Abstract
The spread of chloroquine-resistant Plasmodium falciparum calls for a constant search for new drugs. The in vitro activity of piperaquine, a new Chinese synthetic drug belonging to the bisquinolines, was evaluated in 103 fresh clinical isolates of P. falciparum in Cameroon, Central Africa, and compared with that of other 4-aminoquinoline and Mannich base derivatives and dihydroartemisinin. Piperaquine was highly active (geometric mean 50% inhibitory concentration, 38.9 nmol/liter; range, 7.76 to 78.3 nmol/liter) and equally active (P > 0.05) against the chloroquine-sensitive and the chloroquine-resistant isolates. There was a significant but low correlation of response between chloroquine and piperaquine (r = 0.257, P < 0.05). These results suggest that further development of piperaquine, in combination with dihydroartemisinin, holds promise for use in chloroquine-resistant regions of endemicity.
Bisquinolines are compounds with two quinoline nuclei bound by a covalent aliphatic or aromatic link. Several of these compounds, including piperaquine, were identified as promising candidates during drug screening programs in the 1960s. However, because these drugs were thought to confer no major advantages over chloroquine, their development was not pursued outside China. During the last two decades, the potential of old and new bisquinolines to treat chloroquine-resistant Plasmodium falciparum has received renewed attention. Old bisquinolines, such as 12,494RP, dichlorquinazine (12,278RP), hydroxypiperaquine, and piperaquine, were reevaluated and shown to be active in vitro against chloroquine-resistant P. falciparum isolates (11). During the same period, Chinese researchers continued to develop piperaquine and hydroxypiperaquine for clinical use (23). Furthermore, several novel series of bisquinolines were synthesized during the 1990s (9, 16, 21).
First synthesized in France (Rhone-Poulenc [currently Aventis]), piperaquine (13,228RP) underwent early clinical evaluation in Africa (20). Piperaquine was also synthesized independently by the Shanghai Research Institute of Pharmaceutical Industry in 1966. In the preliminary clinical studies conducted in Hainan Island in the early 1970s, more than 4,000 adults and children >6 years of age received monthly prophylactic doses of piperaquine for 4 months and 43 P. falciparum-infected patients rapidly cleared fever and parasitemia after piperaquine therapy (total dose for adults, 1.8 g in divided doses over 2 days) (2). The results of these early studies indicated good tolerance, mild side effects, high-level prophylactic efficacy for 3 weeks after a single oral administration, and rapid blood schizonticidal action against P. falciparum. Subsequent clinical studies in China have shown the efficacy of piperaquine in the treatment of chloroquine-resistant falciparum malaria (1, 7). Piperaquine was registered in 1978 in China and has been used as the first-line treatment for chloroquine-resistant malaria in southern China.
Because of the increasing spread of chloroquine-resistant P. falciparum, new antimalarial drug candidates need to be developed and introduced to areas of endemicity at a regular pace. Of these candidates, piperaquine is one whose final clinical development may be accelerated because of existing preclinical and clinical data in the Chinese literature. Unlike their performance in other areas of endemicity in Asia and South America, 4-aminoquinoline and Mannich base derivatives such as amodiaquine and pyronaridine remain clinically effective in Africa even when administered as monotherapy, possibly due to a high level of acquired immunity and a generally lower level of chloroquine resistance (18, 19). However, introduction of piperaquine for use as a monotherapy in Africa is not recommended due to the rapid emergence of clinical resistance observed in China (1, 8, 10, 15). The current strong advocacy for drug combinations to delay the emergence of multidrug-resistant malaria parasites calls for a search for suitable partners of existing and new drugs. In the case of piperaquine, dihydroartemisinin has been chosen as the best drug partner. Despite these promising perspectives for the rapid deployment of the piperaquine-dihydroartemisinin combination, a number of issues concerning its efficacy and safety need to be addressed. As an initial step to answer some of these questions, the present study was conducted to determine the baseline in vitro response of African clinical isolates to piperaquine and to evaluate the possible existence of cross-resistance between chloroquine and piperaquine.
MATERIALS AND METHODS
Parasites.
Venous blood samples (5 to 10 ml) were collected on EDTA-coated tubes from symptomatic Cameroonian patients ≥12 years of age in 2001 to 2002 at the Nlongkak Catholic missionary dispensary in Yaoundé when the following criteria are met: informed consent, monoinfection with P. falciparum, minimal initial parasitemia of 0.1%, and negative Saker-Solomons urine test for 4-aminoquinolines. Pregnant women and patients presenting signs and symptoms of severe and complicated malaria were excluded. Enrolled patients were treated with amodiaquine, which is the first-line treatment officially recommended by the Cameroonian Ministry of Public Health. This study was approved by the Cameroonian National Ethics Committee and Cameroonian Ministry of Public Health.
Test compounds.
Chloroquine sulfate was obtained from Aventis (formerly Rhone Poulenc; Antony, France). Monodesethylamodiaquine dihydrochloride was supplied by Parke-Davis (Dakar, Senegal). Piperaquine tetraphosphate was kindly provided by Li Quoqiao (Institute of Tropical Medicine, Guangzhou University of Traditional Chinese Medicine, Guangzhou, China). Pyronaridine tetraphosphate and dihydroartemisinin were obtained from Shin Poong Pharmaceutical Co., Ltd. (Seoul, South Korea). Stock solutions and dilutions of chloroquine, monodesethylamodiaquine, and pyronaridine were prepared in sterile distilled water. The stock solution and dilutions of dihydroartemisinin were prepared in methanol. Piperaquine tetraphosphate is slightly soluble in water at neutral pH and almost insoluble in absolute ethanol. To dissolve piperaquine in a culture-compatible solvent, a stock solution of piperaquine tetraphosphate was prepared in 0.5% lactic acid (Sigma Chemical Company, St. Louis, Mo.), and further dilutions were made in sterile water as kindly suggested by Yang Henglin (Yunnan Institute of Malaria Control, Simao, Yunnan, China). The final concentration of lactic acid was ≤0.00014%, which did not affect parasite growth. The following final concentrations were tested in triplicate: 25 to 1,600 nmol/liter for chloroquine, 5 to 320 nmol/liter for monodesethylamodiaquine, 1.25 to 80 nmol/liter for pyronaridine, 6.25 to 400 nmol/liter for piperaquine, and 0.25 to 16 nmol/liter for dihydroartemisinin.
In vitro assay.
Venous blood samples were washed with RPMI 1640 culture medium three times by centrifugation (2,000 rpm × 10 min) within 2 h after blood collection. The in vitro drug sensitivity assay was performed according to the isotopic microtest assay described by Desjardins et al. (4). The culture plates were incubated for 42 h at 37°C in 5% CO2 in an incubator. The incorporation of [3H]hypoxanthine was measured by a liquid scintillation counter (Wallac 1409; Pharmacia, Stockholm, Sweden). The in vitro activities of test compounds were expressed as the 50% inhibitory concentration (IC50), defined as the drug concentration at which 50% of the incorporation of [3H]hypoxanthine is inhibited compared with the incorporation in the drug-free wells. Parasite growth was plotted against drug concentration, and the best-fitting sigmoid curve was traced using Prism software (Graphpad Software, San Diego, Calif.) to determine the IC50 value.
Statistical analysis.
The in vitro activity of test compounds was expressed as the geometric mean of the IC50s for all isolates. To measure the possible difference in the levels of activity against the chloroquine-sensitive and the chloroquine-resistant isolates, the mean logarithmic IC50 values of the test compounds were compared by the two-tailed unpaired Student t test. The potential for in vitro cross-resistance was evaluated by linear regression. For all statistical tests, the significance level (P) was set at 0.05.
RESULTS
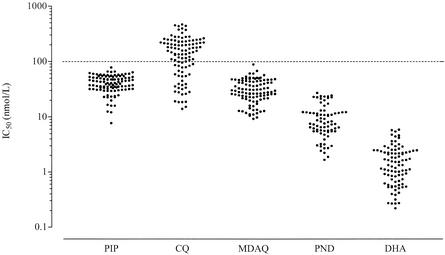
A total of 103 fresh clinical isolates were tested to determine the patterns of sensitivity to piperaquine and other test compounds. Piperaquine was highly active (geometric mean IC50, 38.9 nmol/liter; range, 7.76 to 78.3 nmol/liter) against all Cameroonian isolates. Piperaquine exhibited a strong inhibitory action at 50 or 100 nmol/liter for all isolates, leading to a sharp decline in parasite growth at these concentrations. The mean IC50s (range) of monodesethylamodiaquine, pyronaridine, and dihydroartemisinin were 29.0 nmol/liter (9.27 to 88.8 nmol/liter), 8.01 nmol/liter (1.67 to 27.1 nmol/liter), and 1.29 nmol/liter (0.220 to 5.88 nmol/liter), respectively. The distribution of IC50s for each compound is illustrated in Fig. 1. Of 103 isolates, 34 (33%) were chloroquine sensitive (IC50 < 100 nmol/liter), and 69 (67%) were chloroquine resistant (IC50 ≥ 100 nmol/liter). The geometric mean IC50s (range) of chloroquine for sensitive and resistant isolates were 41.6 nmol/liter (14.0 to 98.8 nmol/liter) and 201 nmol/liter (101 to 466 nmol/liter), respectively. There was thus a fivefold difference in drug IC50 levels for these two groups of isolates.
FIG. 1.
Distribution of IC50s for each of the test compounds. PIP, piperaquine; CQ, chloroquine; MDAQ, monodesethylamodiaquine; PND, pyronaridine; DHA, dihydroartemisinin. The dotted line corresponds to the threshold value for in vitro chloroquine resistance (100 nmol/liter).
Data analysis for isolates classified into chloroquine-sensitive and chloroquine-resistant groups showed that the in vitro activities of piperaquine (35.5 versus 40.7 nmol/liter) and dihydroartemisinin (1.12 versus 1.39 nmol/liter) against the chloroquine-sensitive isolates and the chloroquine-resistant isolates were not significantly different (P > 0.05). By contrast, monodesethylamodiaquine (17.8 versus 37.4 nmol/liter) and pyronaridine (5.61 versus 9.86 nmol/liter) were more active (P < 0.05) against the chloroquine-sensitive isolates than against the chloroquine-resistant isolates.
The in vitro responses to chloroquine and monodesethylamodiaquine were highly correlated (r = 0.851, P < 0.001). The other drug pairs were correlated to a lower degree. There were slight correlations between chloroquine and piperaquine (r = 0.257, P = 0.0085), between monodesethylamodiaquine and piperaquine (r = 0.366, P = 0.0002), and between pyronaridine and piperaquine (r = 0.653, P < 0.0001). Likewise, the correlation coefficients between other 4-aminoquinoline derivatives were not elevated (r = 0.473 and P < 0.0001 for chloroquine versus pyronaridine).
DISCUSSION
In earlier studies based on the microscopic count of schizont formation, the chloroquine-sensitive FCC-1/Hainan strain (chloroquine IC50, 81 nmol/liter; piperaquine IC50, 59 nmol/liter) and the chloroquine-resistant Cambodian I strain (chloroquine IC50, 563 nmol/liter; piperaquine IC50, 61 nmol/liter) showed similar levels of in vitro response to piperaquine (6). Furthermore, highly chloroquine-resistant laboratory strains from Brazil and West Africa were inhibited at IC50s between 62 and 94 nmol/liter (11). In another study using the isotopic microtest, the in vitro activity of piperaquine was higher against the chloroquine-sensitive D-6/Sierra Leone reference clone (piperaquine IC50, 8.3 nmol/liter) than against the chloroquine-resistant W-2/Indochina reference clone (piperaquine IC50, 16 nmol/liter) (21). In the latter study, the slightly higher level of in vitro activity of piperaquine (compared to our results) might have been due to the use of dimethyl sulfoxide to dissolve piperaquine. In our study, a much less toxic solvent was used. Our results suggest that piperaquine is highly and equally active against the chloroquine-sensitive and the chloroquine-resistant clinical isolates.
In a study on field isolates from Madagascar, the IC50s of piperaquine were widely dispersed, ranging from <12.5 nmol/liter to >250 nmol/liter, with values of <100 nmol/liter for the majority (83%) of isolates (3). By contrast, our study showed the high level of activity of piperaquine within a narrower range (7.76 to 78.3 nmol/liter), with IC50s of <100 nmol/liter against all isolates. The differences in results between these in vitro studies are probably due to methodological differences rather than being inherent in different drug sensitivity patterns between isolates from Madagascar and Cameroon. In fact, the results of their study are not comparable to those of other studies because of a high level of hematocrit (5% versus 1.5% in the standard microtest), a low rate of interpretable results (84 of 124 [68%] versus >90% in our study), which introduces an important bias, and evaluation of schizont maturation by microscope counting, which leads to an IC50 estimation that is two to three times higher than that determined by isotope incorporation (22).
In vitro sensitivity to piperaquine has been regularly monitored during the 1980s and 1990s in southern China by using the WHO microtest (5, 14, 25-27). Chloroquine had been massively employed in this region in the past for prophylaxis and presumptive treatment, and chloroquine-resistant P. falciparum has become highly prevalent. Although the in vitro monitoring was not conducted by the same research group at the same site, the results suggest a general tendency towards an increasing proportion of piperaquine-resistant isolates, from 18 to 43% during the late 1980s to 48 to 98% during the 1990s. In parallel, it has been reported that the clinical efficacy of piperaquine has declined. Until the mid-1980s, the majority of P. falciparum-infected patients were cured with piperaquine given as monotherapy, and most of the recrudescent cases were at RI level (1, 7, 10). However, by the late 1980s, cases of RII and RIII resistance were reported (8, 15). These observations have prompted the Chinese investigators to resort to combination therapy to delay the emergence of piperaquine-resistant P. falciparum. The existing combination undergoing clinical development is based on piperaquine-dihydroartemisinin, to which trimethoprim (triple combination) and primaquine (quadruple combination) had been added in some past studies (24). Should piperaquine be introduced massively in Africa, the observations made by the Chinese investigators should prompt researchers working in that continent to set up a surveillance network that serves as an early warning system for possible emergence and spread of 4-aminoquinoline resistance.
The Chinese literature suggests the existence of some degree of cross-resistance between chloroquine and piperaquine. It has been noted that in piperaquine-resistant strains of P. berghei obtained by serial passage under drug pressure in a rodent malaria model, cross-resistance occurred at a high level between piperaquine and mefloquine or artemisinin derivatives but the level of cross-resistance between piperaquine and chloroquine or pyronaridine was low to nonexistent, depending on the parasite strain (12, 13). In vitro, many but not all of the chloroquine-resistant Chinese P. falciparum isolates were equally piperaquine resistant (5, 14, 25-27). However, because the proportion of chloroquine-resistant P. falciparum strains has attained a high level in southern China, the potential for in vitro cross-resistance between chloroquine and piperaquine has become difficult to assess. In Cameroon, both in vivo and in vitro chloroquine resistance occurs in approximately 50 to 60% of patients and isolates, respectively, and our monitoring over the past 8 years in Yaoundé attests to a stabilization of this proportion (17). This feature allows a more accurate evaluation of the relation between the in vitro responses to chloroquine and other antimalarial drugs. In our study, there was a slight but statistically significant correlation (r = 0.257) between chloroquine and piperaquine. At this low coefficient correlation level, in vitro cross-resistance is unlikely to occur. This interpretation is supported by our finding that overall, piperaquine is equally active against chloroquine-sensitive and chloroquine-resistant isolates.
Our data show that piperaquine is highly active in vitro against both chloroquine-sensitive and chloroquine-resistant Cameroonian clinical isolates of P. falciparum, with IC50s of <100 nmol/liter. There was only a slight correlation between chloroquine and piperaquine, minimizing the risk for in vitro cross-resistance between these drugs, at least in Central Africa. These features, together with the fact that it is now administered in combination with dihydroartemisinin, suggest that piperaquine is a highly promising candidate for further development and rapid deployment in chloroquine-resistant areas of endemicity. It needs to be reemphasized that, given the Chinese experience, piperaquine monotherapy is probably not a viable option for long-term use of this drug and that piperaquine should be administered as a combination therapy to maximize its clinical utility.
Acknowledgments
This work was supported by Technical Service Agreement no. 4983.00 from the World Health Organization, Geneva, Switzerland.
We are grateful to Sister Marie-Solange Oko and her staff at the Nlongkak Catholic missionary dispensary, Yaoundé, for recruiting patients.
REFERENCES
- 1.Chen, L. 1991. Recent studies on antimalarial efficacy of piperaquine and hydroxypiperaquine. Chinese Med. J. 104:161-163. [PubMed] [Google Scholar]
- 2.Chen, L., F. Y. Qu, and Y. C. Zhou. 1982. Field observations on the antimalarial piperaquine. Chinese Med. J. 95:281-286. [PubMed] [Google Scholar]
- 3.Deloron, P., J. Le Bras, J. A. Ramanamirija, and P. Coulanges. 1985. Plasmodium falciparum in Madagascar: in vivo and in vitro sensitivity to seven drugs. Ann. Trop. Med. Parasitol. 79:357-365. [DOI] [PubMed] [Google Scholar]
- 4.Desjardins, R. E., C. J. Canfield, J. D. Haynes, and J. D. Chulay. 1979. Quantitative assessment of antimalarial activity in vitro by a semi-automated microdilution technique. Antimicrob. Agents Chemother. 16:710-718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fan, B., W. Zhao, X. W. Ma, Z. M. Huang, Y. S. Wen, J. Q. Yang, and Z. X. Yang. 1998. In vitro sensitivity of Plasmodium falciparum to chloroquine, piperaquine, pyronaridine and artesunate in Yuxi prefecture of Yunnan province. Chinese J. Parasitol. Parasit. Dis. 16:460-462. [PubMed] [Google Scholar]
- 6.Guan, W. B., W. J. Huang, Y. C. Zhou, and W. Q. Pan. 1983. Effect of piperaquine and hydroxypiperaquine on chloroquine-resistant strain of Plasmodium falciparum. Chinese J. Parasitol. Parasit. Dis. 1:88-90. [PubMed] [Google Scholar]
- 7.Guo, X. B., L. C. Fu, Y. X. Fu, J. Z. Lin, H. M. Su, D. C. Xie, and G. Q. Li.1989. Comparative study of artemisinin suppositories and piperaquine phosphate for the treatment of falciparum malaria. Chinese J. Integr. Trad. Western Med. 9:475-477. [PubMed] [Google Scholar]
- 8.Guo, X. B., L. C. Fu, Y. X. Fu, B. S. Qian, and G. Q. Li. 1993. Randomized comparison of the treatment of falciparum malaria with dihydroartemisinin and piperaquine. Nat. Med. J. China 73:602-604. [PubMed] [Google Scholar]
- 9.Ismail, F. M. D., M. J. Dascombe, P. Carr, and S. E. North. 1996. An exploration of the structure-activity relationships of 4-aminoquinolines: novel antimalarials with activity in vivo. J. Pharm. Pharmacol. 48:841-850. [DOI] [PubMed] [Google Scholar]
- 10.Lan, C. X., X. Lin, Z. S. Huang, Y. S. Chen, and R. N. Guo. 1989. In vivo sensitivity of Plasmodium falciparum to piperaquine phosphate assayed in Linshui and Baisha counties, Hainan province. Chinese J. Parasitol. Parasit. Dis. 7:163-165. [PubMed] [Google Scholar]
- 11.Le Bras, J., P. Deloron, and G. Charmot. 1983. Dichlorquinazine (a 4-aminoquinoline) effective in vitro against chloroquine-resistant Plasmodium falciparum. Lancet i:73-74. [DOI] [PubMed] [Google Scholar]
- 12.Li, G. D. 1985. Development of piperaquine-resistant line of Plasmodium berghei K173 strain. Acta Pharmaceutica Sinica 20:412-417. [PubMed] [Google Scholar]
- 13.Li, G. D., F. Y. Qu, and L. Chen. 1985. Development of piperaquine-resistant line of Plasmodium berghei ANKA strain. Chinese J. Parasitol. Parasit. Dis. 3:189-192. [PubMed] [Google Scholar]
- 14.Liu, D. Q., R. J. Liu, C. Y. Zhang, X. Z. Cai, X. Tang, H. L. Yang, P. F. Yang, and Y. Dong. 1996. Present status of the sensitivity of Plasmodium falciparum to antimalarials in China. Chinese J. Parasitol. Parasit. Dis. 14:37-41. [Google Scholar]
- 15.Pang, X. J., J. Liu, S. G. Fu, Q. Y. Chen, Y. H. Chen, and X. L. Su. 1989. Two cases of piperaquine-resistant Plasmodium falciparum in Hainan. Chinese J. Prevention Treatment Parasit. Dis. 2:18. [Google Scholar]
- 16.Raynes, K. J., D. Galatis, A. F. Cowman, L. Tilley, and L. W. Deady. 1995. Synthesis and activity of some antimalarial bisquinolines. J. Med. Chem. 38:204-206. [DOI] [PubMed] [Google Scholar]
- 17.Ringwald, P., and L. K. Basco. 1999. Comparison of in vivo and in vitro tests of resistance in patients treated with chloroquine in Yaounde, Cameroon. Bull. W. H. O. 77:34-43. [PMC free article] [PubMed] [Google Scholar]
- 18.Ringwald, P., J. Bickii, and L. K. Basco. 1996. Randomised trial of pyronaridine versus chloroquine for acute uncomplicated falciparum malaria in Africa. Lancet 347:24-28. [DOI] [PubMed] [Google Scholar]
- 19.Ringwald, P., A. Keundjian, A. Same Ekobo, and L. K. Basco. 2000. Chemoresistance of Plasmodium falciparum in the urban region of Yaounde, Cameroon. Part 2: evaluation of the efficacy of amodiaquine and sulfadoxine-pyrimethamine combination in the treatment of uncomplicated Plasmodium falciparum malaria in Yaounde, Cameroon. Trop. Med. Int. Health 5:620-627. [DOI] [PubMed] [Google Scholar]
- 20.Thompson, P. E., and L. M. Werbel. 1972. Antimalarial agents: chemistry and pharmacology. Academic Press, New York, N.Y.
- 21.Vennerstrom, J. L., W. Y. Ellis, A. L. Ager, S. L. Andersen, L. Gerena, and W. K. Milhous. 1992. Bisquinolines. 1. N,N-bis(7-chloroquinolin-4-yl)alkanediamines with potential against chloroquine-resistant malaria. J. Med. Chem. 35:2129-2134. [DOI] [PubMed] [Google Scholar]
- 22.Wernsdorfer, W. H., and D. Payne. 1988. Drug sensitivity tests in malaria parasites, p. 1765-1800. In W. H. Wernsdorfer and I. A. McGregor (ed.), Malaria: principles and practice of malariology, vol. 2. Churchill Livingstone, Edinburgh, Scotland.
- 23.World Health Organization. 1984. Advances in antimalarial chemotherapy. Technical report series no. 711. World Health Organization, Geneva, Switzerland.
- 24.World Health Organization. 2001. Antimalarial drug combination therapy. Report of a W. H. O. Technical Consultation, W. H. O./CDS/RBM/2001.35. World Health Organization, Geneva, Switzerland.
- 25.Yang, H. L., D. Q. Liu, K. G. Huang, Y. M. Yang, P. F. Yang, M. Z. Liao, and C. Y. Zhang. 1999. Assay of sensitivity of Plasmodium falciparum to chloroquine, amodiaquine, piperaquine, mefloquine and quinine in Yunnan province. Chinese J. Parasitol. Parasit. Dis. 17:43-45. [PubMed] [Google Scholar]
- 26.Yang, H. L., P. F. Yang, D. Q. Liu, R. J. Liu, Y. Dong, C. Y. Zhang, D. Q. Cao, and H. He. 1992. Sensitivity in vitro of Plasmodium falciparum to chloroquine, pyronaridine, artesunate and piperaquine in south Yunnan. Chinese J. Parasitol. Parasit. Dis. 10:198-200. [PubMed] [Google Scholar]
- 27.Zhang, K. Y., J. X. Zhou, Z. Wu, and Q. J. Huang. 1987. Susceptibility of Plasmodium falciparum to chloroquine, piperaquine, amodiaquine, mefloquine and quinine with in vitro microtechnique in Hainan Island. Chinese J. Parasitol. Parasit. Dis. 5:165-169. [PubMed] [Google Scholar]