Abstract
Hyperhomocysteinaemia is an independent risk factor for cardiovascular diseases due to atherosclerosis. The development of atherosclerosis involves reactive oxygen species-induced oxidative stress in vascular cells. Our previous study [Wang and O (2001) Biochem. J. 357, 233–240] demonstrated that Hcy (homocysteine) treatment caused a significant elevation of intracellular superoxide anion, leading to increased expression of chemokine receptor in monocytes. NADPH oxidase is primarily responsible for superoxide anion production in monocytes. In the present study, we investigated the molecular mechanism of Hcy-induced superoxide anion production in monocytes. Hcy treatment (20–100 μM) caused an activation of NADPH oxidase and an increase in the superoxide anion level in monocytes (THP-1, a human monocytic cell line). Transfection of cells with p47phox siRNA (small interfering RNA) abolished Hcy-induced superoxide anion production, indicating the involvement of NADPH oxidase. Hcy treatment resulted in phosphorylation and subsequently membrane translocation of p47phox and p67phox subunits leading to NADPH oxidase activation. Pretreatment of cells with PKC (protein kinase C) inhibitors Ro-32-0432 (bisindolylmaleimide XI hydrochloride) (selective for PKCα, PKCβ and PKCγ) abolished Hcy-induced phosphorylation of p47phox and p67phox subunits in monocytes. Transfection of cells with antisense PKCβ oligonucleotide, but not antisense PKCα oligonucleotide, completely blocked Hcy-induced phosphorylation of p47phox and p67phox subunits as well as superoxide anion production. Pretreatment of cells with LY333531, a PKCβ inhibitor, abolished Hcy-induced superoxide anion production. Taken together, these results indicate that Hcy-stimulated superoxide anion production in monocytes is regulated through PKC-dependent phosphorylation of p47phox and p67phox subunits of NADPH oxidase. Increased superoxide anion production via NADPH oxidase may play an important role in Hcy-induced inflammatory response during atherogenesis.
Keywords: atherosclerosis, homocysteine, monocyte, NADPH oxidase, reactive oxygen species, superoxide anion
Abbreviations: CCR2, chemoattractant protein-1 receptor 2; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; Hcy, homocysteine; L-NAME, NG-nitro-L-arginine methyl ester; MCP-1, monocyte chemoattractant protein-1; NBT, Nitro Blue Tetrazolium; NF-κB, nuclear factor κB; PKC, protein kinase C; cPKC, conventional PKC; ROS, reactive oxygen species; RT, reverse transcriptase; siRNA, small interfering RNA; SOD, superoxide dismutase; PEG–SOD, poly(ethylene glycol)-bound SOD
INTRODUCTION
Hyperhomocysteinaemia, a condition of elevated blood Hcy (homocysteine) levels, is a common and independent risk factor for cardiovascular diseases [1–4]. Abnormal elevation in Hcy levels up to 100–250 μM in the blood has been reported in patients with hyperhomocysteinaemia [5,6]. Hcy is a thiol-containing amino acid formed during the metabolism of methionine. A significant proportion of patients (up to 30–40%) with coronary artery disease had elevated blood Hcy levels [7]. Although the precise mechanisms of Hcy-associated atherosclerosis remain unclear, inflammatory reaction in vascular cells due to oxidative stress is considered to be one of the important mechanisms contributing to vessel injury [8,9].
Many risk factors for atherosclerosis share a common feature of generating intracellular ROS (reactive oxygen species) causing oxidative stress [10–12]. Dysregulation of superoxide anion production can lead to cell injury. NADPH oxidase is primarily responsible for superoxide anion generation in phagocytes such as neutrophils and monocytes [13,14]. In phagocytes, this oxidase is an enzyme complex consisting of membrane-bound components, termed flavocytochrome b558 (p22phox and gp91phox subunits) and cytosolic components (p47phox, p67phox and p40phox). The membrane-associated subunits p22phox and gp91phox (the catalytic subunit of the oxidase) are present as a heterodimer-containing NADPH-binding site, whereas the subunits p47phox, p67phox and p40phox reside in the cytosol as a complex [15–17]. Activation of NADPH oxidase requires the assembly of cytosolic subunits with p22phox and gp91phox subunits in the membrane. It is generally believed that phosphorylation and subsequent translocation of cytosolic subunits to the membrane are essential steps for the activation of NADPH oxidase [15–17]. Upon stimulation, p47phox is rapidly phosphorylated at several serine residues and undergoes a conformational change. Phosphorylated p47phox then interacts with p22phox and gp91phox to promote the oxidase assembly and subsequent activation [16,18]. Phosphorylation of p67phox is also involved in the activation of NADPH oxidase [18]. NADPH oxidase activity can also be regulated at the gene level of oxidase subunits. It has been shown that NADPH oxidase activity is significantly elevated in atherosclerotic lesions, leading to increased superoxide anion production [19,20]. The activation of PKC (protein kinase C) is associated with increased production of superoxide anions in phagocytes as well as in other vascular cells [21–24].
In our previous study, Hcy treatment resulted in an elevation of superoxide anion levels in THP-1 monocytes [25] and in endothelial cells [26]. We further demonstrated that increased intracellular level of superoxide anions was responsible for enhanced mRNA expression of MCP-1 (monocyte chemoattractant protein-1) [25] and its receptor CCR2 (chemoattractant protein-1 receptor 2) in monocytes [27]. Our results suggest that Hcy-induced MCP-1 and CCR2 expression due to increased oxidative stress may facilitate the recruitment of monocytes into the arterial wall [25,27]. Hcy was able to activate NF-κB (nuclear factor κB), an oxidative stress-responsive transcription factor, in endothelial cells via oxidative stress [26]. We also observed that the PKC signalling pathway and oxidative stress played a role in Hcy-induced NF-κB activation in vascular smooth muscle cells [28]. Monocytes/macrophages serve as a major source of superoxide anions contributing to the progression of atherosclerosis [29]. However, the mechanism through which Hcy stimulates superoxide anion production in monocytes is not well understood. In the present study, we investigated the mechanism of Hcy-induced superoxide anion production via NADPH oxidase in human monocytic cells. Our results clearly demonstrated that PKCβ played an important role in Hcy-induced NADPH oxidase activation, leading to dysregulation of superoxide anion production in monocytes.
EXPERIMENTAL
Reagents
Goat polyclonal antibodies against NADPH oxidase subunits (p47phox, p67phox, p22phox and gp91phox), rabbit polyclonal antibodies against PKC isoenzymes (PKCα, PKCβ and PKCγ), goat polyclonal antibodies against actin and p47phox siRNA (small interfering RNA) duplex were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, U.S.A.). Mouse anti-phosphoserine monoclonal antibody was purchased from Sigma–Aldrich (St. Louis, MO, U.S.A.). The corresponding secondary antibodies were purchased from Zymed (Carlsbad, CA, U.S.A.). Phosphorothioate-modified oligonucleotides that were purified by HPLC were purchased from Sigma–Genosys (St. Louis, MO, U.S.A.). All other reagents were from Sigma–Aldrich, except where specified. L-Hcy was prepared from L-Hcy thiolactone as described previously [30,31]. In brief, L-Hcy thiolactone was dissolved and hydrolysed in potassium hydroxide (5 M) to remove the thiolactone group, followed by neutralization with potassium phosphate. The concentration of L-Hcy in the preparation was quantified with the IMx Hcy assay (Abbott Laboratories, Abbott Park, IL, U.S.A.). Freshly prepared L-Hcy was used in all of the experiments.
Cell culture
A human monocytic cell line THP-1 was purchased from the American Type Culture Collection (Rockville, MD, U.S.A.). Cells were cultured in RPMI 1640 medium containing 10% (v/v) low-endotoxin fetal bovine serum as described previously [27]. For experiments, cells were cultured in the RPMI 1640 medium and the cell density was maintained at less than 5×105 cells/ml to prevent cell differentiation [27].
Determination of superoxide anion levels
After cells were incubated with Hcy and other defined compounds for various time periods, the intracellular superoxide anion level was measured by the NBT (Nitro Blue Tetrazolium) reduction assay described previously [25–27]. In brief, cells were incubated in Krebs–Henseleit buffer (120 mM NaCl, 25 mM NaHCO3, 4.7 mM KCl, 1.2 mM MgSO4·7H2O, 1.2 mM NaH2PO4, 1.27 mM CaCl2·2H2O and 5.5 mM D-glucose, pH 7.4) in the presence of NBT (1.0 mg/ml) for 60 min. The formazan was generated by the reduction of NBT in the presence of superoxide anions, which was proportional to the amount of superoxide anion generated intracellularly. At the end of the incubation period, the culture medium was removed and cells were washed with warm (37 °C) Krebs–Henseleit buffer. Cells were lysed with a phosphate buffer (6.8 mM NaH2PO4 and 73.2 mM Na2HPO4, pH 7.8) containing 5% (w/v) SDS and 0.45% gelatin. The cell lysate was centrifuged for 5 min at 13000 g. The supernatant was used for the measurement of the absorbance at 540 nm (formazan) and 450 nm. The relative concentration of superoxide anion was calculated based on the amount of formazan formed. In control experiments, Hcy (100 μM) was added to the cell lysis buffer and the absorbance values at 540 nm (formazan) and 450 nm were measured. In addition, experiments were performed using SOD (superoxide dismutase)-inhibitable cytochrome c reduction assay [32,33]. After cells were incubated with Hcy or other compounds for a certain period of time, cytochrome c (80 μM) with or without the superoxide anion scavenger SOD (100 units/ml) was added to the collected culture medium and the absorbance at 550 nm was measured using a spectrophotometer. The amount of superoxide anions secreted by cells was calculated. Both NBT reduction assay and cytochrome c reduction assay were also performed in the culture medium containing Hcy (100 μM) in the absence of monocytes. Results were used as a control for the autoxidation of Hcy to generate superoxide. Effects of Hcy on superoxide anion levels were calculated by subtracting the data obtained from Hcy-treated cells from the control data for the autoxidation of Hcy.
Determination of NADPH oxidase activity
NADPH oxidase activity was measured using lucigenin chemiluminescence assay [34]. After cells were incubated with Hcy or other compounds, lucigenin (5 μM) was added and equilibrated for 2 min. After equilibration, 100 μM NADPH was added and the chemiluminescent signal was measured every 15 s for 3 min using a luminometer (Lumet LB9507; Berthold Technologies, Bad Wildbad, Germany). The chemiluminescent signal yielded in the samples was corrected for background in each measurement. A standard curve was prepared with xanthine (100 μM) and xanthine oxidase (Sigma–Aldrich) at various concentrations [32]. NADPH oxidase activity was calculated based on the amount of superoxide anion produced in the reaction mixture [34].
Western-blot analysis
Cell lysate was prepared by homogenizing cells in a buffer containing 10 mM Tris/HCl (pH 7.5), 5 mM EDTA, 10 mM EGTA, 0.2 mM PMSF, 20 μM leupeptin, 0.5 mM dithiothreitol and 0.15 μM pepstatin A. Western-blot analysis was performed to determine cellular protein levels of NADPH oxidase subunits p47phox, p67phox, p22phox and gp91phox as well as PKC isoenzymes. The membrane fraction of cell lysate was prepared by ultracentrifugation. In addition, the protein levels of p47phox and p67phox in the membrane fraction were also determined by Western-blot analysis. In brief, proteins in the cell lysate or in the membrane fraction were separated by SDS/10% PAGE. The proteins on the gel were transferred on to a nitrocellulose membrane. The primary and secondary antibodies were used at the dilution of 1:1000 and 1:2000 respectively. Bands corresponding to individual NADPH oxidase subunits or PKC isoforms were visualized with ECL® (enhanced chemiluminescence) reagent (Amersham Biosciences, Little Chalfont, Bucks., U.K.) and exposed to Kodak X-Omat Blue XB-1 films. The results were analysed with Quantity One image analysis software (version 4.2.1; Bio-Rad, Hercules, CA, U.S.A.).
Measurement of NADPH oxidase mRNA
Total RNAs were isolated from cultured cells with TRIzol® reagent (Invitrogen, Carlsbad, CA, U.S.A.). The mRNA expression of p22phox, gp91phox, p47phox and p67phox were examined by semiquantitative RT (reverse transcriptase)–PCR analysis. The primers used for p22phox in the PCR reactions were: (i) 5′-GTTTGTGTGCCTGCTGGAGT-3′ and (ii) 5′-TGGGCGGCTGCTTGATGGT-3′. The primers used for gp91phox were: (i) 5′-GCTGTTCAATGCTTGTGGCT-3′ and (ii) 5′-TCTCCTCATCATGGTGCACA-3′. The primers used for p47phox were: (i) 5′-ACCCAGCCAGCACTATGTGT-3′ and (ii) 5′-AGTAGCCTGTGACGTCGTCT-3′. The primers used for p67phox were: (i) 5′-CGAGGGAACCAGCTGATAGA-3′ and (ii) 5′-CATGGGAACACTGAGCTTCA-3′. All primers were synthesized by Invitrogen. In the reverse transcription reaction, 2 μg of total RNA was converted into cDNA. The reaction mixture of PCR contained 0.2 mM dNTP, 0.4 μM 5′- and 3′-primers, 2 units of Taq DNA polymerase, 1.5 mM MgCl2 and 2 μg of cDNA product from the reverse transcription reaction. The numbers of cycles of PCR amplification were 30 cycles for all the subunits (95 °C for 55 s, 60 °C for 55 s and 72 °C for 90 s). An additional 7 min extension was carried out at 72 °C. The PCR products were separated by agarose gel electrophoresis (1.5% agarose containing ethidium bromide) and visualized under UV light with a gel documentation system (Bio-Rad Gel Doc 1000). Human GAPDH (glyceraldehyde-3-phosphate dehydrogenase) mRNA was used as an internal standard to verify equal PCR product loading for each experiment. The values were expressed as a ratio of p47phox, p67phox, p22phox or gp91phox to GAPDH.
Transfection of cells with p47phox siRNA
Cells were transfected with p47phox siRNA duplex (Santa Cruz Biotechnology). In brief, THP-1 cells were seeded in a six-well plate (2×105 cells/well) and transfected with p47phox siRNA duplex (sc-29422) according to the manufacturer's instructions. For a negative control, cells were transfected with a control siRNA duplex (sc-36869) consisting of a scrambled sequence that did not knock down cellular RNA (Santa Cruz Biotechnology). At 48 h after transfection, cells were washed three times with RPMI 1640 medium. Cells were incubated in the absence or presence of Hcy for 30 min. The intracellular superoxide anion level was measured by NBT reduction assay as described in the ‘Determination of superoxide anion levels’ subsection. The mRNA and protein levels of p47phox in transfected cells were determined by RT–PCR and Western-blot analysis respectively.
Immunoprecipitation and immunoblotting analysis
Immunoprecipitation followed by immunoblotting analysis was performed to detect the phosphorylation status of p47phox and p67phox subunits. In brief, cells were lysed in a buffer containing 10 mM Tris/HCl (pH 7.4), 150 mM NaCl, 10 mM EGTA, 10 mM EDTA, 10 mM sodium pyrophosphate, 1 mM sodium orthovanadate, 50 mM sodium fluoride, 1% (v/v) Triton X-100, 20 μM leupeptin, 1 mM PMSF and 0.15 μM pepstatin. Immunoprecipitation was performed by incubating cell lysate with goat polyclonal anti-p47phox or anti-p67phox antibodies for 2 h, followed by precipitation with agarose-immobilized Protein A. The immunoprecipitated proteins were then subjected to Western-blot analysis using a mouse monoclonal anti-phosphoserine antibody. The protein levels of p47phox and p67phox in the immunoprecipitates were also determined by Western-blot analysis using goat polyclonal anti-p47phox and anti-p67phox antibodies. Phosphorylated p47phox and p67phox protein levels were normalized to the total p47phox and p67phox proteins detected in the immunoprecipitates.
Transfection of cells with sense and antisense oligonucleotides
Phosphorothioate-modified oligonucleotides were used in the present study to minimize degradation. The antisense and sense oligonucleotides were derived from published results [22]. The sequence for PKCα isoenzyme-specific antisense oligonucleotide was 5′-CGCCGTGGAGTCGTTGCCCG-3′ and its control sense sequence was 5′-CGGGCAACGACTCCACCGCG-3′. The sequence for PKCβ isoenzyme-specific antisense oligonucleotide was 5′-AGCGCACGGTGCTCTCCTCG-3′ and its control sense sequence was 5′-CGAGGAGAGCACCGTGCGCT-3′. For experiments, individual phosphorothioate-modified oligonucleotides (4–8 μM) were delivered into cells with Oligofectamine reagent according to the manufacturer's instructions (Invitrogen). The transfection efficiency was determined 48 h later by measuring protein levels of individual PKC isoenzymes in transfected cells using Western-blot analysis.
Assay for PKC activity
PKC activity was measured with an assay kit purchased from Upstate Biotechnology (Charlottesville, VA, U.S.A.). The assay was based on the phosphorylation of a specific substrate peptide (QKRPSQRSKYL) catalysed by PKC. In brief, after treatment with Hcy and other defined compounds, cells were harvested in lysis buffer containing 50 mM Hepes (pH 7.5), 0.2% Triton X-100, 1 mM EDTA, 1 mM EGTA, 1 mM dithiothreitol, 85 μM leupeptin, 2 mM benzamidine and 0.4 mM PMSF. The cell lysate was centrifuged at 100000 g for 20 min to obtain a detergent-soluble fraction (supernatant) [35]. An aliquot of the supernatant (10 μl containing 40 μg of proteins) was incubated with a reaction mixture containing 100 μM [γ-32P]ATP (PerkinElmer Life Sciences, Boston, MA, U.S.A.) for 10 min at 30 °C. At the end of the incubation, the reaction mixture was transferred to P81 ion-exchange chromatography paper, followed by four washes with 0.75% (v/v) phosphoric acid and one wash with acetone, according to the manufacturer's instructions. The radioactivity incorporation into the peptide substrate was determined by a liquid-scintillation counter.
Statistics
Results were analysed using ANOVA followed by Dunnett's post-hoc test. Results are means±S.E.M. P<0.05 was considered significant.
RESULTS
Effect of Hcy on intracellular levels of superoxide anions
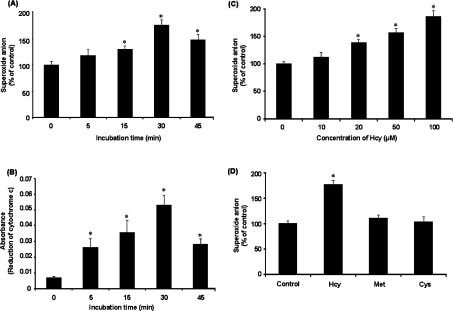
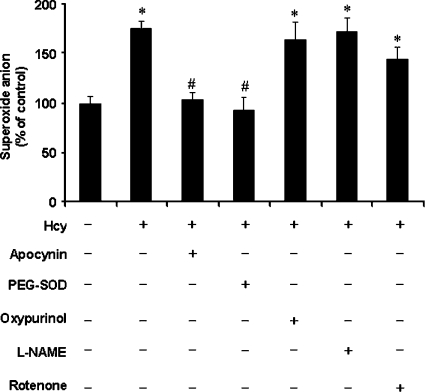
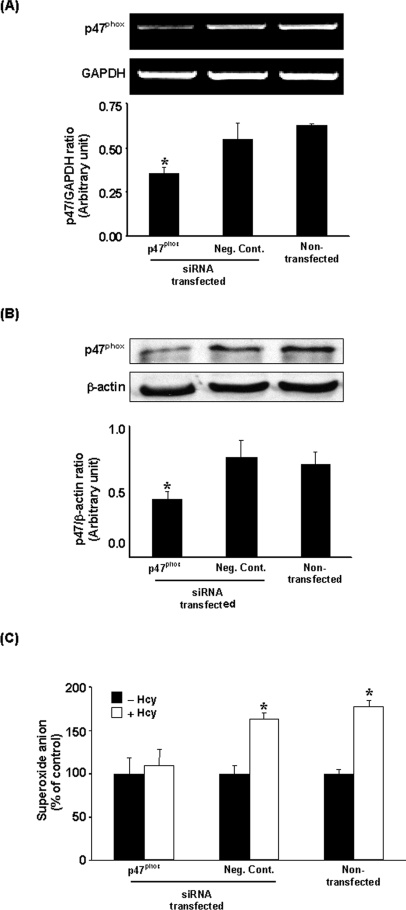
Hcy elicited a time-dependent elevation in superoxide anion level in monocytes, which reached a maximum at 30 min of incubation (Figure 1A). The production of superoxide anion by monocytes was also determined using cytochrome c reduction assay (Figure 1B). A significant elevation in superoxide anion level was observed in cells treated with Hcy in a concentration-dependent manner (Figure 1C). To determine whether such a stimulatory effect was not a generalized effect of thiol-containing amino acids but related specifically to Hcy, the effect of other thiol-containing amino acids, namely cysteine and methionine, on superoxide anion production in monocytes was also examined. There was no significant change in the level of superoxide anions in cells incubated with either cysteine (100 μM) or methionine (100 μM) (Figure 1D). These results indicated that the stimulatory effect on superoxide anion production was related specifically to Hcy. Next, to examine the possible source of increased superoxide anion levels in Hcy-treated cells, several inhibitors of superoxide-producing enzymes were added to the culture medium. Pretreatment of these cells with a specific inhibitor (apocynin) for the NADPH oxidase abolished Hcy-induced elevation in intracellular superoxide anion levels, indicating that the NADPH oxidase system might be activated upon Hcy treatment (Figure 2). There was a significant increase in NADPH oxidase activity in cells incubated with Hcy for 30 min [203.43±37.50 μmol·min−1·(106 cells)−1 versus 3.02±0.50 μmol·min−1·(106 cells)−1 in the control group]. Addition of apocynin to cultured cells completely blocked Hcy-induced activation of NADPH oxidase [2.75±0.45 μmol·min−1·(106 cells)−1 versus 203.43±37.50 μmol·min−1·(106 cells)−1 in the Hcy-treated group]. In addition, cells were pretreated with inhibitors specific for other candidate enzymes followed by incubation with Hcy. Pretreatment of cells with oxypurinol (an inhibitor for xanthine oxidase), L-NAME (NG-nitro-L-arginine methyl ester; an inhibitor of nitric oxide synthase) or rotenone (an inhibitor of mitochondrial respiratory chain complex I) did not affect intracellular superoxide anion levels upon Hcy treatment (Figure 2). In contrast, pretreatment of monocytes with cell-permeant PEG–SOD [poly(ethylene glycol)-bound SOD], a well-known superoxide scavenger, significantly reduced intracellular superoxide anion (Figure 2). To further confirm the involvement of NADPH oxidase in Hcy-induced superoxide anion production in monocytes, cells were transfected with p47phox siRNA. mRNA and protein levels of p47phox were markedly reduced in cells transfected with p47phox siRNA compared with cells transfected with control siRNA (Figures 3A and 3B). Inhibition of p47phox expression abolished Hcy-induced superoxide anion production (Figure 3C).
Figure 1. Superoxide anion levels in monocytes.
Cells were incubated with or without Hcy (100 μM) for various time periods. At the end of the incubation, the intracellular superoxide anion was measured by NBT reduction assay (A) and the extracellular superoxide anion was measured using cytochrome c reduction assay (B). (C) Cells were incubated with Hcy (10–100 μM) or without Hcy for 30 min. (D) Cells were incubated for 30 min in the absence (Control) or presence of Hcy (100 μM), cysteine (Cys; 100 μM) or methionine (Met; 100 μM). In (C and D), the levels of superoxide anions in these cells were determined by NBT reduction assay. The level of superoxide anions in control cells was expressed as 100%. Results were expressed as means±S.E.M. for five separate experiments each performed in duplicate. *Significantly different from the control value obtained from cells incubated in the absence of Hcy (P<0.05).
Figure 2. Effect of inhibitors on intracellular superoxide anion levels.
Cells were pre-incubated with apocynin (300 μM), PEG–SOD (300 units/ml), oxypurinol (10 μM), L-NAME (100 μM) or rotenone (1 μM) for 30 min, followed by incubation with Hcy (100 μM) for another 30 min. At the end of incubation, levels of intracellular superoxide anions were determined by the NBT reduction assay. Results are expressed as means±S.E.M. for five separate experiments each performed in duplicate. The level of superoxide anions in control cells (cultured in the absence of inhibitors and Hcy) was expressed as 100%. *Significantly different from the control value (P<0.05). #Significantly different from the value obtained with Hcy-treated cells (P<0.05).
Figure 3. Effect of siRNA transfection on p47phox expression and superoxide anion production.
At 48 h after transfection of cells with p47phox siRNA or a negative control siRNA (Neg. Cont.), the mRNA levels of p47phox were determined by RT–PCR analysis (A). GAPDH mRNA was used as an internal standard to verify equal PCR product loading for each experiment. (B) The protein levels of p47phox were determined by Western-blot analysis. β-Actin was used to confirm an equal amount of protein loading for each sample. Representative blots were obtained from four separate experiments. *Significantly different from the value obtained from cells transfected with negative control siRNA (P<0.05). (C) In different sets of experiments, transfected and non-transfected cells were incubated with Hcy (100 μM; +Hcy) or without Hcy (−Hcy) for 30 min. The intracellular superoxide anion was measured by the NBT reduction assay. Results are expressed as means±S.E.M. for five separate experiments each performed in duplicate. *Significantly different from the value obtained from cells incubated in the absence of Hcy (−Hcy) (P<0.05).
Mechanism of Hcy-induced NADPH oxidase activation in monocytes
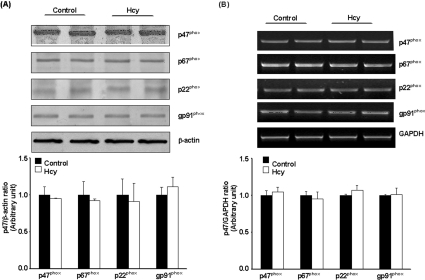
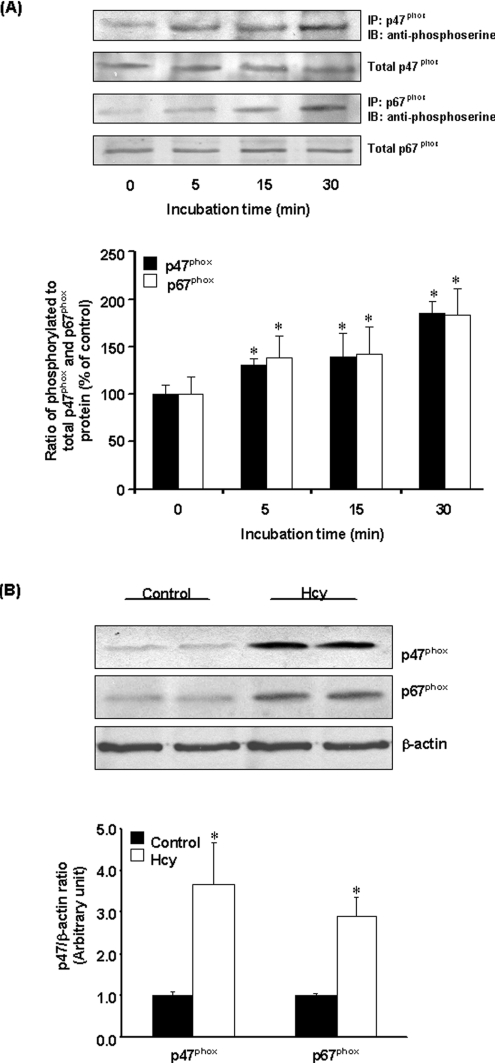
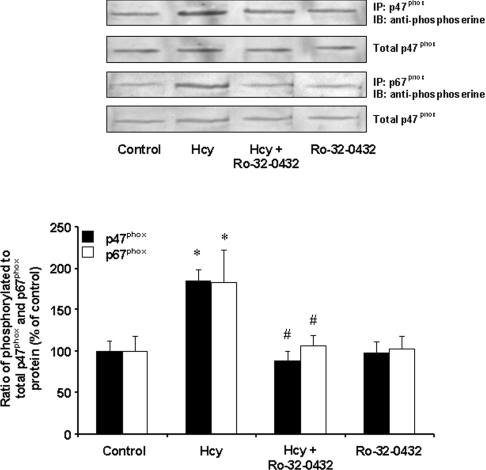
To determine whether increased NADPH oxidase-mediated superoxide anion production was due to an increase in the protein levels of NADPH oxidase, Western-blot analysis was performed. There was no change in the protein levels of p47phox, p67phox, p22phox and gp91phox in Hcy-treated cells (Figure 4A). Further analysis revealed that there was no change in the mRNA levels of individual oxidase subunits (Figure 4B). These results suggested that activation of NADPH oxidase in Hcy-treated cells was not due to an increase in the enzyme protein levels. To further investigate the mechanism by which Hcy treatment activated NADPH oxidase-dependent superoxide anion generation in monocytes, the phosphorylation status of the oxidase subunits was examined. Immunoprecipitation and immunoblotting analyses were carried out to detect the phosphorylation status of p47phox and p67phox subunits. There was a significant increase in serine phosphorylation of p47phox and p67phox subunits in Hcy-treated cells (Figure 5A). The levels of p47phox and p67phox proteins were also found to be increased in the membrane fraction of Hcy-treated cells as compared with control cells (Figure 5B). Hcy-induced phosphorylation of p47phox and p67phox subunits was completely abolished by a selective PKC inhibitor, Ro-32-0432 (bisindolylmaleimide XI hydrochloride) (Figure 6). These results indicated that Hcy-induced NADPH oxidase activation in monocytes was due to increased phosphorylation and subsequent membrane translocation of p47phox and p67phox.
Figure 4. NADPH oxidase subunit protein and mRNA levels in monocytes.
Cells were incubated for 30 min in the absence (Control) or presence of Hcy (100 μM). (A) Cellular extracts were prepared and the protein levels of p47phox, p67phox, p22phox and gp91phox were determined by Western-blot analysis. β-Actin was used to confirm equal amount of protein loading for each sample. (B) Total mRNA was isolated and the mRNA levels of individual NADPH oxidase subunits were analysed by RT–PCR. GAPDH mRNA was used as an internal standard to verify equal PCR product loading for each experiment. Representative photos were obtained from three separate experiments. Results are expressed as means±S.E.M.
Figure 5. Phosphorylation of p47phox and p67phox subunits.
(A) Cells were incubated with Hcy (100 μM) or without Hcy for various time periods. p47phox and p67phox proteins were immunoprecipitated (IP) using goat anti-p47phox or p67phox antibodies followed by Western-blot analysis (IB) using anti-phosphoserine antibodies to detect serine-phosphorylated proteins. The immunoblots were analysed by densitometry. Results were normalized to total p47phox and p67phox proteins in the immunoprecipitates and are means±S.E.M. for five separate experiments. The relative densitometric units for the ratio of phosphorylated to total p47phox or p67phox proteins in the control cells (incubated in the absence of Hcy) were expressed as 100%. (B) Cells were incubated for 30 min in the absence (Control) or presence of Hcy (100 μM). The membrane fraction was prepared for detection of p47phox or p67phox proteins by Western-blot analysis. β-Actin was used to confirm equal amount of protein loading for each sample. Representative blots were obtained from five separate experiments. *Significantly different from the control value obtained from cells incubated in the absence of Hcy (P<0.05).
Figure 6. Effect of PKC inhibition on the phosphorylation of p47phox and p67phox subunits.
Cells were incubated for 30 min in the absence (Control) or presence of Hcy (100 μM). In some experiments, cells were pre-incubated with a PKC inhibitor (Ro-32-0432; 100 nM) for 30 min, followed by incubation with Hcy (100 μM) or without Hcy for another 30 min. The cell lysate was prepared for immunoprecipitation by antibodies against p47phox or p67phox protein. Serine-phosphorylated p47phox and p67phox proteins in the immunoprecipitates were determined by Western-blot analysis using anti-phosphoserine antibodies. Results were normalized to total p47phox and p67phox proteins in the immunoprecipitates and are means±S.E.M. for five separate experiments. The relative densitometric units for the control levels were expressed as 100%. *Significantly different from the control values (P<0.05). #Significantly different from the values obtained from Hcy-treated cells (P<0.05).
Role of PKC in Hcy-stimulated NADPH oxidase activation
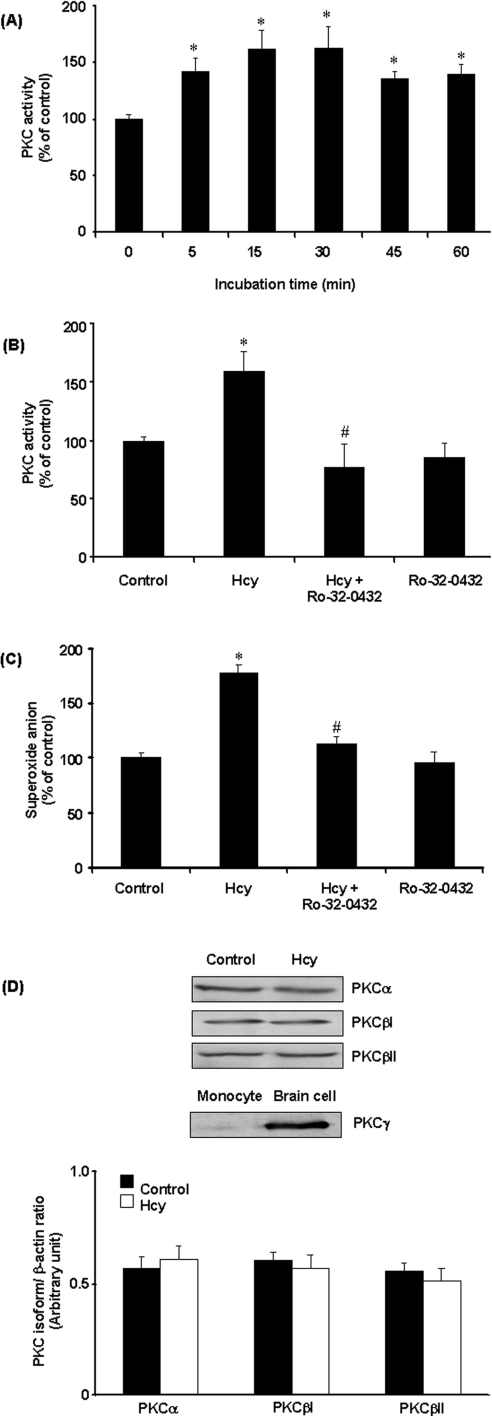
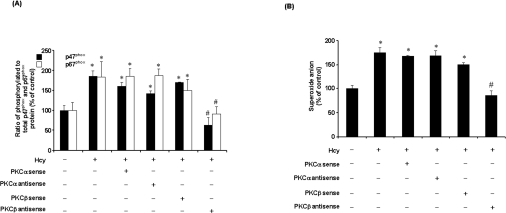
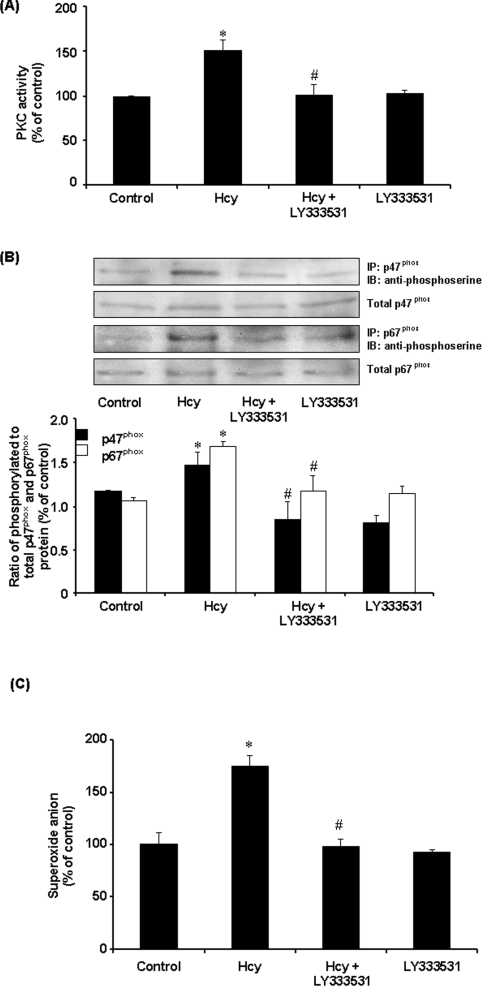
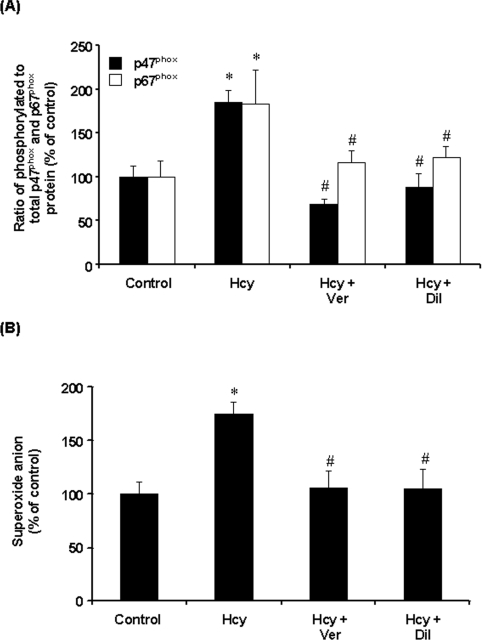
To investigate the involvement of PKC in Hcy-induced NADPH oxidase activation, PKC activity was measured in monocytes. PKC activity was significantly increased in cells treated with Hcy in a time-dependent manner (Figure 7A). Such a stimulatory effect was completely blocked by the PKC inhibitor Ro-32-0432 (Figure 7B). In accordance with these results, pretreatment of cells with Ro-32-0432 also abolished Hcy-induced superoxide anion production in these cells (Figure 7C). Together with the fact that inhibition of PKC activation could abolish Hcy-induced phosphorylation of p47phox and p67phox subunits in monocytes, these results suggested that PKC signalling pathway might play an important role in Hcy-induced NADPH oxidase activation. Ro-32-0432 is a selective cell-permeant inhibitor of PKCα, PKCβ and PKCγ isoenzymes. Addition of this inhibitor to the culture medium could abolish NADPH oxidase-dependent superoxide anion production, indicating that activation of PKC was a prerequisite for Hcy-induced phosphorylation of p47phox and p67phox. It has been suggested that PKCα and PKCβ (PKCβI and PKCβII) but not PKCγ are present in monocytes [29]. Consistent with these findings, only PKCα and PKCβ, but not PKCγ, were detected in THP-1 monocytes (Figure 7D). Hcy treatment did not alter the intracellular protein levels of PKCα, PKCβI and PKCβII (Figure 7D). To identify whether PKCα and/or PKCβ were responsible for Hcy-induced phosphorylation of NADPH oxidase subunits, cells were transfected with PKCα or PKCβ antisense oligonucleotides. In cells transfected with PKCα or PKCβ antisense oligonucleotides, Western-blot analysis revealed a 50, 65 and 60% reduction in PKCα, PKCβI and PKCβII protein levels respectively. On the other hand, protein levels of PKCα or PKCβ were not affected in cells transfected with PKCα and PKCβ sense oligonucleotides. Furthermore, transfection of antisense PKCβ oligonucleotides effectively inhibited Hcy-induced phosphorylation of p47phox and p67phox (Figure 8A) as well as preventing Hcy-induced superoxide anion production in monocytes (Figure 8B). However, no significant changes in NADPH oxidase subunit phosphorylation and superoxide anion production were observed in cells transfected with PKCα antisense oligonucleotide or PKCα and PKCβ sense oligonucleotides (Figure 8). To further confirm the role of PKCβ in Hcy-induced superoxide anion production in monocytes, cells were treated with a specific PKCβ inhibitor (LY333531). In the presence of LY333531, the stimulatory effect of Hcy on PKC activity was completely abolished (Figure 9A). Furthermore, LY333531 treatment also blocked Hcy-induced phosphorylation of p47phox and p67phox (Figure 9B). Addition of LY333531 to the culture medium completely abolished Hcy-induced superoxide anion production in monocytes (Figure 9C). These results clearly indicated that PKCβ played an essential role in Hcy-induced NADPH oxidase activation in monocytes via phosphorylation of both p47phox and p67phox subunits leading to increased production of superoxide anions. To determine whether Hcy-induced phosphorylation of both p47phox and p67phox subunits via PKC signalling pathway was calcium-dependent, cells were pretreated with verapamil, a calcium channel blocker, prior to incubation with Hcy. Such a treatment completely reversed Hcy-induced phosphorylation of p47phox and p67phox (Figure 10A). Similar results were obtained after cells were treated with another calcium channel blocker, diltiazem (Figure 10A). Inhibition of calcium influx by calcium channel blockers also antagonized Hcy-induced superoxide anion production (Figure 10B). These results suggested that changes in calcium influx affected Hcy-induced phosphorylation of p47phox and p67phox and subsequently increased superoxide anion generation in monocytes.
Figure 7. Effect of Hcy on PKC activity in monocytes.
(A) Cells were incubated in the presence of Hcy (100 μM) for various time periods. (B) Cells were incubated for 30 min in the absence (Control) or presence of Hcy (100 μM). In some experiments, cells were pre-incubated with Ro-32-0432 (100 nM) for 30 min, followed by incubation with Hcy (100 μM) or without Hcy for another 30 min. PKC activity in control cells was expressed as 100% (151.9±20.06 pmol·min−1·mg of protein−1). (C) Intracellular superoxide anion levels were measured by the NBT reduction assay. (D) The cellular protein levels of PKCα, PKCβ and PKCγ isoenzymes were determined by Western-blot analysis. Proteins prepared from primary cortical cells of rat brain were used as a positive control for PKCγ. The results are means±S.E.M. for five experiments each performed in duplicate. *Significantly different from the control value (P<0.05). #Significantly different from the value obtained from Hcy-treated cells (P<0.05).
Figure 8. Effect of sense and antisense PKC isoenzyme oligonucleotide transfections on NADPH oxidase subunit phosphorylation.
(A) After 48 h of transfection, cells were incubated with Hcy (100 μM) for 30 min and the levels of serine-phosphorylated p47phox and p67phox proteins were determined by immunoprecipitation and Western-blot analysis. Results were normalized to total p47phox and p67phox proteins in the immunoprecipitates. The relative densitometric units for the control levels were expressed as 100%. (B) Intracellular superoxide anion levels were measured by the NBT reduction assay after cells were transfected with sense or antisense PKC isoenzyme oligonucleotide for 48 h. The level of superoxide anions in control cells was expressed as 100%. Results are means±S.E.M. for three experiments each performed in duplicate. *Significantly different from the control value (P<0.05). #Significantly different from the value obtained from Hcy-treated cells (P<0.05).
Figure 9. Effect of LY333531 on PKC activity, NADPH oxidase subunit phosphorylation and superoxide anion levels in monocytes.
(A) Cells were incubated in the absence (Control) or presence of Hcy (100 μM) for 30 min. In some experiments, cells were pre-incubated with a PKC inhibitor (LY333531; 5 nM) for 30 min, followed by incubation with Hcy (100 μM) or without Hcy for another 30 min. PKC activity was measured. (B) Serine-phosphorylated p47phox and p67phox protein subunits were determined by immunoprecipitation and Western-blot analysis. Results are expressed as means±S.E.M. for three separate experiments. (C) Intracellular superoxide anion levels were measured by the NBT reduction assay. The level of superoxide anions in control cells was expressed as 100%. Results are expressed as means±S.E.M. for four separate experiments each performed in duplicate. The values obtained from control cells were expressed as an arbitrary unit of 100%. *Significantly different from the control value (P<0.05). #Significantly different from the value obtained from Hcy-treated cells (P<0.05).
Figure 10. Effect of calcium channel blockers.
Cells were incubated in the absence (Control) or presence of Hcy (100 μM) for 30 min. In some experiments, cells were pre-incubated with verapamil (Ver; 10 nM) or diltiazem (Dil; 100 nM) for 30 min, followed by incubation with Hcy (100 μM) or without Hcy for another 30 min. (A) Serine-phosphorylated p47phox and p67phox proteins were determined by immunoprecipitation and immunoblotting analysis. Results were normalized to total p47phox and p67phox proteins in the immunoprecipitates. (B) Intracellular superoxide anion levels were measured by the NBT reduction assay. The level of superoxide anions in control cells was expressed as 100%. Results are expressed as means±S.E.M. for four separate experiments each performed in duplicate. *Significantly different from the control value (P<0.05). #Significantly different from the value obtained from Hcy-treated cells (P<0.05).
DISCUSSION
The present study has clearly demonstrated that Hcy activates NADPH oxidase in monocytes. The PKC signalling pathway is responsible for NADPH oxidase activation in monocytes upon Hcy treatment. PKCβ appears to play an important role in stimulating phosphorylation of p47phox and p67phox subunits leading to the activation of NADPH oxidase in Hcy-treated cells.
Previous studies have suggested that oxidative stress is involved in Hcy-mediated cardiovascular risk [9,11,12]. The Hcy-induced ROS production has been indicated as one of the mechanisms causing cell injury in the vasculature [26–28]. One of the early inflammatory responses in monocytes is the production of superoxide anions. NADPH oxidase is considered to be the major source of superoxide anions in monocytes. In the present study, several lines of evidence suggest that NADPH oxidase is responsible for Hcy-induced elevation of superoxide anion levels in monocytes. First, there was a significant increase in NADPH oxidase activity in cells treated with Hcy. Addition of a specific NADPH oxidase inhibitor (apocynin) to cultured cells completely blocked Hcy-induced activation of NADPH oxidase and reduced superoxide anion levels to that of the control. Secondly, phosphorylation of p47phox and p67phox is one of the important mechanisms for NADPH oxidase activation. Indeed, we observed a significant increase in the levels of phosphorylated p47phox and p67phox proteins in Hcy-treated cells. Thirdly, phosphorylation of p47phox and p67phox by PKC is an important mechanism for NADPH oxidase activation in monocytes. Inhibition of PKC activity by either PKC inhibitors or PKCβ antisense oligonucleotides not only attenuated Hcy-induced phosphorylation of p47phox and p67phox, but also blocked Hcy-induced superoxide anion production in these cells. Addition of inhibitors specific for other superoxide anion-producing enzymes to cultured cells failed to inhibit Hcy-induced superoxide anion production, indicating that enzymes other than NADPH oxidase were not directly involved in Hcy-stimulated superoxide anion production in monocytes. Furthermore, inhibition of p47phox expression by siRNA abolished Hcy-induced superoxide anion production. Taken together, these results suggest that NADPH oxidase is the major source of superoxide anion production in monocytes upon Hcy treatment.
We also investigated the possible mechanism by which Hcy activated NADPH oxidase in monocytes. Phosphorylation of p47phox and p67phox subunits is thought to be a key step that precedes membrane translocation of the cytosolic subunits. In the present study, there was a significant increase in phosphorylation of p47phox and p67phox subunits in monocytes upon Hcy treatment. We further investigated the molecular mechanism of increased phosphorylation of these two subunits via PKC signalling pathway. Several PKC isoforms, such as PKCα, PKCβI, PKCβII, PKCϵ, PKCδ and PKCζ, have been found in monocytes [29,36]. The first three isoforms (PKCα, PKCβI and PKCβII) belong to the classic or cPKC (conventional PKC) subfamily and their activation is calcium-dependent. The cPKCs expressed in monocytes are PKCα, PKCβI and PKCβII isoforms. In the present study, several lines of evidence suggested that PKCβ was involved in Hcy-induced phosphorylation of p47phox and p67phox in monocytes. First, when cells were pretreated with an inhibitor (Ro-32-0432) that specifically inhibited PKCα, PKCβ and PKCγ activities, Hcy-induced phosphorylation of p47phox and p67phox was completely abolished (Figure 6). Secondly, Western-blot analysis revealed that PKCα, PKCβI and PKCβII were present in monocytes, whereas little PKCγ was detected (Figure 7D). These results suggested that PKCα and PKCβ might be the potential candidates playing an important role in Hcy-induced phosphorylation of p47phox and p67phox subunits. Thirdly, to differentiate whether PKCα and/or PKCβ were involved in Hcy-induced phosphorylation of p47phox and p67phox, an antisense strategy was used to down-regulate PKCα and PKCβ expression prior to Hcy treatment. Suppression of PKCβ, but not PKCα, expression by antisense oligonucleotides completely abolished Hcy-induced serine phosphorylation of both p47phox and p67phox subunits in monocytes (Figure 8A). As a result, Hcy-induced superoxide anion production was prevented (Figure 8B). Finally, to further confirm the role of PKCβ in Hcy-induced NADPH oxidase activation, a specific PKCβ inhibitor (LY333531) was used. Inhibition of PKCβ not only abolished Hcy-induced PKC activation (Figure 9A), but also prevented Hcy-induced phosphorylation of p47phox and p67phox in monocytes (Figure 8B). Taken together, these results indicated that PKC activation was a prerequisite for Hcy-induced NADPH oxidase activation via phosphorylation of p47phox and p67phox subunits. Based on the results obtained from the present study, we propose the following mechanism by which Hcy induces superoxide anion production in monocytes: (i) Hcy activates PKCβ in monocytes and, once activated, PKCβ is responsible for serine phosphorylation of p47phox and p67phox subunits; and (ii) phosphorylation followed by membrane translocation of cytosolic subunits results in the activation of NADPH oxidase and superoxide anion production in these cells. Although our results suggest that Hcy-induced phosphorylation of p47phox and p67phox in monocytes appears to be mediated via PKCβ, it does not exclude the possibility that other PKC isoforms can also phosphorylate p47phox and p67phox in monocytes activated by other stimuli. The involvement of these isoforms in Hcy-induced oxidative stress remains to be investigated in future studies.
In summary, the present study has clearly demonstrated that Hcy stimulates superoxide anion production in monocytes via activation of NADPH oxidase. PKCβ plays an important role in phosphorylation of p47phox and p67phox leading to NADPH oxidase activation in monocytes. Dysregulation of superoxide anion levels in monocytes via NADPH oxidase may represent one of the mechanisms that contribute to Hcy-associated athero sclerosis.
Acknowledgments
This study was supported by the Heart and Stroke Foundation, and the Natural Sciences and Engineering Research Council of Canada.
References
- 1.Clarke R., Daly L., Robinson K., Naughten E., Cahalane S., Fowler B., Graham I. Hyperhomocysteinemia: an independent risk factor for vascular disease. N. Engl. J. Med. 1991;324:1149–1155. doi: 10.1056/NEJM199104253241701. [DOI] [PubMed] [Google Scholar]
- 2.Duell P. B., Malinow M. R. Homocyst(e)ine: an important risk factor for atherosclerotic vascular disease. Curr. Opin. Lipidol. 1997;8:28–34. doi: 10.1097/00041433-199702000-00007. [DOI] [PubMed] [Google Scholar]
- 3.Refsum H., Ueland P. M., Nygard O., Vollset S. E. Homocysteine and cardiovascular disease. Annu. Rev. Med. 1998;49:31–62. doi: 10.1146/annurev.med.49.1.31. [DOI] [PubMed] [Google Scholar]
- 4.Welch G. N., Loscalzo J. Homocysteine and atherothrombosis. N. Engl. J. Med. 1998;338:1042–1050. doi: 10.1056/NEJM199804093381507. [DOI] [PubMed] [Google Scholar]
- 5.Harker L. A., Slichter S. J., Scott C. R., Ross R. Homocystinemia: vascular injury and arterial thrombosis. N. Engl. J. Med. 1974;291:537–543. doi: 10.1056/NEJM197409122911101. [DOI] [PubMed] [Google Scholar]
- 6.Mainlow M. R. Hyperhomocysteinemia: a common and easily reversible risk factor for occlusive atherosclerosis. Circulation. 1990;81:2004–2006. doi: 10.1161/01.cir.81.6.2004. [DOI] [PubMed] [Google Scholar]
- 7.Nygard O., Nordrehaug J. E., Refsum H., Ueland P. M., Farstad M., Vollset S. E. Plasma homocysteine levels and mortality in patients with coronary artery disease. N. Engl. J. Med. 1997;337:230–236. doi: 10.1056/NEJM199707243370403. [DOI] [PubMed] [Google Scholar]
- 8.Khatri J. J., Johnson C., Magid R., Lessner S. M., Laude K. M., Dikalov S. I., Harrison D. G., Sung H. J., Rong Y., Galis Z. S. Vascular oxidant stress enhances progression and angiogenesis of experimental atheroma. Circulation. 2004;109:520–525. doi: 10.1161/01.CIR.0000109698.70638.2B. [DOI] [PubMed] [Google Scholar]
- 9.Weiss N., Heydrick S., Zhang Y. Y., Bierl C., Cap A., Loscalzo J. Cellular redox state and endothelial dysfunction in mildly hyperhomocysteinemic cystathionine beta-synthase-deficient mice. Arterioscler. Thromb. Vasc. Biol. 2002;22:34–41. doi: 10.1161/hq1201.100456. [DOI] [PubMed] [Google Scholar]
- 10.Dayal S., Brown K. L., Weydert C. J., Oberley L. W., Arning E., Bottiglieri T., Faraci F. M., Lentz S. R. Deficiency of glutathione peroxidase-1 sensitizes hyperhomocysteinemic mice to endothelial dysfunction. Arterioscler. Thromb. Vasc. Biol. 2002;22:1996–2002. doi: 10.1161/01.atv.0000041629.92741.dc. [DOI] [PubMed] [Google Scholar]
- 11.Kanani P. M., Sinkey C. A., Browning R. L., Allaman M., Knapp H. R., Haynes W. G. Role of oxidant stress in endothelial dysfunction produced by experimental hyperhomocystinemia in humans. Circulation. 1999;100:1161–1168. doi: 10.1161/01.cir.100.11.1161. [DOI] [PubMed] [Google Scholar]
- 12.Upchurch G. R., Jr, Welch G. N., Fabian A. J., Freedman J. E., Johnson J. L., Keaney J. F., Jr, Loscalzo J. Homocyst(e)ine decreases bioavailable nitric oxide by a mechanism involving glutathione peroxidase. J. Biol. Chem. 1997;272:17012–17017. doi: 10.1074/jbc.272.27.17012. [DOI] [PubMed] [Google Scholar]
- 13.Bey E. A., Xu B., Bhattacharjee A., Oldfield C. M., Zhao X., Li Q., Subbulakshmi V., Feldman G. M., Wientjes F. B., Cathcart M. K. Protein kinase Cδ is required for p47phox phosphorylation and translocation in activated human monocytes. J. Immunol. 2004;173:5730–5738. doi: 10.4049/jimmunol.173.9.5730. [DOI] [PubMed] [Google Scholar]
- 14.Van Heerebeek L., Meischl C., Stooker W., Meijer C. J., Niessen H. W., Roos D. NADPH oxidase(s): new source(s) of reactive oxygen species in the vascular system? J. Clin. Pathol. 2002;55:561–568. doi: 10.1136/jcp.55.8.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Babior B. M. NADPH oxidase: an update. Blood. 1999;93:1464–1476. [PubMed] [Google Scholar]
- 16.Groemping Y., Lapouge K., Smerdon S. J., Rittinger K. Molecular basis of phosphorylation-induced activation of the NADPH oxidase. Cell. 2003;113:343–355. doi: 10.1016/s0092-8674(03)00314-3. [DOI] [PubMed] [Google Scholar]
- 17.Robinson J. M., Ohira T., Badwey J. A. Regulation of the NADPH-oxidase complex of phagocytic leukocytes. Recent insights from structural biology, molecular genetics, and microscopy. Histochem. Cell Biol. 2004;122:293–304. doi: 10.1007/s00418-004-0672-2. [DOI] [PubMed] [Google Scholar]
- 18.Benna J. E., Dang P. M., Gaudry M., Fay M., Morel F., Hakim J., Gougerot-Pocidalo M. A. Phosphorylation of the respiratory burst oxidase subunit p67phox during human neutrophil activation. Regulation by protein kinase C-dependent and independent pathways. J. Biol. Chem. 1997;272:17204–17208. doi: 10.1074/jbc.272.27.17204. [DOI] [PubMed] [Google Scholar]
- 19.Kalinina N., Agrotis A., Tararak E., Antropova Y., Kanellakis P., Ilyinskaya O., Quinn M. T., Smirnov V., Bobik A. Cytochrome b558-dependent NAD(P)H oxidase-phox units in smooth muscle and macrophages of atherosclerotic lesions. Arterioscler. Thromb. Vasc. Biol. 2002;22:2037–2043. doi: 10.1161/01.atv.0000040222.02255.0f. [DOI] [PubMed] [Google Scholar]
- 20.Sorescu D., Weiss D., Lassegue B., Clempus R. E., Szocs K., Sorescu G. P., Valppu L., Quinn M. T., Lambeth J. D., Vega J. D., et al. Superoxide production and expression of nox family proteins in human atherosclerosis. Circulation. 2002;105:1429–1435. doi: 10.1161/01.cir.0000012917.74432.66. [DOI] [PubMed] [Google Scholar]
- 21.Frey R. S., Rahman A., Kefer J. C., Minshall R. D., Malik A. B. PKCζ regulates TNF-alpha-induced activation of NADPH oxidase in endothelial cells. Circ. Res. 2002;90:1012–1019. doi: 10.1161/01.res.0000017631.28815.8e. [DOI] [PubMed] [Google Scholar]
- 22.Li Q., Subbulakshmi V., Fields A. P., Murray N. R., Cathcart M. K. Protein kinase Cα regulates human monocyte O·2 production and low density lipoprotein oxidation. J. Biol. Chem. 1999;274:3764–3771. doi: 10.1074/jbc.274.6.3764. [DOI] [PubMed] [Google Scholar]
- 23.Kitada M., Koya D., Sugimoto T., Isono M., Araki S., Kashiwagi A., Haneda M. Translocation of glomerular p47phox and p67phox by protein kinase C-β activation is required for oxidative stress in diabetic nephropathy. Diabetes. 2003;52:2603–2614. doi: 10.2337/diabetes.52.10.2603. [DOI] [PubMed] [Google Scholar]
- 24.Venugopal S. K., Devaraj S., Yang T., Jialal I. Alpha-tocopherol decreases superoxide anion release in human monocytes under hyperglycemic conditions via inhibition of protein kinase C-alpha. Diabetes. 2002;51:3049–3054. doi: 10.2337/diabetes.51.10.3049. [DOI] [PubMed] [Google Scholar]
- 25.Wang G., Siow Y. L., O K. Homocysteine-induced monocytes chemoattractant protein-1 expression in human monocytic cells is mediated by nuclear factor-κB activation. Am. J. Physiol. Heart Circ. Physiol. 2001;280:H2840–H2847. doi: 10.1152/ajpheart.2001.280.6.H2840. [DOI] [PubMed] [Google Scholar]
- 26.Au-Yeung K. K., Woo C. W., Sung F. L., Yip J. C., Siow Y. L., O K. Hyperhomocysteinemia activates nuclear factor-κB in endothelial cells via oxidative stress. Circ. Res. 2004;94:28–36. doi: 10.1161/01.RES.0000108264.67601.2C. [DOI] [PubMed] [Google Scholar]
- 27.Wang G., O K. Homocysteine stimulates the expression of monocyte chemoattractant protein-1 receptor (CCR2) in human monocytes: possible involvement of oxygen free radicals. Biochem. J. 2001;357:233–240. doi: 10.1042/0264-6021:3570233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang G., Siow Y. L., O K. Homocysteine stimulates nuclear factor κB activity and monocyte chemoattractant protein-1 expression in vascular smooth-muscle cells: a possible role for protein kinase C. Biochem. J. 2000;352:817–826. [PMC free article] [PubMed] [Google Scholar]
- 29.Cathcart M. K. Regulation of superoxide anion production by NADPH oxidase in monocytes/macrophages: contributions to atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2004;24:23–28. doi: 10.1161/01.ATV.0000097769.47306.12. [DOI] [PubMed] [Google Scholar]
- 30.Uerre J. A., Miller C. H. Preparation of L-homocysteine from L-homocysteine thiolactone. Anal. Biochem. 1966;17:310–315. doi: 10.1016/0003-2697(66)90209-0. [DOI] [PubMed] [Google Scholar]
- 31.Woo C. W., Siow Y. L., Pierce G. N., Choy P. C., Minuk G. Y., Mymin D., O K. Hyperhomocysteinemia induces hepatic cholesterol biosynthesis and lipid accumulation via activation of transcription factors. Am. J. Physiol. Endocrinol. Metab. 2005;288:E1002–E1010. doi: 10.1152/ajpendo.00518.2004. [DOI] [PubMed] [Google Scholar]
- 32.Barbacanne M. A., Souchard J. P., Darblade B., Iliou J. P., Nepveu F., Pipy B., Bayard F., Arnal J. F. Detection of superoxide anion released extracellularly by endothelial cells using cytochrome c reduction, ESR, fluorescence and lucigenin-enhanced chemiluminescence techniques. Free Radical Biol. Med. 2000;29:388–396. doi: 10.1016/s0891-5849(00)00336-1. [DOI] [PubMed] [Google Scholar]
- 33.El Bekay R., Alvarez M., Carballo M., Martin-Nieto J., Monteseirin J., Pintado E., Bedoya F. J., Sobrino F. Activation of phagocytic cell NADPH oxidase by norfloxacin: a potential mechanism to explain its bactericidal action. J. Leukocyte Biol. 2002;71:255–261. [PubMed] [Google Scholar]
- 34.Chen X., Touyz R. M., Park J. B., Schiffrin E. L. Antioxidant effects of vitamins C and E are associated with altered activation of vascular NADPH oxidase and superoxide dismutase in stroke-prone SHR. Hypertension. 2001;38:606–611. doi: 10.1161/hy09t1.094005. [DOI] [PubMed] [Google Scholar]
- 35.Lynn E. G., Siow Y. L., O K. Very low-density lipoprotein stimulates the expression of monocyte chemoattractant protein-1 in mesangial cells. Kidney Int. 2000;57:1472–1483. doi: 10.1046/j.1523-1755.2000.00992.x. [DOI] [PubMed] [Google Scholar]
- 36.Tan S.-L., Parker P. J. Emerging and diverse roles of protein kinase C in immune cell signalling. Biochem. J. 2003;376:545–552. doi: 10.1042/BJ20031406. [DOI] [PMC free article] [PubMed] [Google Scholar]