Abstract
Mitochondrial dysfunction during acute metabolic crises is considered an important pathomechanism in inherited disorders of propionate metabolism, i.e. propionic and methylmalonic acidurias. Biochemically, these disorders are characterized by accumulation of propionyl-CoA and metabolites of alternative propionate oxidation. In the present study, we demonstrate uncompetitive inhibition of PDHc (pyruvate dehydrogenase complex) by propionyl-CoA in purified porcine enzyme and in submitochondrial particles from bovine heart being in the same range as the inhibition induced by acetyl-CoA, the physiological product and known inhibitor of PDHc. Evaluation of similar monocarboxylic CoA esters showed a chain-length specificity for PDHc inhibition. In contrast with CoA esters, non-esterified fatty acids did not inhibit PDHc activity. In addition to PDHc inhibition, analysis of respiratory chain and tricarboxylic acid cycle enzymes also revealed an inhibition by propionyl-CoA on respiratory chain complex III and α-ketoglutarate dehydrogenase complex. To test whether impairment of mitochondrial energy metabolism is involved in the pathogenesis of propionic aciduria, we performed a thorough bioenergetic analysis in muscle biopsy specimens of two patients. In line with the in vitro results, oxidative phosphorylation was severely compromised in both patients. Furthermore, expression of respiratory chain complexes I–IV and the amount of mitochondrial DNA were strongly decreased, and ultrastructural mitochondrial abnormalities were found, highlighting severe mitochondrial dysfunction. In conclusion, our results favour the hypothesis that toxic metabolites, in particular propionyl-CoA, are involved in the pathogenesis of inherited disorders of propionate metabolism, sharing mechanistic similarities with propionate toxicity in micro-organisms.
Keywords: mitochondrial dysfunction, mitochondrial toxin, oxidative phosphorylation, propionic aciduria, propionyl-CoA, pyruvate dehydrogenase complex
Abbreviations: BN-PAGE, blue native PAGE; E1, pyruvate decarboxylase; E3, dihydrolipoyl dehydrogenase; KGDHc, α-ketoglutarate dehydrogenase complex; mtDNA, mitochondrial DNA; ND6, NADH dehydrogenase 6; OXPHOS, oxidative phosphorylation; PA, propionic aciduria; PDHc, pyruvate dehydrogenase complex; SMP, submitochondrial particle
INTRODUCTION
Propionyl-CoA is an intermediate metabolite in the final common catabolic pathways of the amino acids L-isoleucine, L-methionine, L-threonine and L-valine as well as odd-chain fatty acids and cholesterol. It is carboxylated by propionyl-CoA carboxylase (EC 6.4.1.3) to methylmalonyl-CoA and then is converted by methylmalonyl-CoA mutase (EC 5.4.99.2) into succinyl-CoA, subsequently being fed as anaplerotic precursor into the tricarboxylic acid cycle. Inherited deficiency of propionyl-CoA carboxylase, i.e. PA (propionic aciduria), is biochemically characterized by an accumulation of propionate, 3-hydroxypropionate, 2-methylcitrate and propionylglycine, demonstrating alternative propionate oxidation in these patients. Furthermore, metabolic acidosis, ketosis, increased lactate concentrations, hypoglycaemia and hyperammonaemia are found during metabolic derangement, highlighting secondarily compromised energy metabolism and ammonia detoxification [1]. Clinically, PA is complicated by acute life-threatening metabolic crises, which are precipitated by catabolic state and result in multiple organ failure or even death if untreated [1]. Like other organic acidurias (e.g. methylmalonic aciduria), age at clinical onset and disease course may vary between individual patients presenting with neonatal metabolic encephalopathy (neonatal onset), recurrent episodes of ketoacidotic coma or Reye-like syndromes (chronic intermittent form), or psychomotor retardation and failure to thrive in the absence of acute crises (chronic progressive form). Similarly to neonatal onset of PA patients, PCCA−/− mice, a transgenic mouse model for PA, develop lethal ketoacidosis shortly after birth [2].
Despite improvements in the diagnostic work-up and management of affected patients during the last decades, the neurological outcome remains disappointing in PA patients [1,3]. In particular, mental retardation and movement disorders secondary to cortical atrophy, leucoencephalopathy and basal ganglia injury are still frequently found even in early diagnosed children. The biochemical abnormalities found during acute metabolic crises have led to the suggestion that impairment of OXPHOS (oxidative phosphorylation) is crucial for the pathogenesis of this disease. Notably, accumulating metabolites of alternative propionate oxidation have been suggested to act as endogenous inhibitors of energy metabolism in different in vitro models [4–7]; however, the pathophysiological impact of these findings on PA still remains unclear.
Here, we report severe disturbance of mitochondrial energy metabolism in muscle tissues from two PA patients and demonstrate in vitro that propionyl-CoA-induced mitochondrial dysfunction plays a central role in this scenario.
EXPERIMENTAL
Patient 1
This girl was born at term as the second child of non-consanguineous Caucasian parents. At the third day of life, she was admitted because of progressive feeding refusal, lethargy and abnormal breathing. Laboratory investigations revealed a severe metabolic acidosis [pH 7.01; pCO2 18 mmHg (1 mmHg=0.133 kPa); bicarbonate 5 mM; base excess 25 mM] and hyperammonaemia (830 μM; normal <80 μM). The amino acid analyses in plasma revealed no abnormalities. Analysis of urine organic acids by GC/MS showed biochemical abnormalities characteristic for PA with increased concentrations of β-hydroxybutyric acid (6373 mmol/mol of creatinine; control range: 0–45 mmol/mol of creatinine), β-hydroxypropionic acid (21461 mmol/mol of creatinine; control range: 0–160 mmol/mol of creatinine), β-hydroxyisovaleric acid (176 mmol/mol of creatinine; control range: 0–10 mmol/mol of creatinine), lactic acid (776 mmol/mol of creatinine; control range: 0–270 mmol/mol of creatinine), propionylglycine, methylcitrate and tiglylglycine. The last three metabolites were not quantified. The concentration of free carnitine in plasma was decreased (10 μM; control range 20–200 μM), and the concentration of total carnitine was below normal. A carnitine profiling was not done at the time of admission, but later on it repeatedly showed increased concentrations of propionylcarnitine varying between 40 and 80 μM (normal <4 μM).
Extracorporal detoxification was performed. Besides a high caloric intake and transient stop of protein intake, metronidazol, lactulose, L-carnitine and biotin were added in the acute treatment phase. At age 10 days, she was dismissed from the neonatal intensive care unit and then was nursed in the metabolic ward. The only abnormal clinical sign at that time was a slight axial hypotonia. Maintenance treatment consisted of a natural protein intake (1.0 g/kg per day) supplemented with an amino acid mixture lacking precursor amino acids of propionyl-CoA (i.e. L-isoleucine, L-methionine, L-threonine and L-valine) and application of L-carnitine and biotin. Analysis of propionyl-CoA carboxylase activity in leucocytes showed deficient activity of this enzyme (0.1 nmol·h−1·mg of protein−1; control: 2.3 nmol·h−1·mg of protein−1) and thus confirmed the diagnosis of PA. She was admitted to hospital numerous times due to metabolic derangements, the last one, at age 5 years, being fatal. The child had a severe myopathic appearance during life, which was, in combination with repeatedly increased blood lactate concentrations (2.0–7.2 mM; normal <2.1 mM), a reason to perform a muscle biopsy for mitochondrial studies.
Patient 2
This boy was born at term following an uncomplicated pregnancy and delivery. At the third day of life, tachypnoea and metabolic acidosis led to a detailed metabolic investigation. The investigation of amino acids in plasma revealed an increased concentration of glycine (722 μM; 80–440 μM). As for patient 1, urine organic acid analysis showed an increased concentration of β-hydroxybutyric acid (17818 mmol/mol of creatinine), β-hydroxypropionic acid (25096 mmol/mol of creatinine), β-hydroxyisovaleric acid (34 mmol/mol of creatinine), lactic acid (1322 mmol/mol of creatinine), propionylglycine, methylcitrate and tiglylglycine. Propionyl-CoA carboxylase activity in cultured skin fibroblasts was strongly decreased (0.3 nmol·h−1·mg of protein−1), confirming the diagnosis of PA that was treated according to standard methods (see also the Patient 1 subsection).
The child always had a myopathic appearance and exercise intolerance. Standard exercise testing at age 5 years showed an increase in blood lactate from normal concentrations at rest to 20 mM lactate after a short run of approx. 50 m. This was the reason for further bioenergetic analyses in muscle tissue (Table 1).
Table 1. Bioenergetic analysis in muscle tissue of PA patients.
Radiochemical analysis of coupled mitochondria (A) using [1-14C]pyruvate, [U-14C]malate and [1,4-14C]succinate. Spectrophotometric analyses of single enzymes of the respiratory chain, tricarboxylic acid cycle, PDHc (B), ATP and phosphocreatine production rate (C), and citrate synthase (D). Analyses were performed in muscle biopsy specimens of both patients. Activities are expressed as nmol·h−1·mg of protein−1 (A), m-unit/unit of citrate synthase (B), nmol·h−1·m-unit−1 citrate synthase (C) and m-unit/mg of protein (D). The production of ATP and phosphocreatine was determined to establish the total mitochondrial energy-generating capacity of the muscle mitochondria of both patients. n.m., not measured.
| Experiment | Patient 1 | Patient 2 | Control range |
|---|---|---|---|
| A. Oxidation of pyruvate, malate and succinate | |||
| [1-14C]Pyruvate+malate | 0.13 | 0.56 | 3.61–7.48 |
| [1-14C]Pyruvate+carnitine | 0.21 | 0.59 | 2.84–8.24 |
| [U-14C]Malate+pyruvate+malonate | 0.18 | 0.32 | 4.68–9.62 |
| [U-14C]Malate+acetylcarnitine+malonate | 0.46 | 1.32 | 3.43–7.30 |
| [U-14C]Malate+acetylcarnitine+arsenite | 0.43 | 1.45 | 2.05–6.39 |
| [1,4-14C]Succinate+acetylcarnitine | 0.27 | 0.82 | 2.54–6.39 |
| B. Single enzyme activity | |||
| Complex I | 16 | 34 | 70–250 |
| Complex II | 59 | 38 | 67–177 |
| Complex III | 446 | 989 | 2200–6610 |
| Complex IV | 507 | 593 | 810–3120 |
| Complex II+coenzyme Q+complex III | 87 | 118 | 300–970 |
| PDHc | 6 | 10 | 34–122 |
| PDHc-E1 | 1.08 | 1.52 | 1.23–4.13 |
| PDHc-E3 | 353 | 533 | 760–2630 |
| KGDHc | n.m. | 3 | 23–49 |
| C. ATP and phosphocreatine | |||
| ATP+phosphocreatine | 10 | 0.0 | 42–81 |
| D. Mitochondrial reference enzyme | |||
| Citrate synthase | 81 | 147 | 37–162 |
Muscle and skin biopsies
Muscle biopsies (musculus vastus lateralis dexter) and forearm skin punch biopsies were performed in both patients. Furthermore, fibroblast cultures from patients with confirmed diagnosis of PDHc (pyruvate dehydrogenase complex) deficiency (n=3) and healthy volunteers (n=12) were used. Biopsies and subsequent experiments were performed after receipt of informed consent. The study was approved by the Institutional Review Board (Medical Faculty of Heidelberg, Heidelberg, Germany). Further investigations were performed in muscle tissue (musculus quadriceps femoris) of wild-type C57Bl/6 mice. Animal care followed the official governmental guidelines and was approved by the government ethics committee.
Human skin fibroblast cultures
Human skin fibroblasts from forearm skin biopsies were cultivated under standard conditions in Dulbecco's modified Eagle's medium at 37 °C supplemented with 10% (v/v) fetal calf serum, 100 μg/ml penicillin, 100 μg/ml streptomycin, 2.5 μg/ml of fungizone and 200 μM uridine until confluency [8]. Medium was changed twice a week. Each cell culture was tested for contamination with Mycoplasma spp. before enzyme analysis.
Preparation of tissue extracts
Fibroblast and muscle homogenates as well as SMPs (submitochondrial particles) from bovine heart were prepared as previously described [8–10].
PDHc activity
Spectrophotometric analysis of PDHc activity [E1 (pyruvate decarboxylase), EC 4.1.1.1; E2 (dihydrolipoyl transacetylase), EC 2.3.1.12; E3 (dihydrolipoyl dehydrogenase), EC 1.8.1.4] was performed in purified porcine PDHc (Sigma–Aldrich, Schnelldorf, Germany), in SMP, and in homogenates from human skin fibroblasts and quadriceps muscle biopsy specimens using a p-Iodonitrotetrazolium Violet-coupled system as previously described [8]. In brief, steady-state activities of PDHc were recorded using a computer tuneable spectrophotometer (SPECTRAmax Plus 384 microplate reader; Molecular Devices, Sunnyvale, CA, U.S.A.) operating in the dual wavelength mode. Samples were analysed in temperature-controlled 96-well plates in a final volume of 300 μl. PDHc (purified enzyme, 160 m-units/ml; SMP, 1.2 mg of protein/ml; fibroblast homogenate, 4 mg of protein/ml) was assayed in a buffer containing 0.05 M potassium phosphate, 2.5 mM NAD+, 5 mM L-carnitine, 0.2 mM thiamine pyrophosphate, 0.1 mM CoA, 0.1% (v/v) Triton X-100, 1 mM MgCl2, 1 mg/ml BSA, 0.6 mM p-Iodonitrotetrazolium Violet and 6.5 μM phenazine methosulphate, which was adjusted to pH 7.5 at 25 °C. PDHc activity was determined as p-Iodonitrotetrazolium Violet reduction at a wavelength of λ=500–750 nm. The specificity of this assay was confirmed by complete inhibition of PDHc activity by the specific E1 inhibitor 3-fluoropyruvate (5 mM).
To investigate the effect of acetyl-CoA and propionyl-CoA, we varied the concentrations of these CoA esters (0–1 mM) and pyruvate (0–0.1 mM). Additional experiments were performed using short-chain (acetyl-CoA [C2], propionyl-CoA [C3] and butyryl-CoA [C4]), medium-chain (hexanoyl-CoA [C6], octanoyl-CoA [C8] and decanoyl-CoA [C10]) and long-chain (myr-istoyl-CoA [C14] and palmitoyl-CoA[C16]) acyl-CoA esters as well as corresponding short- and medium-chain fatty acids (up to 1 mM; all adjusted to pH 7.5).
Spectrophotometric analysis of KGDHc (α-ketoglutarate dehydrogenase complex) activity
Spectrophotometric analyses of KGDHc [KGDHc subunits: E1k (α-ketoglutarate dehydrogenase), EC 1.2.4.2; E2k (dihydrolipoyl succinyltransferase), EC 2.3.1.61; and E3] were performed in SMP and muscle homogenates as previously described [10]. In brief, KGDHc (650 m-units/ml) was assayed in a buffer containing 35 mM potassium phosphate, 5 mM MgCl2, 0.5 mM EDTA, 0.5 mM NAD+, 0.2 mM thiamine pyrophosphate, 0.04 mM CoA-SH (where SH is thiol group) and 2 mM α-ketoglutarate, which was adjusted to pH 7.4 at 30 °C. KGDHc activity was determined as NAD+ reduction at a wavelength of λ=340–400 nm. The effect of propionyl-CoA on KGDHc activity was tested in analogy to PDHc (n=8).
Spectrophotometric analysis of OXPHOS complexes I–V
The catalytic activities of respiratory chain complexes I–V in SMP and muscle homogenates were investigated as previously described [9–14]. The addition of standard respiratory chain inhibitors [complex I, 2-n-decylquinazolin-4-yl-amine (1 μM); complex II, thenoyltrifluoroacetone (8 mM); complex III, antimycin A (1 μM); complex IV, NaCN (2 mM); complex V, oligomycin (80 μM)] revealed good inhibitory responses (93–100% of control activity, P<0.001 versus controls), confirming a high specific enzyme activity in our assay system. Acyl-CoA esters (C2, C3, C4 and C6) and corresponding fatty acids (all adjusted to pH 7.4) were added and effects on single respiratory chain complexes I–V were determined at concentrations up to 1 mM each (complex I, 0.5 mg/ml of protein; complex II, 0.3 mg/ml of protein; complex III, 0.1 mg/ml of protein, complex IV, 0.01 mg/ml of protein; complex V, 1.1 mg/ml of protein). By analogy, complex III activity was also investigated in isolated complex III from bovine heart (0.1 mg/ml of protein).
Radiometric analysis of mitochondrial OXPHOS
Radiometric analysis was performed in freshly prepared quadriceps-muscle biopsy specimens from PA patients. Supernatants (600 g) were prepared and oxidation rates of [1-14C]pyruvate, [U-14C]malate and [1,4-14C]succinate were determined as previously described [14–16].
ATP production
Spectrophotometric analysis of ATP production was determined in muscle biopsies of PA patients using unlabelled pyruvate, malate and succinate as described previously [14]. In brief, spectrophotometric analysis of ATP production is coupled with the formation of NADPH (λ=320–400 nm, 25 °C). The test principle consists of two enzyme reactions. In the first step, glucose (30.3 mM) and ATP are catalysed to glucose 6-phosphate and ADP by hexokinase (EC 2.7.1.1). Subsequently, glucose-6-phosphate dehydrogenase (EC 1.1.1.49) catalyses glucose 6-phosphate and NADP+ to 6-phosphogluconolacton and NADPH.
Protein electrophoresis of muscle tissue
One- and two-dimensional BN-PAGE (blue native PAGE) and subsequent in-gel activity assays and Western-blot analysis were performed in muscle biopsies of PA patients as described previously [17]. Antibodies against OXPHOS complex I [subunits 39 kDa and ND6 (NADH dehydrogenase 6)], complex II (subunit 70 kDa) and complex III (subunit Core 2) were obtained from Molecular Probes (Eugene, OR, U.S.A.), and against cyclophilin B, from Affinity Bioreagents (Golden, CO, U.S.A.).
Electron microscopy of muscle tissue
Electron microscopical examination of muscle biopsies of PA patients was done according to standard methods.
Data analysis
Enzyme activities were normalized to citrate synthase (EC 2.3.3.1) activity and the protein concentration of the same sample [18,19]. Results were expressed as means±S.D. for at least three independent experiments. ANOVA followed by post hoc Bonferroni's multiple comparison test (for three or more groups) or Student's t test (for two groups) were used to calculate statistical differences between groups. Results are presented as the means±S.D. if not indicated differently. Statistics were calculated using SPSS for Windows 12.0 software. P<0.05 was considered significant.
RESULTS
Propionyl-CoA inhibits PDHc
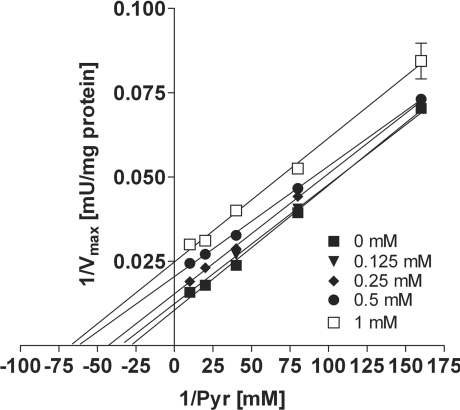
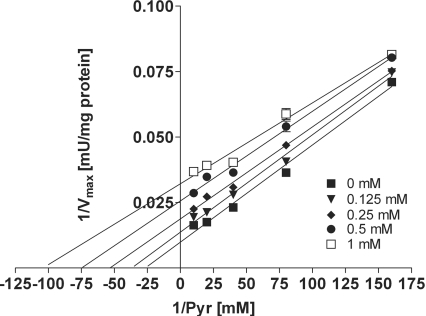
In purified porcine PDHc, propionyl-CoA showed an inhibition of PDHc activity, being uncompetitive with respect to pyruvate (Figure 1). Notably, this inhibitory effect was in the same range as for acetyl-CoA, the physiological product and known inhibitor of PDHc (Figure 2). For propionyl-CoA (0.125–1 mM), the inhibition constant (Ki) ranged from 101 to 422 μM [3–12 times Km (Michaelis–Menten constant)] and for acetyl-CoA (0.125–1 mM), it ranged from 90 to 256 μM (2–7 times Km). The Km was 35±0.5 μM for pyruvate. Similar results were found in SMP from bovine heart (results not shown).
Figure 1. Inhibition of PDHc by propionyl-CoA.
Spectrophotometric analysis in purified PDHc from porcine heart demonstrates uncompetitive inhibition of PDHc activity by propionyl-CoA with respect to pyruvate (Pyr) as demonstrated by a Lineweaver–Burk plot. Values are given as the means±S.D. (where no error bars are visible the S.D. is within the symbol). Experiments were performed at least in triplicate.
Figure 2. Inhibition of PDHc by acetyl-CoA.
Spectrophotometric analysis in purified PDHc from porcine heart demonstrates uncompetitive inhibition of PDHc activity by acetyl-CoA with respect to pyruvate (Pyr) as demonstrated by a Lineweaver–Burk plot. Values are given as the means±S.D. (where no error bars are visible the S.D. is within the symbol). Experiments were performed at least in triplicate.
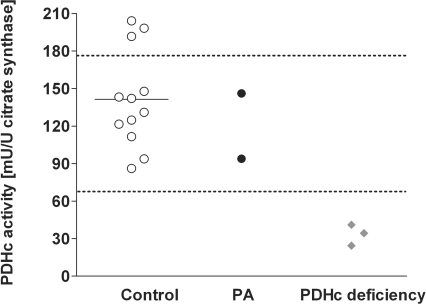
Next, we determined PDHc activity in fibroblast homogenates from two PA patients. Assay conditions allowed enzyme analysis in the absence of intracellularly formed propionyl-CoA. Notably, fibroblasts from both patients revealed PDHc activities within the control range, confirming our hypothesis of secondary PDHc inhibition induced by propionyl-CoA (Figure 3). As expected, patients with confirmed PDHc deficiency showed a reduced PDHc activity in fibroblasts (Figure 3).
Figure 3. Spectrophotometric analysis of PDHc activity in human skin fibroblasts.
Spectrophotometric analysis of PDHc activity was performed in human skin fibroblasts from healthy volunteers (n=12), patients with PA (n=2) and primary PDHc deficiency (n=3). The horizontal bar represents the mean for the control group and the dotted lines indicate the range of the control group.
Acyl-CoA esters inhibit PDHc in a chain-length-specific manner
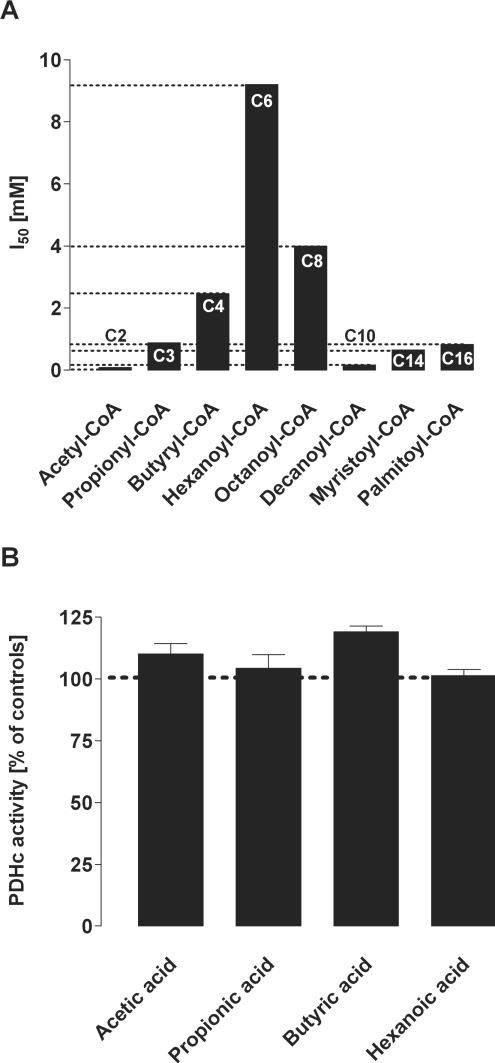
To investigate whether PDHc inhibition was dependent on the chain length of acyl-CoA esters, we investigated the inhibitory effect of similar monocarboxylic CoA esters ranging from C4 to C16 in purified porcine PDHc. In fact, PDHc was also effectively inhibited by C4, C10, C14 and C16 but less effectively by C6 and C8 esters (Figure 4A). In contrast, short-chain fatty acids (up to 1 mM) did not inhibit of PDHc (Figure 4B). These results were confirmed in SMP from bovine heart (results not shown).
Figure 4. Chain-length-specific effects of acyl-CoA esters (A) and fatty acids (B) on PDHc activity.
(A) Acyl-CoA esters: spectrophotometric analysis of PDHc activity was performed in purified enzyme from porcine heart using short-, medium- and long-chain monocarboxylic acyl-CoA esters. The Figure summarizes the I50 values of acyl-CoA esters. Values are given as means. Experiments were performed at least in triplicate. (B) Fatty acids: in contrast with acyl-CoA esters, corresponding short- and medium-chain fatty acids (up to 1 mM) did not inhibit PDHc activity in purified enzyme from porcine heart. PDHc activity was determined under standard conditions. Values were normalized to 100% (dotted line) and are given as the means±S.D. Experiments were performed at least in triplicate.
Alternative effects of propionyl-CoA on energy metabolism
Bioenergetic investigations in muscle tissue of both PA patients showed strongly reduced oxidation of pyruvate and PDHc activity as well as a diminished ATP plus phosphocreatine production (Table 1). In addition, decreased oxidation of succinate and malate as well as of almost all measured enzymes and enzyme complexes of mitochondrial respiratory chain and tricarboxylic acid cycle were found in these patients (Table 1).
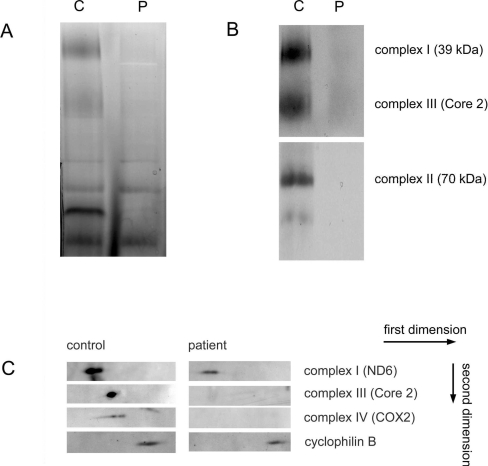
Consistent with the enzymatic measurements is the decrease in the OXPHOS complexes I–IV in patient 2 observed with one- and two-dimensional BN-PAGE in combination with Western blotting and a complex I in-gel activity assay (Figure 5).
Figure 5. BN-PAGE of OXPHOS complexes in muscle homogenates.
Proteins (40 μg) of solubilized muscle homogenates from patient 2 (P) and control (C) were analysed by BN-PAGE (5–15% gel) for the separation of multisubunit complexes. (A) In-gel activity assay of mitochondrial complex I confirming a decrease in activities of patient 2 compared with control samples. (B) A second gel was run in duplicate and Western-blot analysis was performed using antibodies against complex I subunit 39 kDa, complex II subunit 70 kDa and complex III subunit Core 2. (C) A second dimension was run and Western-blot analysis was performed using antibodies against OXPHOS complexes I (ND6), III (Core 2) and IV [COX2 (cytochrome oxidase 2)] and cyclophilin B as a loading control. Arrows indicate the first and second dimensions.
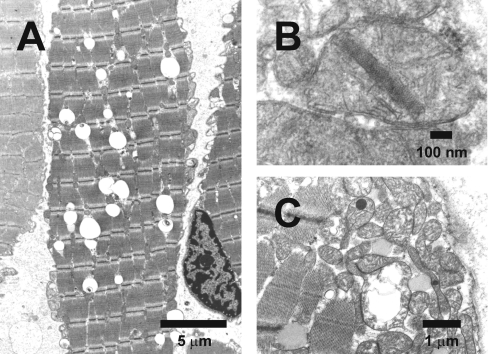
The general decrease in the amount of these OXPHOS complexes suggests a destabilization and increased breakdown of mitochondria. The observed reduction in mtDNA (mitochondrial DNA) content of about approx. 50% in patients 1 and 2 is in line with this suggestion. By analogy to these biochemical results, histopathology of muscle biopsies showed numerous signs of mitochondrial alteration indicating disturbance of mitochondrial energy metabolism, such as inclusion of many lipid droplets in muscle fibres of patient 1 (Figure 6A), crystalline inclusions (Figure 6B) and large swollen mitochondria with dark globular inclusions (Figure 6C).
Figure 6. Ultrastructural changes in muscle tissue of PA patients.
Electron microscopy of muscle biopsy specimens (musculus vastus lateralis dexter) of both PA patients was performed. (A) Patient 1: muscle fibre with many lipid droplets (the large clear globules). (B) Patient 1: mitochondrion with a crystalline inclusion. (C) Patient 2: large swollen mitochondrion with disrupted cristae and two mitochondria with a dark globular inclusion.
Since bioenergetic analyses in both PA patients suggested additional mechanisms acting in concert with propionyl-CoA-induced PDHc inhibition, we investigated whether propionyl-CoA and other short-chain acyl-CoA esters revealed inhibitory effects on respiratory chain and tricarboxylic acid cycle enzymes. We found no effect of short-chain acyl-CoA esters and fatty acids on respiratory complexes I, II, IV and V in SMP (results not shown). However, by analogy to muscle tissue of PA patients, propionyl-CoA (1 mM) induced a mild inhibition of complex III activity in SMP (72±5% of controls) and in isolated complex III from bovine heart (81±8% of controls). In addition, the activity of KGDHc, a rate-limiting tricarboxylic acid cycle enzyme complex, was also decreased by propionyl-CoA (1 mM propionyl-CoA: 38±3% of controls).
DISCUSSION
The accumulation of metabolites from the alternative oxidative pathways of propionyl-CoA is the biochemical hallmark of PA. During metabolic crisis, additional biochemical abnormalities such as lactic acidosis, hyperketosis and hypoglycaemia indicate the development of severe mitochondrial dysfunction resulting in impairment of energy metabolism [1]. The present study significantly adds to the understanding of these mechanisms demonstrating (i) a severe bioenergetic disturbance and ultrastructural changes of mitochondria in muscle tissue of PA patients and (ii) propionyl-CoA-induced synergistic inhibition of PDHc, KGDHc and complex III in vitro. These results support the hypothesis that secondary mitochondrial dysfunction induced by accumulating toxic metabolites plays a major role in the pathomechanisms of PA [7]. We clearly demonstrated that propionyl-CoA, but not propionate, is the major toxic metabolite involved in this scenario.
PDHc is a bioenergetic bottleneck coupling cytosolic anaerobic glycolysis with the mitochondrial tricarboxylic acid cycle and OXPHOS. Not surprisingly, inherited PDHc deficiency results in a severe disturbance of energy metabolism and frequently in a fatal disease course [20]. In analogy, secondary inhibition of PDHc by propionyl-CoA can be suggested as an important mechanism inducing energy failure in PA, which is confirmed by severely reduced ATP and phosphocreatine concentrations in muscle tissue of PA patients and increased serum lactate concentrations. In line with this, multiple organ failure in PA patients predominantly manifests in tissues with a high energy demand, such as the central nervous system (encephalopathy, movement disorders and developmental retardation), skeletal muscle (myopathy), heart muscle (cardiomyopathy) and bone marrow (pancytopenia) [1,3]. Apart from mammalian species, propionyl-CoA-induced inhibition of PDHc has recently been suggested as a major mechanism underlying the antibacterial and antifungal properties of propionate [4]. Thus propionyl-CoA-induced mitochondrial dysfunction can be regarded as a common final pathway involved in different conditions, i.e. inherited disorders of propionate metabolism and propionate toxicity. Future studies should focus on concentration-dependent effects of propionyl-CoA in tissues of children affected with PA. However, since the amount of a muscle biopsy specimen in children is always very limited because of ethical and functional considerations, this important aspect could not be investigated additionally in the present study.
In addition to PDHc inhibition, alternative mechanisms for propionyl-CoA and metabolites of alternative propionate oxidation should be considered as relevant. In particular, inhibition of respiratory chain complex III (the present study) and the tricarboxylic acid cycle enzymes KGDHc (the present study), citrate synthase [5] and succinyl-CoA synthetase [4] may also contribute to impaired energy metabolism in this disease. Interestingly, inherited deficiency of succinyl-CoA synthetase caused by deleterious mutations in the SUCLA2 gene has recently been associated with encephalomyopathy and mtDNA depletion [21], linking the tricarboxylic acid cycle with mtDNA homoeostasis [21,22]. In addition, increased oxidative stress, which has been demonstrated in an in vitro model for disorders of propionate metabolism, induces mtDNA damage [23,24]. Interestingly, the amount of mtDNA and the activities of OXPHOS complexes I, III and IV, which are partially encoded by mtDNA, were significantly decreased in muscle tissue of both PA patients; however, it remains unclear whether this result reflects a causal link. Besides mtDNA homoeostasis, other secondary or tertiary targets might be involved but have not yet been identified.
PA shares a variety of biochemical and clinical similarities with methylmalonic aciduria, which is caused by inherited deficiency of methylmalonyl-CoA mutase or the synthesis or transport of its cofactor, 5′-adenosylcobalamin [1]. We have recently hypothesized that propionyl-CoA and metabolites deriving from propionyl-CoA, such as 2-methylcitrate, might act as endogenous neurotoxins also in this disease, whereas methylmalonate most likely plays a minor role [13,14,25]. Since the manifestation of secondary metabolic blocks is pathophysiologically relevant in PA and methylmalonic aciduria, it is of interest to investigate whether alternative energy substrates such as succinate and citrate might be beneficial for metabolic maintenance treatment and intensified emergency treatment of these patients helping to restore mitochondrial energy metabolism and to prevent multiple organ failure.
Acknowledgments
This study was supported by a research grant from the University of Heidelberg to M.A.S. (no. 19/2003) and a grant from the Deutsche Forschungsgemeinschaft (SCHW1367/1-1). We are grateful to Roel Smeets and Sonja Exner-Camps for excellent technical support.
References
- 1.Fenton W. A., Gravel R. A., Rosenblatt D. S. Disorders of propionate and methylmalonate metabolism. In: Scriver C. R., Beaudet A. L., Valle A. D., Sly W. S., editors. The Metabolic and Molecular Bases of Inherited Disease. New York: McGraw-Hill; 2001. pp. 2165–2193. [Google Scholar]
- 2.Miyazaki T., Ohura T., Kobayashi M., Shigematsu Y., Yamaguchi S., Suzuki Y., Hata I., Aoki Y., Yang X., Minjares C., et al. Fatal propionic acidemia in mice lacking propionyl-CoA carboxylase and its rescue by postnatal, liver-specific supplementation via a transgene. J. Biol. Chem. 2001;276:35995–35999. doi: 10.1074/jbc.M105467200. [DOI] [PubMed] [Google Scholar]
- 3.Brismar J., Ozand P. T. CT and MR of the brain in disorders of the propionate and methylmalonate metabolism. Am. J. Neuroradiol. 1994;15:1459–1473. [PMC free article] [PubMed] [Google Scholar]
- 4.Brock M., Buckel W. On the mechanism of action of the antifungal agent propionate. Eur. J. Biochem. 2004;271:3227–3241. doi: 10.1111/j.1432-1033.2004.04255.x. [DOI] [PubMed] [Google Scholar]
- 5.Cheema-Dhadli S., Leznoff C. C., Halperin M. L. Effect of 2-methylcitrate on citrate metabolism: implications for the management of patients with propionic acidemia and methylmalonic aciduria. Pediatr. Res. 1975;9:905–908. doi: 10.1203/00006450-197512000-00008. [DOI] [PubMed] [Google Scholar]
- 6.Horswill A. R., Dudding A. R., Escalante-Semerena J. C. Studies of propionate toxicity in Salmonella enterica identify 2-methylcitrate as a potent inhibitor of cell growth. J. Biol. Chem. 2001;276:19094–19101. doi: 10.1074/jbc.M100244200. [DOI] [PubMed] [Google Scholar]
- 7.Gregersen N. The specific inhibition of the pyruvate dehydrogenase complex from pig kidney by propionyl-CoA and isovaleryl-CoA. Biochem. Med. 1981;26:20–27. doi: 10.1016/0006-2944(81)90026-0. [DOI] [PubMed] [Google Scholar]
- 8.Schwab M. A., Kölker S., van den Heuvel L. P., Sauer S., Wolf N. I., Rating D., Hoffmann G. F., Smeitink J. A., Okun J. G. Optimized spectrophotometric assay for the completely activated pyruvate dehydrogenase complex in fibroblasts. Clin. Chem. 2005;51:151–160. doi: 10.1373/clinchem.2004.033852. [DOI] [PubMed] [Google Scholar]
- 9.Okun J. G., Lümmen P., Brandt U. Three classes of inhibitors share a common binding domain in mitochondrial complex I (NADH:ubiqinone oxidoreductase) J. Biol. Chem. 1999;27:2625–2630. doi: 10.1074/jbc.274.5.2625. [DOI] [PubMed] [Google Scholar]
- 10.Sauer S. W., Okun J. G., Schwab M. A., Crnic L. R., Hoffmann G. F., Goodman S. I., Koeller D. M., Kölker S. Bioenergetics in glutaryl-coenzyme A dehydrogenase deficiency, a role for glutaryl-coenzyme A. J. Biol. Chem. 2005;280:21830–21836. doi: 10.1074/jbc.M502845200. [DOI] [PubMed] [Google Scholar]
- 11.Schägger H., Link T. A., Engel W. D., von Jagow G. Isolation of the eleven protein subunits of the bc1 complex from beef heart. Methods Enzymol. 1986;126:224–237. doi: 10.1016/s0076-6879(86)26024-3. [DOI] [PubMed] [Google Scholar]
- 12.Brandt U., Okun J. G. Role of deprotonation events in ubihydroquinone:cytochrome c oxidoreductase from bovine heart and yeast mitochondria. Biochemistry. 1997;36:11234–11240. doi: 10.1021/bi970968g. [DOI] [PubMed] [Google Scholar]
- 13.Okun J. G., Hörster F., Farkas L. M., Feyh P., Hinz A., Sauer S., Hoffmann G. F., Unsicker K., Mayatepek E., Kölker S. Neurodegeneration in methylmalonic aciduria involves inhibition of complex II and the tricarboxylic acid cycle, and synergistically acting excitotoxicity. J. Biol. Chem. 2002;277:14674–14680. doi: 10.1074/jbc.M200997200. [DOI] [PubMed] [Google Scholar]
- 14.Kölker S., Schwab M., Hörster F., Sauer S., Hinz A., Wolf N. I., Mayatepek E., Hoffmann G. F., Smeitink J. A. M., Okun J. G. Methylmalonic acid, a biochemical hallmark of methylmalonic acidurias but no inhibitor of mitochondrial respiratory chain. J. Biol. Chem. 2003;278:47388–47393. doi: 10.1074/jbc.M308861200. [DOI] [PubMed] [Google Scholar]
- 15.Trijbels J. M., Sengers R. C., Ruitenbeek W., Fischer J. C., Bakkeren J. A., Janssen A. J. Disorders of the mitochondrial respiratory chain: clinical manifestations and diagnostic approach. Eur. J. Pediatr. 1988;148:92–97. doi: 10.1007/BF00445910. [DOI] [PubMed] [Google Scholar]
- 16.Sperl W., Trijbels J. M. F., Ruitenbeek W., Van Laack H. L., Janssen A. J., Kerkhof C. M., Sengers R. C. Measurement of totally activated pyruvate dehydrogenase complex activity in human muscle: evaluation of a useful assay. Enzyme Protein. 1993;47:37–46. doi: 10.1159/000468654. [DOI] [PubMed] [Google Scholar]
- 17.Nijtmans L., Henderson N., Holt I. Blue native electrophoresis to study mitochondrial and other protein complexes. Methods. 2002;26:327–334. doi: 10.1016/S1046-2023(02)00038-5. [DOI] [PubMed] [Google Scholar]
- 18.Srere P. A. Citrate synthase, EC 4.1.3.7, citrate oxaloacetate-lyase (CoA-acetylating) In: Löwenstein J. M., editor. Methods in Enzymology. London: Academic Press; 1969. pp. 3–11. [Google Scholar]
- 19.Lowry O. H., Rosebrough N. R., Farr A. J. Protein measurement with the folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 20.DeMeirleir L. Defects of pyruvate metabolism and the Krebs cycle. J. Child Neurol. 2002;17(Suppl. 3):26–33. [PubMed] [Google Scholar]
- 21.Elpeleg O., Miller C., Hershkovitz E., Bitner-Glindzicz M., Bondi-Rubenstein G., Rahman S., Pagnamenta A., Eshhar S., Saada A. Deficiency of the ADP-forming succinyl-CoA synthase activity is associated with encephalomyopathy and mitochondrial DNA depletion. Am. J. Hum. Genet. 2005;76:1081–1086. doi: 10.1086/430843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saada A. Deoxyribonucleotides and disorders of mitochondrial DNA integrity. Cell Biol. 2004;23:797–806. doi: 10.1089/dna.2004.23.797. [DOI] [PubMed] [Google Scholar]
- 23.Fontella F. U., Pulrolnik V., Gassen E., Wannmacher C. M., Klein A. B., Wajner M., Dutra-Filho C. S. Propionic and L-methylmalonic acids induce oxidative stress in brain of young rats. Neuroreport. 2000;11:541–544. doi: 10.1097/00001756-200002280-00023. [DOI] [PubMed] [Google Scholar]
- 24.Wallace D. C. The mitochondrial genome in human adaptive radiation and disease: on the road to therapeutics and performance enhancement. Gene. 2005;354:169–180. doi: 10.1016/j.gene.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 25.Kölker S., Okun J. G. Methylmalonic acid – an endogenous toxin? Cell. Mol. Life Sci. 2005;62:621–624. doi: 10.1007/s00018-005-4463-2. [DOI] [PubMed] [Google Scholar]