI] [PubMed] [Google Scholar]
- 14.Starr, S. E., C. V. Fletcher, S. A. Spector, F. H. Yong, T. Fenton, R. C. Brundage, D. Manion, N. Ruiz, M. Gersten, M. Becker, J. McNamara, L. M. Mofenson, L. Purdue, S. Siminski, B. Graham, D. M. Kornhauser, W. Fiske, C. Vincent, H. W. Lischner, W. M. Dankner, et al. 1999. Combination therapy with efavirenz, nelfinavir, and nucleoside reverse-transcriptase inhibitors in children infected with human immunodeficiency virus type 1. N. Engl. J. Med. 341:1874-1881. [DOI] [PubMed] [Google Scholar]
- 15.Tanabe, T., and J. K. Yamamoto. 2001. Phenotypic and functional characteristics of FIV infection in the bone marrow stroma. Virology 282:113-122. [DOI] [PubMed] [Google Scholar]
- 16.Uckun, F. M., E. K. Onur, X. P. Liu, and C.-L. Chen. 1999. In vivo toxicity and pharmacokinetic features of the janus kinase 3 inhibitor WHI-P131 [(4′-hydroxylphenyl)-amino-6,7-dimethoxyquinazoline]. Clin. Cancer Res. 5:2954-2962. [PubMed] [Google Scholar]
- 17.Uckun, F. M., K. Bellomy, K. O'Neill, Y. Messinger, T. Johnson, and C.-L. Chen. 1999. Pharmacokinetics of TXU(Anti-CD7)-pokeweed antiviral protein in chimpanzees and adult patients infected with human immunodeficiency virus (HIV)-1. J. Pharmacol. Exp. Ther. 291:1301-1307. [PubMed] [Google Scholar]
- 18.Uckun, F. M., and R. Vig. February 2000. Aryl phosphate derivatives of D4T having anti-HIV activity. U.S. patent 6,030,957.
- 19.Uckun, F. M., C.-L. Chen, E. Lisowski, G. C. Mitcheltree, T. K. Venkatachalam, D. Erbeck, H. Chen, and B. Waurzyniak. Toxicity and pharmacokinetics of stampidine in mice and rats. Arzneim.-Forsch., in press. [DOI] [PubMed]
- 20.Uckun, F. M., P. Samuel, S. Qazi, C. Chen, S. Pendergrass, and T. K. Venkatachalam. 2002. Effects of aryl substituents on the anti-HIV activity of the arylphosphoramidate derivatives of stavudine. Antivir. Chem. Chemother. 13(3):197-203. [DOI] [PubMed] [Google Scholar]
- 21.Uckun, F. M., S. Pendergrass, T. K. Venkatachalam, S. Qazi, and D. Richman. 2002. Stampidine is a potent inhibitor of zidovudine- and nucleoside analog reverse transcriptase inhibitor-resistant primary clinical human immunodeficiency virus type 1 isolates with thymidine analog mutations. Antimicrob. Agents Chemother. 46:3613-3616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Uckun, F. M., S. Qazi, S. Pendergrass, E. Lisowski, B. Waurzyniak, C.-L. Chen, and T. K. Venkatachalam. 2002. In vivo toxicity, pharmacokinetics, and anti-human immunodeficiency virus activity of stavudine-5′-(p-bromophenyl methoxyalaninyl phosphate) (stampidine) in mice. Antimicrob. Agents Chemother. 46:3428-3436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Venkatachalam, T. K., H. L. Tai, R. Vig, C.-L. Chen, S. T. Jan, and F. M. Uckun. 1998. Enhanced effects of a mono-bromo substitution at the para position of the phenyl moiety on the metabolism and anti-HIV activity of D4T-phenyl methoxyalaninyl phosphate derivatives. Bioorg. Med. Chem. Lett. 8:3121-3126. [DOI] [PubMed] [Google Scholar]
- 24.Venturi, G., L. Romano, M. Catucci, M. L. Riccio, A. De Milito, A. Gonnelli, M. Rubino, P. E. Valensin, and M. Zazzi. 1999. Genotypic resistance to zidovudine as a predictor of failure of subsequent therapy with human immunodeficiency virus type-1 nucleoside reverse-transcriptase inhibitors. Eur. J. Clin. Microbiol. Infect. Dis. 18:274-282. [DOI] [PubMed] [Google Scholar]
- 25.Vig, R., T. K. Venkatachalam, and F. M. Uckun. 1998. D4T-5′-[p-bromophenyl methoxyalaninyl phosphate] as a potent and non-toxic anti-human immunodeficiency virus agent. Antivir. Chem. Chemother. 9:445-448. [PubMed] [Google Scholar]
- 26.Yamamoto, J. K., N. C. Pederson, E. W. Ho, T. Okuda, and G. H. Theilen. 1988. Feline immunodeficiency syndrome—a comparison between feline T-lymphotropic lentivirus and feline leukemia virus. Leukemia 2:204S-215S. [PubMed] [Google Scholar]