Abstract
Regulation of PIPK (phosphatidylinositol phosphate kinase) and PtdIns(4,5)P2 signalling by small G-proteins and their effectors is key to many biological functions. Through selective recruitment and activation of different PIPK isoforms, small G-proteins such as Rho, Rac and Cdc42 modulate actin dynamics and cytoskeleton-dependent cellular events in response to extracellular signalling. These activities affect a number of processes, including endocytosis, bacterial penetration into host cells and cytolytic granule-mediated targeted cell killing. Small G-proteins and their modulators are also regulated by phosphoinositides through translocation and conformational changes. Arf family small G-proteins act at multiple sites as regulators of membrane trafficking and actin cytoskeletal remodelling, and regulate a feedback loop comprising phospholipase D, phosphatidic acid, PIPKs and PtdIns(4,5)P2, contributing to enhancement of PtdIns(4,5)P2-mediated cellular events and receptor signalling. Na+, Kir (inwardly rectifying K+), Ca2+ and TRP (transient receptor potential) ion channels are regulated by small G-proteins and membrane pools of PtdIns(4,5)P2. Yeast phosphatidylinositol 4-phosphate 5-kinases Mss4 and Its3 are involved in resistance against disturbance of sphingolipid biosynthesis and maintenance of cell integrity through the synthesis of PtdIns(4,5)P2 and downstream signalling through the Rom2/Rho2 and Rgf1/Rho pathways. Here, we review models for regulated intracellular targeting of PIPKs by small G-proteins and other modulators in response to extracellular signalling. We also describe the spatial and temporal cross-regulation of PIPKs and small G-proteins that is critical for a number of cellular functions.
Keywords: ADP-ribosylation factor 1/6 (Arf1/Arf6); phosphatidylinositol phosphate kinase (PIPK); PtdIns(4,5)P2; phosphatidic acid; Rho/Rac/Cdc42; small G-protein
Abbreviations: Arf, ADP-ribosylation factor; Arp2/3, actin-related protein 2/3; DH, Dbl homology; DHR, discs large homology region; EGF, epidermal growth factor; ENaC, epithelial Na+ channel; ERM, ezrin/radixin/meosin; GAP, GTPase-activating protein; GDI, GDP-dissociation inhibitor; GEF, guanine nucleotide-exchange factor; GIRK, G-protein-activated inwardly rectifying K+; GPCR, G-protein-coupled receptor; HEK-293, human embryonic kidney; KATP, ATP-sensitive K+ channel; Kir, inwardly rectifying K+ channel; Kv, voltage-gated K+ channel; LPA, lysophosphatidic acid; PA, phosphatidic acid; PDGF, platelet-derived growth factor; PH, pleckstrin homology; PI4K, phosphatidylinositol 4-kinase; PI4P5K, phosphatidylinositol 4-phosphate 5-kinase; PIPK, phosphatidylinositol phosphate kinase; PIPKI, type I PIPK; PIPKII, type II PIPK; PLC, phospholipase C; PLD, phospholipase D; PTX, pertussis toxin; ROCK, Rho kinase; (N-)WASP, (neuronal) Wiskott–Aldrich syndrome protein
INTRODUCTION
PtdIns(4,5)P2 is a major cellular phosphoinositide that exists in discrete and compartmentalized pools in membranes and vesicles. In membranes, PtdIns(4,5)P2 is a precursor to second messengers and is a key signalling and targeting molecule [1]. PtdIns(4,5)P2 is involved in regulation of a number of cellular activities, including cell motility [2–4], gating of ion channels [5,6], exocytosis [7–9] and vesicular trafficking [10,11].
There are two subfamilies of PIPKs (phosphatidylinositol phosphate kinases), types I and II, that generate PtdIns(4,5)P2 from distinct substrate pools. PIPKIs (type I PIPKs) use PtdIns4P as a substrate [12], whereas PIPKIIs (type II PIPKs) use PtdIns5P [12,13]. PIPKIs also use PtdIns3P as a substrate, with lower in vitro efficacy, and, interestingly, generate PtdIns(3,4,5)P3 by a concerted double phosphorylation [13]. The role of PtdIns3P as substrate for generation of PtdIns(3,4,5)P3 by PIPKI in vivo has not yet been defined. The PIPKI and PIPKII subfamilies include three major isoforms, α, β and γ (the nomenclature for PIPKIα and PIPKIβ is reversed in mice). The PIPKI isoforms are also alternatively spliced [14–18]. These PIPK isotypes synthesize PtdIns(4,5)P2 in diverse cellular compartments and are key regulators of PtdIns(4,5)P2-mediated cellular signalling events.
The activation loops of PIPKI and PIPKII differ in a key amino acid residue, a glutamate (PIPKIs) or an alanine (PIPKIIs), that defines the substrate specificity for PtdIns4P compared with PtdIns5P and determines the hydroxy residue on the inositol ring that is phosphorylated [19]. The substrate specificity conferred by the activation loop, as well as differential localization of distinct PIPK isoforms, allows for PtdIns(4,5)P2-mediated regulation of non-redundant biochemical pathways by specific PIPKs. In this model, targeting of PIPKs by regulated protein–protein interactions controls PtdIns(4,5)P2 generation in a spatial and temporal fashion [19]. There is evidence that specific spatial and temporal generation of PtdIns(4,5)P2 is key for regulation of specific cellular events.
Although the regulation of PIPK spatial targeting is only beginning to be defined, it is understood that stereospecific interactions between a PIPK and its substrate are important. When the specificity-conferring activation loops are swapped between PIPKI and PIPKII, a change of the PIPK intracellular targeting of the overexpressed enzyme that corresponds to that of the PIPK that donated the loop is observed [19]. Clearly, there are additional requirements for PIPK targeting, as PIPKI isoforms with virtually identical specificity loops have dramatically different intracellular locations [20]. In addition to substrates, other targeting factors determine the intracellular location of PIPKs, and some of these factors are likely to be small G-proteins as well as their regulators, which are dynamically regulated by upstream signalling pathways (Figure 1).
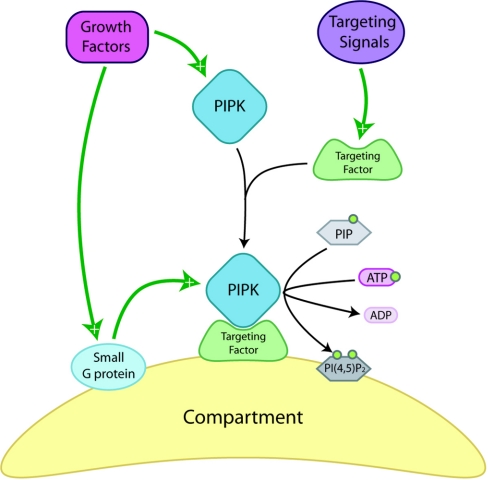
Figure 1. Spatial control of PIPK signalling and local PtdIns(4,5)P2 levels through small G-proteins and compartment-specific targeting factors.
In response to extracellular signals, PIPKs are targeted to specific cellular localizations, most often membranous structures, by specific targeting molecules where interactions with small G-proteins contribute to increased local PtdIns(4,5)P2 [PI(4,5)P2] availability downstream. PIP, PtdInsP.
Individual PIPK isoforms have been identified as downstream effectors of small G-protein signalling that produce PtdIns(4,5)P2 at specific cellular sites [20,21]. Functional coupling of small G-proteins and PIPKs regulates the generation of PtdIns(4,5)P2 critical to regulation of cytoskeletal-dependent functions, including platelet activation and membrane ruffle formation required for cell migration [22–24]. The synthesis of PtdIns(4,5)P2 by PIPKs has the potential to be regulated by a feedback loop involving small G-proteins, PLD (phospholipase D) and its reaction product, PA (phosphatidic acid) [25–29].
Emerging evidence supports the concept that PtdIns(4,5)P2 is generated in highly specific pools or by channelling PtdIns(4,5)P2 directly to effectors [30]. In this model, the interaction of PIPKI isoforms with components for targeting to specific cellular compartments modulates highly specific PtdIns(4,5)P2 synthesis and channelling to effectors. For example, PIPKIγ661 targeting to focal adhesions by an association with talin results in spatially generated PtdIns(4,5)P2 that strengthens the binding of talin to β-integrin [30]. Understanding how PIPKs are regulated by interactions with their binding partners, as well as the mechanisms by which their product, PtdIns(4,5)P2, exerts its effects on cellular processes is critical to understanding the co-ordinated assembly of cellular signalling complexes and pathways. In this review, we describe and critically examine significant findings in the field surrounding regulation of PIPKs by small G-proteins and their associated regulators, as well as the roles of the PIPKs and PtdIns(4,5)P2 in co-ordinated stimuli-driven signalling.
SMALL G-PROTEINS
The superfamily of small G-proteins can be classified into five subfamilies: Ras, Rho, Arf (ADP-ribosylation factor), Rab and Ran. Commonly referred to as monomeric G-proteins, members of this superfamily share monomeric structure and contain GTPase domains ranging in size from 20 to 40 kDa. Monomeric G-proteins act as molecular switches to spatially and temporally modulate a variety of cellular functions through diverse signalling networks, including activities or targeting of PIPKs [31]. Small G-proteins are shuttled between membranes and the cytosol, and their ability to modulate downstream signalling processes depends on the finely tuned regulation of their activation and inhibition through interactions with regulators. Through regulation of downstream signalling processes, small G-proteins co-ordinate activities of effector molecules to elicit specific physiological responses to stimuli.
Regulation of the Rho and Arf families of small G-proteins
Most small G-proteins can be found in the cytosol or on membranes and are modified by C-terminal lipid conjugation, which is essential for binding to membranes, interactions with regulators and signalling to downstream effectors [32,33]. Biochemical activities of the Rho family of small G-proteins are subject to modulation by GEFs (guanine nucleotide-exchange factors) and GAPs (GTPase-activating proteins), as well as by GDIs (GDP-dissociation inhibitors) [34–37]. Through interactions with these factors, Rho family members are regulated by catalytic and membrane association cycling [31,37]. Rho GEFs contain PH (pleckstrin homology) and DHR (discs large homology region) domains, which are important for regulation of their activities through interactions with factors including phosphoinositides [34,38]. The Dbl family of GEFs, which contain DH (Dbl homology)/PH tandem repeats, have been established as the major canonical Rho protein GEFs [39]. One member of this family, Tiam1, is recruited to membranes and is activated by PtdIns-(3,4,5)P3 binding to its PH domain [40]. More recently, the CZH family of Rho GEFs, lacking tandem DH/PH domains, has been characterized [34]. One member of this family, DOCK180, binds to PtdIns(3,4,5)P3 via its DHR-1 domain and couples signalling to GTP loading of Rac via its DHR-2 domain [41]. There is a feedback mechanism modulating signalling between Rho family small G-proteins and phosphoinositides. For example, Rac GEFs are stimulated by PtdIns(4,5)P2 and PtdIns(3,4,5)P3, products of Rac activation of PIPKI and PI3K [39,40,42]. Activities of Rho family GAPs are regulated by protein phosphorylation, lipid binding and protein–protein interaction [36]. For instance, one member of the Rac-specific GAPs, chimaerin, is regulated in vitro by diacylglycerol and its analogues, phorbol esters, as well as by phosphatidylinositol lipids [36].
Arf protein cycling is also mediated by specific GEFs and GAPs, but is not subject to regulation by GDIs (Figure 2) [31]. Regulation of Arf proteins by GEFs and GAPs is modulated by interactions of GEFs, GAPs and Arf proteins themselves with PtdIns(4,5)P2 [43–45]. For example, Arno is an Arf GEF whose activation of Arf1 depends on its binding to PtdIns(4,5)P2 and subsequent translocation to the plasma membrane, as shown in Figure 2 [44,45]. Arf GAP activity depends on PtdIns(4,5)P2 binding directly to Arf, also illustrated in Figure 2 [43]. Collectively, regulation of the Rho and Arf families of small G-proteins by phosphoinositides occurs by two major mechanisms: alteration of activity and physical translocation.
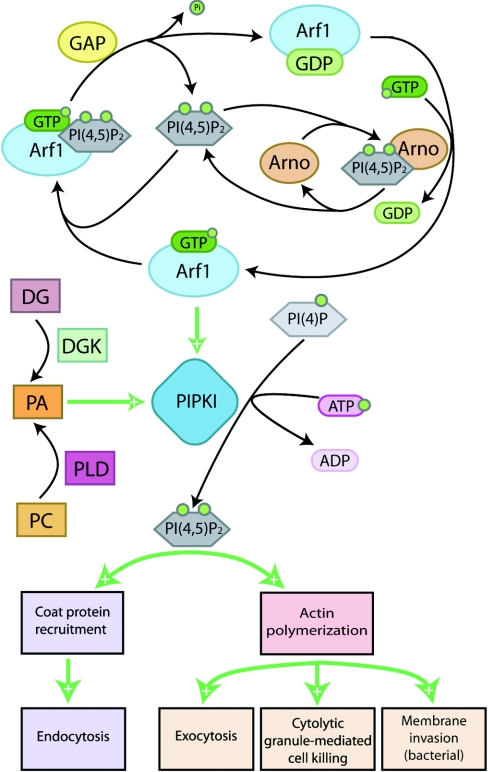
Figure 2. Arf protein signalling through PIPKIs affects both cellular trafficking and motility.
Arf1 and Arf6 signal through PIPKIs to control intracellular trafficking and actin polymerization. A positive-feedback loop involving PtdIns(4,5)P2 [PI(4,5)P2], PLD and its product PA intensify Arf effects on PtdIns(4,5)P2 levels. See the text for detailed pathway descriptions. DG, diacylglycerol; DGK, DG kinase; PC, phosphatidylcholine; PI(4)P, PtdIns4P.
Rho
Members of the Rho family of small G-proteins are important for regulation of the formation of actin stress fibres and focal adhesion complexes [46]. The specific role of Rho in these processes is in actin filament reorganization, and, accordingly, cells treated with Rho inhibitors show significant disruption of stress fibres [46].
A wide variety of downstream effectors of Rho proteins have been identified, and their regulatory signalling mechanisms have been characterized. Several PIPKs have been identified as Rho protein interactors and effectors, with PIPKI specifically serving a critical role in Rho-mediated signalling. GTP[S] (guanosine 5′-[γ-thio]triphosphate) stimulation of PIPKI activity in C3H 10T1/2 mouse fibroblast and HEK-293 (human embryonic kidney) cell lysates is blocked by the Rho inhibitor Clostridium botulinum C3 exoenzyme [47,48]. Addition of GTP-activated recombinant RhoA increases PIPKI activity in the same system, providing additional evidence to support the hypothesis that Rho modulates PIPKI activity [47,48]. Furthermore, although microinjection of C3 exoenzyme alone into cells reduces PtdIns(4,5)P2-mediated PDGF- (platelet-derived growth factor) and thrombin-induced Ca2+ mobilization, addition of constitutively active Rho reverses this defect, indicating that Rho modulates PtdIns(4,5)P2 synthesis and modulates PIPK activity [47]. Consistent with this evidence, inactivation of Rho GTPases by Clostridium difficile toxin B and C3 exoenzyme reduces total cellular PtdIns(4,5)P2 levels by 40–50% in HEK-293 cells [49]. The reduction in total cellular PtdIns(4,5)P2 supply results in an inhibition of receptor-mediated inositol phosphate formation by PLC (phospholipase C) and PtdIns(4,5)P2-sensitive PLD [49,50].
Ren et al. [51] established that PIPKI physically associates with Rho independently of Rho being in its active, GTP-bound, form. Although direct interaction was not verified in these experiments, only the GTP-bound form of Rho was found to have the capacity to activate PIPKI, suggesting a model in which Rho-GTP activates a downstream effector that is also required for the activation of PIPKI [51]. This model was supported by identification of ROCK (Rho kinase) as a downstream effector of Rho-GTP that regulates the controlled synthesis of PtdIns(4,5)P2 by PIPKI in HEK-293 cells [48,52]. The existence of a RhoA/ROCK/PIPKI signalling pathway is supported further by the observation that PIPKIα-mediated stress-fibre formation was blocked and PtdIns(4,5)P2 synthesis was inhibited by the ROCK inhibitor Y-27632 [(+)-(R)-trans-4-(1-aminoethyl)-N-(4-pyridyl)cyclohexanecarboxamide] in CV-1 monkey kidney fibroblast cells [53] and human lens epithelial cells [54]. How these events coordinate with the modulation of focal adhesions by the PIPKIγ remains to be defined [30,55].
PIPKI has also been indicated as a downstream effector of the RhoA–ROCK signalling unit during neurite retraction; overproduction of kinase-deficient mutant PIPKIα blocks both LPA (lysophosphatidic acid)-induced and ROCK-induced neurite retraction in N1E-115 neuroblastoma cells, while Y-27632, a ROCK-specific inhibitor, is unable to block the overexpressed PIPKIα-induced neurite remodelling effects. This suggests that PIPKIα plays a role as a downstream effector of the RhoA/ROCK pathway and serves as the mechanism to couple LPA signalling to neurite retraction [56]. More recently, Yang et al. [22] showed that platelet PIPKI is regulated by Rho and ROCK. In their system, Rho-GTP and ROCK-GTP activate PIPKI translocation to the actin cytoskeleton, making it available to initiate actin assembly [22].
In contrast with the above model, evidence exists that PIPKIs are direct effectors of Rho signalling independently of ROCK [57]. ERM (ezrin/radixin/moesin) proteins are normally activated and recruited to the plasma membrane, where they serve to cross-link actin filaments to the plasma membrane [58]. Experimental evidence indicates that ERM protein phosphorylation and the resultant formation of microvilli are up-regulated by constitutively active Rho, but not by ROCK. In addition, ERM phosphorylation induced by introduction of constitutively active RhoA was found to be protected against suppression by the Y-27632, a ROCK-specific inhibitor. PIPKIα expression alone, however, is sufficient for induction of ERM phosphorylation and microvilli formation in NIH 3T3 cells [57]. These results indicate that PIPKI is a direct downstream effector of Rho independently of ROCK signalling.
Rac
Rac has a number of established roles in cell signalling, including regulation of lamellipodium formation, membrane ruffling and actin polymerization [59,60]. Similarly to Rho, several lines of evidence demonstrate a link between Rac and PIPKI [42,61–64]. Rac has also been shown to interact with PIPKI regardless of whether it is bound to GDP or to GTP. The lack of GTP dependence in Rac binding to PIPKI is consistent with the location of the interaction site between Rac and PIPKI, which is separated from the Rac effector domain [31,42,61]. This suggests the possibility that activation of Rac by a GDP-into-GTP conversion results in a conformational change in Rac that allows its effector domain to interact with, and more efficiently activate, already bound PIPKI. The relevance of this interaction was demonstrated in a permeabilized platelet system, where the addition of a constitutively active mutant Rac was shown to activate PtdIns(4,5)P2-mediated F-actin (filamentous actin) uncapping and actin polymerization [65]. Building on these findings [65], overexpression of PIPKIα and overexpression of constitutively active Rac had similar effects on actin filament uncapping and assembly [62]. Together these results provide salient evidence that PIPKIα is an important mediator of Rac-dependent actin assembly. However, overexpression of the PIPKs can be misleading, as this may saturate normal specific targeting mechanisms.
Different PIPKI isoforms have been found to localize to a number of distinct subcellular locations. Endogenous PIPKIα localizes to the nucleus and membrane ruffles [20,66], whereas PIPKIβ is found primarily at cytosolic perinuclear vesicular structures, and one splice variant of PIPKIγ is targeted to focal adhesions [66]. Despite differences in localization, all three PIPKI isoforms physically associate with both Rho and Rac1 [64]. This direct association is guanine-nucleotide-independent and results in increased cellular PtdIns(4,5)P2 levels, and presumably PIPK activity, in HEK-293 cells [64]. Additionally, indirect regulation of PIPKI activity by Rac has also been proposed. Both Rac and Rho have been shown to mediate thrombin receptor activation and thus translocation and activation of PIPKIα. This mediated translocation is dependent on the small G-protein's GTP-bound state. Experiments with dominant-negative and constitutively active mutants of Rac and Rho indicate that the regulation of PIPKI by Rac is indirect in nature and requires engagement of activated Rho. In vivo, this interaction results in membrane-translocated and activated PIPKIα becoming available to phosphorylate PtdIns4P to generate PtdIns(4,5)P2 on the plasma membrane in response to Rac and Rho signalling [67].
Cdc42
Cdc42 has been reported to regulate filopodia formation through localized actin polymerization and function as a sensor at the leading edge of motile cells [68,69]. It has been implicated in up-regulation of PtdIns(4,5)P2 synthesis by all three PIPKI isoforms independently of direct physical interaction between Cdc42 and PIPKI [63,64]. Cdc42 influences actin polymerization through downstream effector, N-WASP (neuronal Wiskott–Aldrich syndrome protein), which is activated by increased local levels of PtdIns(4,5)P2 resulting from Cdc42 activation of PIPKI. N-WASP then activates the Arp2/3 (actin-related protein 2/3) complex to initiate actin filament branching and elongation [70].
All members of the Rho family of small G-proteins increase local PtdIns(4,5)P2 levels through up-regulation of PIPK activity, although only Rac and Rho associate directly with PIPKI isoforms. Local phosphoinositide levels, especially PtdIns(4,5)P2 and PtdIns(3,4,5)P2 in turn affect activity and localization of Rho family members and their effectors. This signalling loop is critically important for co-ordinated regulation of actin stress fibres and focal adhesion complexes.
Arf
Mammalian cells contain six Arf small G-proteins that are categorized into three classes on the basis of primary structure [31,71]. Arf1 and Arf6 are the most extensively characterized members of the Arf family of small G-proteins. Multiple regulated roles for Arf1 and Arf6 have been identified in the regulation of membrane trafficking and actin structural remodelling [71–75]. Activity of the Arf family of GTP-binding proteins is generally mediated through interactions with effector proteins, as shown in Figure 2. All Arf proteins have been shown to be capable of activating PIPKIs as well as PLD, implying that many of the Arf protein functions may be dependent on the products of PIPKI and PLD activation, namely PtdIns(4,5)P2 and PA [23,76,77].
The primary localization of Arf1 in cells is at the Golgi membrane, where it reversibly associates via GDP–GTP cycling and functions in the recruitment of coat proteins for intracellular trafficking [74,78]. Arf1 has also been shown to be required for Golgi body structure and function. Interestingly, PtdIns(4,5)P2 availability appears to be critical for the role of the Golgi compartment in membrane trafficking [79]. Current lines of evidence indicate that Arf1 stimulates PtdIns(4,5)P2 synthesis by mediating the recruitment of PI4Kβ (phosphatidylinositol 4-kinase β) and PIPKI from the cytosol to the Golgi complex [80]. Jones et al. [79] reported that PIPKI isoforms directly interact with Arf1 and this interaction regulates PtdIns(4,5)P2 synthesis in a guanine nucleotide-dependent manner, thus identifying PIPKI as a direct downstream effector of Arf1-GTP. This Arf1-mediated PIPKI activation has been shown to be stimulated by PA derived from PLD activation [81].
Arf6 is primarily associated with the plasma membrane, where it is involved in the formation of PtdIns(4,5)P2 and modulation of the cortical actin cytoskeleton [73,78,82]. Arf6 is required for Rac attachment to plasma membrane ruffles, as well as changes in actin structure at the plasma membrane through Rac translocation and activation [83]. Expression of the Arf6-specific GEF EFA6 in cells generates membrane protrusions and ruffles through activation of Arf6, a Rac-dependent activity [84,85].
Arf6 transforms membrane topography by altering membrane lipid composition, and it has been shown to co-localize with, bind to, and activate PIPKI at the plasma membrane [7,23,86]. Arf-GTP binds to and activates PLD, which cleaves phosphatidylcholine to generate PA [76,77], whereas PtdIns(4,5)P2 stimulates PLD activity and PA stimulates PIPKI activity [25–29]. These combined regulatory loops result in enhanced production of PtdIns(4,5)P2 and PA, lipid messengers that mediate Arf's role in recruiting additional proteins to compartments involve in membrane trafficking and sites of cortical actin cytoskeleton reorganization [72,73,75,82,87–89]. In addition, these lipid messengers may also alter membrane structure and curvature, supplying a partial explanation for Arf6 regulation of membrane trafficking [10,73,82].
In terms of membrane trafficking, Arf6 has been shown to regulate PtdIns(4,5)P2-dependent exocytosis in MIN6 pancreatic cells [82] and PC12 neuroendocrine cells [7]. Furthermore, expression of a constitutively active Arf6 mutant in PC12 cells results in an accumulation of Arf6 mutant protein, PtdIns(4,5)P2, and PIPKIγ on endosomal membranes and their corresponding depletion from the plasma membrane and inhibits Ca2+-dependent exocytosis [7]. Arf6-mediated depletion of plasma membrane PtdIns(4,5)P2 is thought to be due to the re-targeting of PIPKIγ, a major PIPKI isoform in neuroendocrine cells, to endosomal compartments. This PIPKIγ translocation and associated plasma membrane PtdIns(4,5)P2 depletion dampens the PtdIns(4,5)P2-dependent Ca2+-dependent exocytosis response. Direct evidence of Arf6's regulation of PIPKIγ comes from the observation that Arf6 physically associates with PIPKIγ and is able to stimulate human growth hormone exocytosis in PC12 cells in a Ca2+-dependent manner [7]. Association of PIPKIγ and Arf6 has been shown to depend on the phosphorylation status of PIPKIγ, rather than the guanine nucleotide-binding status of Arf6; the association of PIPKIγ with membrane-bound Arf6 may be important for targeting the enzyme to its membrane substrate, PtdIns4P, for spatial generation of PtdIns(4,5)P2 necessary for further signalling.
Another line of evidence regarding Arf6 regulation of PIPKIγ reported by Krauss et al. [86] found that GTP-bound Arf6 and PIPKIγ co-localize upon transfection into COS7 kidney fibroblast cells. Arf6 is known to stimulate PtdIns(4,5)P2 production on synaptic membranes, possibly via activation of PIPKIγ and subsequent clathrin/AP-2 (activator protein 2) coated pit assembly [86]. In this manner, Arf6 is able to regulate endocytosis in synaptic membranes by localized synthesis of PtdIns(4,5)P2 through activation of PIPKIγ.
Honda et al. [23] have suggested that Arf6 activates PIPKIα directly in concert with PA in membrane ruffles. In an in vitro reconstitution assay containing purified Arf6, PIPKIα and PA, they demonstrated that PA and Arf6 synergistically up-regulate PIPKIα activity. In a subcellular localization experiment in HeLa cells, PIPKIα was found to translocate to membrane ruffles with Arf6 upon AlF4− and EGF (epidermal growth factor) stimulation of G-proteins, although both proteins normally localize throughout the cytoplasm and plasma membrane. The observed translocation of PIPKIα could be completely inhibited by expression of a dominant-negative Arf6 mutant [23]. PA is a known activator of all PIPKI isoforms and, upon EGF stimulation, overexpressed PLD2, which produces PA, is translocated to the ruffling membrane where it co-localizes with PIPKIα. The PA produced by PLD in ruffles then functions with Arf6 as a co-activator of PIPKIα [23,92]. Additionally, Arf6 stimulates actin dynamics through activation of PIPKIα and regulates growth cone motility in the developing nervous system [90]. These results suggest that Arf6 is a physiological downstream activator of PIPKIα [23,90].
Another body of evidence supporting a functional link between Arf activation and PIPKI has recently been reported. Both Chlamydia trachomatis [91] and Yersinia pseudotuberculosis [92] bacteria have been shown to utilize the Arf6 GTPase–PIPKI signalling unit to penetrate host epithelial cells. The proposed mechanism is initiated by the recruitment to and activation of Arf6 and PIPKI at the site of bacterial entry into the host cell. There, the resultant locally produced PtdIns(4,5)P2 causes extensive actin remodelling to allow for more efficient entry at the invasion site, highlighting further how activation of small G-proteins can affect localized PtdIns(4,5)P2, leading to spatially defined cellular changes [92].
Functional coupling of Arf6 and PIPKI has also been implicated in immune cell function [93]. In this capacity, Arf6 has been indicated to have a role in FcγRIIIA lymphocyte-mediated cytotoxicity. Specifically, aggregation of the FcγRIIIA receptor for the Fc portion of IgG is thought to induce Arf6 activation, which subsequently activates PIPKI. This is demonstrative of an Arf6-mediated targeting mechanism that regulates spatially generated de novo synthesis of PtdIns(4,5)P2. In natural killer cells, IgG receptor activation mediates signalling through the concerted effort of Arf–PIPKI signalling, generating a localized pool of PtdIns(4,5)P2 necessary for marking and modifying membrane microdomain rafts for specific structural and functional modifications required for granule docking and fusion [10,93].
Two members of the Arf family of small G-proteins, Arf1 and Arf6, play important roles in both membrane trafficking and actin structural remodelling. A number of pathways in these processes have been linked directly to PIPKs or to their products, most notably PtdIns(4,5)P2. Downstream signalling effects of Arf activation of PIPK activity include endocytosis, exocytosis, actin polymerization and membrane ruffle formation. How these events are spatially regulated and the identity of the upstream signals remains an important area of investigation.
Spatial and temporal regulation of PIPKs by small G-proteins
There exists evidence indicating the presence of discrete compartmentalized cellular pools of PtdIns(4,5)P2 [10,94]. These pools are functionally and spatially distinct and are influenced by differentially targeted PIPKI isoforms. For example, PIPKIγ635 is reported to be responsible for generation of PtdIns(4,5)P2 pools that can be mobilized by GPCR (G-protein-coupled receptor)-linked Ca2+ signalling to control activities such as histamine receptor activation [95], mouse PIPKIβ and PIPKIγ are responsible for generating PtdIns(4,5)P2 pools for exocytosis of large dense core vesicles in chromaffin cells [8,9], and PIPKIγ661 targets to focal adhesion sites [30]. The targeting of different PIPK isoforms to various subcellular locations is regulated by small G-protein signalling. Precise spatial and temporal synthesis of PtdIns(4,5)P2 is critical for modulation of various cellular responses such as platelet aggregation, endocytosis, exocytosis and cell migration events including lamellipodia formation, focal adhesions and membrane ruffling [1].
Early studies have shown that the addition of low concentrations of thrombin up-regulates PtdIns(4,5)P2 synthesis [96] and increases PIPK activity associated with cytoskeletal functions [97]. Hinchliffe et al. [98] reported that the integrin-mediated signalling associated with platelet aggregation promotes translocation of PIPKII to the cytoskeleton where it regulates a cytoskeleton-associated pool of PtdIns(4,5)P2. Thrombin activation of PIPKIα is via Rac [62], and PIPKIα is targeted to specific sites in the plasma membrane where rapid actin assembly takes place in a Rac- or Rac/Rho-dependent manner [62,67]. Locally produced PtdIns(4,5)P2 regulates actin polymerization through activation of WASP family proteins in platelets. WASP family proteins activate actin assembly via Arp2/3 (actin-related proteins 2/3), and act to gate this actin assembly process dependent upon local PtdIns(4,5)P2 concentrations [99,100]. Recently, Yang et al. [22] demonstrated that platelet PIPKI is tightly regulated by Rho GTPase by illustrating that inhibition of Rho completely blocks trafficking and activation of PIPKI in thrombin receptor-stimulated platelets. The activated Rho–ROCK signalling unit induces the activation and actin cytoskeleton recruitment of PIPKI to generate PtdIns(4,5)P2. It is unclear, however, how GTP-bound Rho targets PIPKI to the platelet membrane cytoskeleton.
Rac also plays a role in spatial targeting of PIPKIα to the plasma membrane. There are two distinct subcellular localizations of Rac and PIPKIα, on the membrane and in the cytosol [22,67,101]. It is thought that these subcellular locales are not mutually exclusive pools and that shuttling can occur between them. In addition, Rac1 has been shown to be associated with PIPKIα, forming a Rac1–PIPKIα signalling complex in both locations [20,61]. In this complex, membrane-associated Rac activates PIPKIα to produce PtdIns(4,5)P2. Membrane ruffle formation induced by PDGF is increased dramatically by increasing ratios of active Rac to PIPKIα in MG63 cells, indicating that the association of PIPKIα with Rac and the signalling of activated Rac are essential in localized actin remodelling to form cell membrane ruffles (Figure 3). This phenomenon is specific to PIPKIα, as Rac1 has been shown to associate with PIPKIα, but not PIPKIβ, in membrane ruffles upon PDGF receptor stimulation in MG63 cells [20]. From this, it is supposed that simultaneous and synchronized activation of PIPKIα and Rac is required for efficient membrane ruffle formation, which is a critical process in cell migration [20]. Recently, the LIM protein Ajuba has been identified as a positive regulator of PIPKIα and Rac as well as a negative regulator of PIPKIIβ [3]. Ajuba signals through both PIPKIα and Rac to contribute to the formation of membrane ruffles in response to growth factor signalling. This supports the model illustrated in Figure 4, where the PIPKIα is spatially targeted, possibly by an interaction with Ajuba, and then modulated by Rac signalling as well as other signals downstream of PDGF.
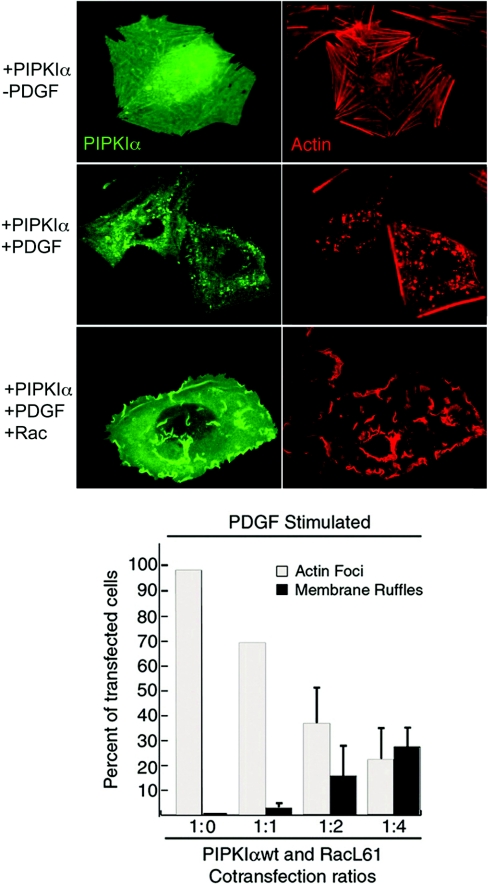
Figure 3. Co-expression of PIPKIα and Rac results in disruption of PIPKIα-induced actin foci and instead increased membrane ruffle formation.
MG63 cells were transiently transfected with epitope-tagged PIPKIα with or without epitope-tagged Rac. At 24 h post-transfection, cells were stimulated with PDGF or control treatment, fixed in 4% (w/v) paraformaldehyde and immunostained to visualize PIPKIα and actin. Immunofluorescence data show that PDGF treatment reorganizes singly expressed PIPKIα to reorganized actin focus structures, while PDGF treatment of cells co-expressing both PIPKIα and Rac results in formation of actin-containing membrane ruffles and translocation of PIPKIα to ruffle structures. This restructuring has been found to be dose-dependent. Quantification of actin morphology is shown in the lower panel, and indicates that focus and membrane ruffle formations are dose-dependent according to the ratio of PIPKIα to active Rac (RacL61). These data show that an increased ratio of Rac to PIPKIα results in a skewing of actin morphology from focus formation towards ruffle formation in response to PDGF stimulation.
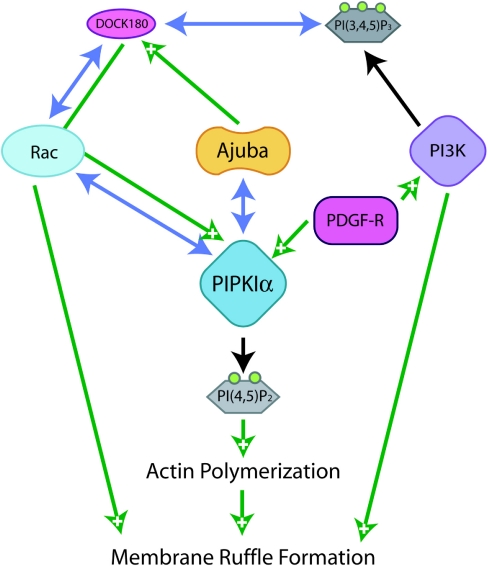
Figure 4. Interaction between Ajuba and PIPKIα in conjunction with Rac signalling increases membrane ruffle formation.
Co-ordinated signalling from extracellular signals through phosphoinositide phosphate kinases PIPK and PI3K in concurrence with interaction with Ajuba alters the localization of PIPKIα to sites of membrane ruffle formation where concurrent translocation of and signalling through Rac allows their collective effect of increased membrane ruffle formation. PDGF-R, PDGF receptor; PI3K, phosphoinositide 3-kinase; PI(3,4,5)P3, PtdIns(3,4,5)P3; PI(4,5)P2, PtdIns(4,5)P2.
Several characteristics of both small G-proteins and their binding partners are implicated in translocation of small G-proteins to the plasma membrane. For example, Rac is prenylated at a C-terminal site, making it more suitable for cell membrane association [32,33]. In the cytosol, Rho GDI interacts with Rac through its prenylated moiety as well as its switch I and II domains [102,103]. Rac has also been shown to exist in a multimeric signalling complex with lipid kinases in the cytosol, complexed with PIPKI in addition to Rho GDI and diacylglycerol kinase [61]. Activation via upstream signalling by integrins, lipids and kinases results in extraction of Rho GDI, which is necessary for shuttling of the Rac–lipid kinase complex to the target site of PtdIns(4,5)P2 production, as has been proposed previously [37,61,104–106]. In a similar scheme of modification, Rho family GTPases have C-termini that are positively charged and resemble phosphoinositide-binding sequences [107]. These modifications are critically important to correct localizations of these small G-proteins, as maintenance of subcellular pools of PtdIns(4,5)P2 is dependent on precise spatial and temporal regulation of PIPKs by these activators.
As previously mentioned, Arf proteins regulate both PLD and PIPKIs [23,76,79,108,109]. PLD isoforms PLD1 and PLD2 have been shown to generate PA via hydrolysis of phosphatidylcholine and through this production activate PIPKI both in vitro and in vivo [25,26]. Both mammalian PLD1 and PLD2 have an absolute requirement for stimulation by PtdIns(4,5)P2 and functional PH domains to regulate their activity and localization [110]. Given these functional links, the possibility of a direct interaction between PLD isoforms and PIPKIα has been postulated [27]. Both PLD1 and PLD2 have been shown to interact with murine PIPKIα, and PLD2 has been demonstrated further to be able to recruit mPIPKIα. In porcine aortic endothelial cells, mPIPKIα localizes to the plasma membrane, cytosol and microtubule-like structures when overexpressed singly. Co-expression with PLD2 results in a relocation of mPIPKIα to the submembranous PLD2-positive patch, indicating that PLD2 is able to recruit mPIPKIα to this compartment [27].
It has been proposed that the PtdIns(4,5)P2 generated by PIPKI acts as a cofactor for PLD activation [28]. If this is correct, then it is plausible that the co-localization observed between overexpressed mPIPKIα and PLD2 mentioned above would lead to a rapid local increase in both PA and PtdIns(4,5)P2 concentrations caused by the positive-feedback loop between increases in PtdIns(4,5)P2 concentration and PA formation by PLD and subsequent PIPKI activation. Both PA and PtdIns(4,5)P2 have been suggested to be important in generation of membrane curvature, vesicle budding and the recruitment and activation of proteins involved in coating of vesicles [10,111–114]. This hypothesis is supported by several different lines of inquiry. First, it has been reported that PLD2-derived PA stimulates PIPKIγ635, and that the subsequent generation of PtdIns(4,5)P2 drives the initial stages of cell adhesion by regulating cell-surface integrins [29]. This model stresses the importance of PA-dependent generation of PtdIns(4,5)P2 by PIPKIγ635, specifically at the plasma membrane. Secondly, it has been demonstrated that there is a comparatively high degree of co-localization between PLD2 and PIPKIγ635 at the plasma membrane of cells in suspension or during the initial stages of adhesion [29]. This spatial and temporal proximity gives credence to the hypothesis that these factors co-operate within the same signalling cascade to affect the initial stages of cell adhesion.
Distinct PtdIns(4,5)P2 pools accumulate and modulated spatially and temporally in cells through regulated PIPK activity. These pools appear to be highly transient, and small G-proteins play roles both in translocation of PIPKs and their effectors to synthesis sites and in their direct stimulation and alter local levels of PtdIns(4,5)P2 production in response to extracellular and intracellular stimuli. Small G-protein-mediated changes in PtdIns(4,5)P2 pools are important for regulated membrane trafficking and a number of processes involved in cell motility.
PIPKs and small G-proteins in yeast
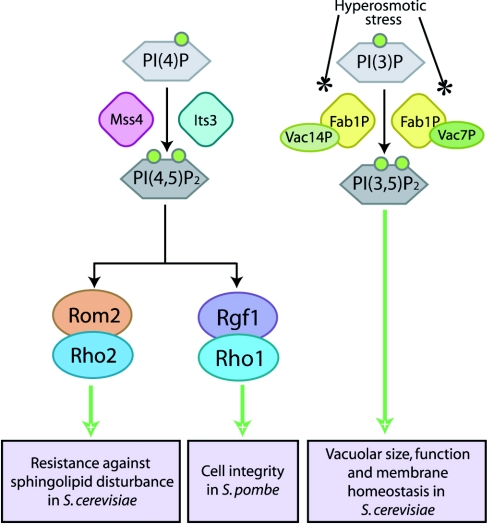
Mss4, the product of the budding yeast Saccharomyces cerevisiae Mss4 gene, shares extensive sequence homology with the mammalian PIPKs [15,18] and has been shown to be the major PI4P5K (phosphatidylinositol 4-phosphate 5-kinase) in budding yeast most similar to mammalian PIPKIs [115]. However, unlike mammalian PIPKIs, Mss4 shows dual substrate specificity in vitro and is able to convert both PtdIns4P into PtdIns(4,5)P2, and PtdIns3P into PtdIns(3,5)P2 [116]. Mss4 has been revealed to control organization of the actin cytoskeleton as part of a signalling pathway involving yeast Rho GTPases, including Rho2, as illustrated in Figure 5 [116]. Recent data suggest that defects in PtdIns(4,5)P2 synthesis reduce Mss4 kinase activity and alter its subcellular localization by preventing recruitment of Rom2 to the membrane, which results in reduced Rho2 activation [117]. The PH domain of Rom2 serves an essential function in vivo [118], as it allows regulation of the Rom2/Rho2 signalling pathway to resist disturbance of sphingolipid biosynthesis by PtdIns(4,5)P2, as shown in Figure 5 [117]. Rho1 and Rho2 are key proteins that are also implicated in construction of cell wall and cell polarity processes in S. cerevisiae [119] and Schizosaccharomyces pombe [120,121].
Figure 5. Yeast PIPKs signal through small G-proteins to mediate structural remodelling and membrane integrity.
Similarly to mammalian PIPK signalling pathways, yeast PIPK isoforms convert PtdIns3P [PI(3)P] and PtdIns4P [PI(4)P] into PtdIns(3,5)P2 [PI(3,5)P2] and PtdIns(4,5)P2 [PI(4,5)P2] respectively to modulate cell integrity and membrane homoeostasis through pathways gated by small G-protein signalling.
The fission yeast Schizosaccharomyces pombe its3+ gene product, Its3, is the budding yeast Mss4 homologue that has been identified as yeast PI4P5K. It has been shown to be required for completion of cytokinesis in concert with calcineurin [122]. Additionally, recent research indicates that the Its3 PI4P5K is involved in regulating fission yeast cell integrity via regulation of the Rgf1, Rho1 and protein kinase C signalling pathway as well as the PLC1-mediated PKC-independent pathway through production of PtdIns(4,5)P2 (Figure 5) [123]. Rgf1, a specific Rho1 GEF is involved in formation of cell wall and septum [124], and co-ordination of cell polarization with cell wall biogenesis [125] in fission yeast.
The yeast FAB1 gene product, Fab1p, was initially proposed as a PI4P5K [126], but now defines a phosphatidylinositol 3-phosphate 5-kinase that synthesizes PtdIns(3,5)P2 using PtdIns3P as a substrate [127]. Complementation analysis of PIPK-deficient yeast mutants indicates that Fab1p and its murine homologue p235 (PIKfyve) comprise a distinct class of type III PIPKs that can exclusively generate PtdIns(3,5)P2 in eukaryotic cells [128]. It has been proposed that Fab1p and its upstream regulator Vac7p form a signalling complex that regulates vacuolar size and membrane homoeostasis via production of PtdIns(3,5)P2 in S. cerevisiae [127,129]. As an extension to this observation, Fab1p is also activated by Vac14p, another novel vacuolar protein, upon hyperosmotic stress and rapidly synthesizes PtdIns(3,5)P2, which induces changes in vacuolar volume to smaller, more highly fragmented vacuoles in S. cerevisiae [130,131]. PtdIns(3,5)P2 is essential for vesicle recycling from vacuole/lysosomal compartments and for protein sorting in the multivesicular body in S. cerevisiae [132–134]. More recently, it has been proposed that levels of both PtdIns(3,5)P2 and PtdIns(4,5)P2 regulate intracellular acidification status in response to environmental pH changes in S. cerevisiae [135].
There is interplay between PIPK and small G-protein signalling pathways in yeast similarly to what has been established in mammalian systems. Although established signalling pathways through Mss4 and Its3 exert effects on cell membrane composition and permeability, it is unknown how Fab1p and PtdIns(3,5)P2 play into signalling pathways to alter vacuolar size, function and membrane homoeostasis. It is likely that at least some of these effects are linked to small G-protein signalling pathways considering the precedent in mammalian systems.
PIPK REGULATION OF ION CHANNELS: IS THERE A ROLE FOR SMALL G-PROTEINS?
Following initial reports that the activities of the Na+/Ca2+ exchanger and KATP channel (ATP-sensitive K+ channel) in giant cardiac membrane patches were affected by changes in membrane PtdIns(4,5)P2 availability [5], a tremendous number of channel activities have been reported to be regulated by phosphoinositides [6]. Small G-proteins have also been implicated in the regulation of membrane ion channel activity, and it is thought that these two types of channel regulation may be interconnected through small G-protein modulation of PIPK activity.
Na+ channels
ENaC (epithelial Na+ channel) is the limiting factor responsible for Na+ reabsorption in distal tubule epithelia. ENaC opening has been linked to membrane PtdIns(4,5)P2 availability through EGF receptor activation, where the resultant decrease in PtdIns(4,5)P2 levels has been shown to decrease Na+ transport [136]. Further reports indicate that PtdIns(4,5)P2 depletion mediates inhibition of ENaC by P2Y2 receptor activation, increasing the body of evidence that PtdIns(4,5)P2 regulates Na+ channel activity [137]. As in Kir channels, PtdIns(4,5)P2 is thought to increase individual channel open probabilities by direct receptor binding and activation or open state stabilization in ENaCs [138]. In addition to this mechanism, PtdIns(4,5)P2 levels are also thought to contribute to channel membrane insertion, thus increasing Na+ transport by increasing total transporting channels [139].
Small G-proteins RhoA and K-Ras have both been linked to ENaC receptor activation through phosphoinositide signalling. The RhoA–ROCK signalling pathway increases membrane PtdIns(4,5)P2 levels through stimulation of PIPKI activity, an effect shown to both increase open probabilities of existing ENaC channels and promote additional channel insertion into the membrane as shown in Figure 6 [139]. K-Ras induces ENaC opening, not through PtdIns(4,5)P2, but through up-regulation of PI3K activity and PtdIns(3,4,5)P3 generation at the membrane [140]. Like PtdIns(4,5)P2, PtdIns(3,4,5)P3 has also been shown to bind directly to a specific ENaC-binding site [141]. This binding has been linked to increased channel open probabilities [141] and provides another mechanism by which small G-proteins regulate ENaC activity.
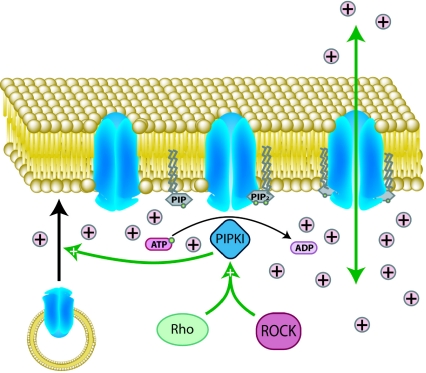
Figure 6. Coupling of Kir family channel transport to membrane PtdIns(4,5)P2 availability.
In the absence of bound PtdIns(4,5)P2, Kir family channels are closed. PIPKI conversion of PtdIns4P into PtdIns(4,5)P2 and subsequent PtdIns(4,5)P2 channel binding is required for the conformational change from the closed to the open state. Additionally, PIPKI activity and increased membrane concentrations of PtdIns(4,5)P2 have been shown to be linked to increases in channel membrane insertion.
K+ channels
The Kir channel family is the one of the earliest and most extensively studied channel families regarding direct interactions between positively charged residues in channel cytoplasmic domains and the anionic inositol head groups of phosphoinositides [142,143]. This family includes Kir2/IRK Kir3/GIRK (G-proteinactivated inwardly rectifying K+), Kir6/KATP and Kv (voltage-gated K+) type channels. The inwardly rectifying K+ channel, Kir3, mediates postsynaptic inhibitory effects of various neurotransmitters in the brain and atrium via GPCRs linked to PTX (pertussis toxin)-sensitive Gi/o family. Kir6 plays a role as a glucose sensor in pancreatic β-cells using association of the Kir subunit with a sulfonylurea receptor.
Kir channel activity is intimately coupled to PtdIns(4,5)P2 availability, as channel opening has been found to be dependent upon PtdIns(4,5)P2 [144,145]. Different members of the Kir channel family have differential sensitivity to PtdIns(4,5)P2. Current research has supported the concept that channels with higher affinity for PtdIns(4,5)P2 overall exist more in open conformations, whereas channels with low PtdIns(4,5)P2 affinity have lower openness probabilities [142] GIRK and Kir2.3 display lower relative affinities for PtdIns(4,5)P2 than do Kir2.1 and Kir1. This presumably has physiological relevance for channel regulation, as a variety of transmembrane signalling systems in diverse cellular environments are able to modulate PtdIns(4,5)P2 concentrations [6,146].
Surprisingly, the small G-protein Rho has been shown to inhibit Kir2.1 channel activity by an unknown mechanism [146a], possibly through alteration of channel membrane targeting or recycling [147]. Rho is a known activator of PIPKIs, and through this mechanism increases local membrane PtdIns(4,5)P2 levels. This indicates that Kir2.1 channel inhibition by Rho is through an alternative pathway, possibly the MAPK (mitogen-activated protein kinase) pathway, which has been implicated previously in channel inhibition [148]. The Kv1.2 channel has been shown to be negatively regulated by Rho as well, and, in this case, GTP-bound RhoA binds directly to the channel to inhibit its activity [149].
Ca2+ channels
It has been reported that PtdIns(4,5)P2 is required for regulation of P/Q type Ca2+ channels [150]. More recently, PtdIns(4,5)P2 has also been shown to be required for stabilization and activation of N-type Ca2+ channels [151]. Muscarinic receptor-mediated suppression of Ca2+ channel activity has been shown to be inhibited by expression of PI(4)K in Xenopus oocytes heterologously expressing Ca2+ channels in an inside-out patch experiment. As indicated by said experiment, PI4K serves to replenish PtdIns4P, the substrate for PtdIns(4,5)P2 synthesis by PIPK, and supplies PtdIns(4,5)P2 into lipid microdomains, where this channel is located. Blockade of PI4K by its pharmacological inhibitor, wortmannin, strongly and selectively attenuates recovery from PTX-insensitive muscarinic modulation. This suggests that Gq/11-mediated depletion of PtdIns(4,5)P2 contributes to Gq/11-mediated muscarinic modulation of N-type Ca2+ channels [151]. Ras family small G-protein Kir/Gem has been indicated in inhibition of voltage-gated Ca2+ channels through direct interaction with the channel's β subunit [152]. As membrane PtdIns(4,5)P2 has been shown to influence channel activity, there is a possibility that another small G-protein signalling pathway could, through PIPK activation, alter membrane PtdIns(4,5)P2 and modulate channel activity.
TRP (transient receptor potential) family ion channels
Plasma membrane PtdIns(4,5)P2 is an important regulator of TRP channels as its breakdown appears to underlie desensitization of several TRP channels, including TRPM4, TRPM5, TRPM8 and TRPV1 [153–156]. In contrast with PtdIns(4,5)P2-mediated activation or stabilization of channel activities as described above, there have been reports that PtdIns(4,5)P2 acts as a negative regulator in some cases and inhibits certain channel activities. PKD2 belongs to the TRP channel superfamily and has been shown to function as an EGF-activated non-selective cation channel in the plasma membrane. Experimental evidence shows that EGF-induced currents were able to be blocked by U73122 [1-(6-{[17-3-methoxyestra-1,3,5(10)-trien-17-yl]amino}hexyl)-1H-pyrrole-2,5-dione], a PLC inhibitor. This is strongly indicative that EGF-induced PKD2 activity requires PLC activity, and suggestive that reduction of PtdIns(4,5)P2 may be linked to activation of PKD2 by EGF. Support for this hypothesis comes from observations that addition of PtdIns(4,5)P2 blocks EGF-induced channel current [157].
Although a direct role for PtdIns(4,5)P2 in channel insertion has yet to be elucidated, PtdIns(4,5)P2 is likely to modulate translocation of the TRPC5 channel to the plasma membrane in addition to playing a role in regulation of TRP channel activity. In HEK-293 cells, Rac1 has been shown to mediate EGF stimulation of PtdIns(4,5)P2 synthesis through activation of PIPKIα and facilitate translocation of TRPC5-containing vesicles to the plasma membrane [158]. To summarize, Rac1 and its downstream effector PIPKI are necessary to stimulate TRPC5 insertion into the plasma membrane after EGF stimulation [158].
As with other phosphoinositide-mediated signalling events, small G-proteins and their effectors play important roles in signalling through stimulation of PIPK activity, localized PtdIns(4,5)P2 production and subsequent changes ion channel activity, as well as, in some cases, ion channel insertion at the plasma membrane.
SUMMARY AND COMMENTS
Understanding how phosphoinositide signalling and small G-protein signalling are connected is critical for the understanding of the functional dynamics of many cellular signal transduction pathways. In this review, we propose a model for regulated intracellular targeting of PIPKs by small G-proteins and other modulators in response to extracellular signalling. The importance of spatial and temporal regulation of these factors in signal transduction cannot be overstated for in vivo signalling events.
Highly co-ordinated signalling networks operate to control functional integrity of PtdIns(4,5)P2-mediated cellular events such as cellular cytoskeletal changes, exocytosis and endocytosis. Small G-proteins and their effectors play pivotal roles in controlling these signalling events through the regulation of PIPKs and are themselves regulated by phosphoinositides. Regulation of PIPKs by small G-proteins occurs primarily through recruitment and activation of different PIPK isoforms and resultant localized de novo synthesis of PtdIns(4,5)P2 in spatially and temporally distinct intracellular pools. The term ‘pool’, commonly used to describe how PtdIns(4,5)P2 is organized in membranes as a second messenger, does not refer exclusively to free PtdIns(4,5)P2. In fact, an unbound PtdIns(4,5)P2 gradient would be difficult to maintain as there would be rapid diffusion of PtdIns(4,5)P2 as well as hydrolysis by PtdIns(4,5)P2 phosphatases. To date, there is no convincing evidence that PtdIns(4,5)P2 is differentially distributed in discrete concentration pools in a contiguous membrane, although it is conceivable that varied concentrations may occur in membrane vesicles. Pools of PtdIns(4,5)P2 are probably stabilized by binding to effectors. In this model, the G-protein lifeguards may work more like G-protein plumbers in directing the channelling of PtdIns(4,5)P2 to specific effectors.
Acknowledgments
We are grateful to Dr Deane Mosher, Dr Anna Huttenlocher and Dr Patricia Keely at the University of Wisconsin for excellent discussions. We are also grateful to Michael Gonzales, Matthew Wagoner, Jessica Heck and Dr Kun Ling for comments on the manuscript. This work was supported by Basic Research Program of Korea Science and Engineering Foundation Grant (R01-2005-000-10596-0) to C.H.L. This work was supported by National Institutes of Health Grants (R01CA104708 and R01 GM057549) to R.A.A.
References
- 1.Niggli V. Regulation of protein activities by phosphoinositide phosphates. Annu. Rev. Cell Dev. Biol. 2005;21:57–79. doi: 10.1146/annurev.cellbio.21.021704.102317. [DOI] [PubMed] [Google Scholar]
- 2.Insall R. H., Weiner O. D. PIP3, PIP2, and cell movement: similar messages, different meanings? Dev. Cell. 2001;1:743–747. doi: 10.1016/s1534-5807(01)00086-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kisseleva M., Feng Y., Ward M., Song C., Anderson R. A., Longmore G. D. The LIM protein Ajuba regulates phosphatidylinositol 4,5-bisphosphate levels in migrating cells through an interaction with and activation of PIPKIα. Mol. Cell. Biol. 2005;25:3956–3966. doi: 10.1128/MCB.25.10.3956-3966.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Golub T., Caroni P. PI(4,5)P2-dependent microdomain assemblies capture microtubules to promote and control leading edge motility. J. Cell Biol. 2005;169:151–165. doi: 10.1083/jcb.200407058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hilgemann D. W., Ball R. Regulation of cardiac Na+, Ca2+ exchange and KATP potassium channels by PIP2. Science. 1996;273:956–959. doi: 10.1126/science.273.5277.956. [DOI] [PubMed] [Google Scholar]
- 6.Suh B. C., Hille B. Regulation of ion channels by phosphatidylinositol 4,5-bisphosphate. Curr. Opin. Neurobiol. 2005;15:370–378. doi: 10.1016/j.conb.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 7.Aikawa Y., Martin T. F. ARF6 regulates a plasma membrane pool of phosphatidylinositol(4,5)bisphosphate required for regulated exocytosis. J. Cell Biol. 2003;162:647–659. doi: 10.1083/jcb.200212142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gong L. W., Di Paolo G., Diaz E., Cestra G., Diaz M. E., Lindau M., De Camilli P., Toomre D. Phosphatidylinositol phosphate kinase type Iγ regulates dynamics of large dense-core vesicle fusion. Proc. Natl. Acad. Sci. U.S.A. 2005;102:5204–5209. doi: 10.1073/pnas.0501412102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aoyagi K., Sugaya T., Umeda M., Yamamoto S., Terakawa S., Takahashi M. The activation of exocytotic sites by the formation of phosphatidylinositol 4,5-bisphosphate microdomains at syntaxin clusters. J. Biol. Chem. 2005;280:17346–17352. doi: 10.1074/jbc.M413307200. [DOI] [PubMed] [Google Scholar]
- 10.Martin T. F. PI(4,5)P2 regulation of surface membrane traffic. Curr. Opin. Cell Biol. 2001;13:493–499. doi: 10.1016/s0955-0674(00)00241-6. [DOI] [PubMed] [Google Scholar]
- 11.Downes C. P., Gray A., Lucocq J. M. Probing phosphoinositide functions in signaling and membrane trafficking. Trends Cell Biol. 2005;15:259–268. doi: 10.1016/j.tcb.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 12.Rameh L. E., Tolias K. F., Duckworth B. C., Cantley L. C. A new pathway for synthesis of phosphatidylinositol-4,5-bisphosphate. Nature (London) 1997;390:192–196. doi: 10.1038/36621. [DOI] [PubMed] [Google Scholar]
- 13.Zhang X., Loijens J. C., Boronenkov I. V., Parker G. J., Norris F. A., Chen J., Thum O., Prestwich G. D., Majerus P. W., Anderson R. A. Phosphatidylinositol-4-phosphate 5-kinase isozymes catalyze the synthesis of 3-phosphate-containing phosphatidylinositol signaling molecules. J. Biol. Chem. 1997;272:17756–17761. doi: 10.1074/jbc.272.28.17756. [DOI] [PubMed] [Google Scholar]
- 14.Ishihara H., Shibasaki Y., Kizuki N., Katagiri H., Yazaki Y., Asano T., Oka Y. Cloning of cDNAs encoding two isoforms of 68-kDa type I phosphatidylinositol-4-phosphate 5-kinase. J. Biol. Chem. 1996;271:23611–23614. doi: 10.1074/jbc.271.39.23611. [DOI] [PubMed] [Google Scholar]
- 15.Loijens J. C., Anderson R. A. Type I phosphatidylinositol-4-phosphate 5-kinases are distinct members of this novel lipid kinase family. J. Biol. Chem. 1996;271:32937–32943. doi: 10.1074/jbc.271.51.32937. [DOI] [PubMed] [Google Scholar]
- 16.Ishihara H., Shibasaki Y., Kizuki N., Wada T., Yazaki Y., Asano T., Oka Y. Type I phosphatidylinositol-4-phosphate 5-kinases: cloning of the third isoform and deletion/substitution analysis of members of this novel lipid kinase family. J. Biol. Chem. 1998;273:8741–8748. doi: 10.1074/jbc.273.15.8741. [DOI] [PubMed] [Google Scholar]
- 17.Itoh T., Ijuin T., Takenawa T. A novel phosphatidylinositol-5-phosphate 4-kinase (phosphatidylinositol-phosphate kinase IIγ) is phosphorylated in the endoplasmic reticulum in response to mitogenic signals. J. Biol. Chem. 1998;273:20292–20299. doi: 10.1074/jbc.273.32.20292. [DOI] [PubMed] [Google Scholar]
- 18.Boronenkov I. V., Loijens J. C., Umeda M., Anderson R. A. Phosphoinositide signaling pathways in nuclei are associated with nuclear speckles containing pre-mRNA processing factors. Mol. Biol. Cell. 1998;9:3547–3560. doi: 10.1091/mbc.9.12.3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kunz J., Wilson M. P., Kisseleva M., Hurley J. H., Majerus P. W., Anderson R. A. The activation loop of phosphatidylinositol phosphate kinases determines signaling specificity. Mol. Cell. 2000;5:1–11. doi: 10.1016/s1097-2765(00)80398-6. [DOI] [PubMed] [Google Scholar]
- 20.Doughman R. L., Firestone A. J., Wojtasiak M. L., Bunce M. W., Anderson R. A. Membrane ruffling requires coordination between type Iα phosphatidylinositol phosphate kinase and Rac signaling. J. Biol. Chem. 2003;278:23036–23045. doi: 10.1074/jbc.M211397200. [DOI] [PubMed] [Google Scholar]
- 21.Oude Weernink P. A., Schmidt M., Jakobs K. H. Regulation and cellular roles of phosphoinositide 5-kinases. Eur. J. Pharmacol. 2004;500:87–99. doi: 10.1016/j.ejphar.2004.07.014. [DOI] [PubMed] [Google Scholar]
- 22.Yang S. A., Carpenter C. L., Abrams C. S. Rho and Rho-kinase mediate thrombin-induced phosphatidylinositol 4-phosphate 5-kinase trafficking in platelets. J. Biol. Chem. 2004;279:42331–42336. doi: 10.1074/jbc.M404335200. [DOI] [PubMed] [Google Scholar]
- 23.Honda A., Nogami M., Yokozeki T., Yamazaki M., Nakamura H., Watanabe H., Kawamoto K., Nakayama K., Morris A. J., Frohman M. A., Kanaho Y. Phosphatidylinositol 4-phosphate 5-kinase α is a downstream effector of the small G protein ARF6 in membrane ruffle formation. Cell. 1999;99:521–532. doi: 10.1016/s0092-8674(00)81540-8. [DOI] [PubMed] [Google Scholar]
- 24.Nishioka H., Horiuchi H., Tabuchi A., Yoshioka A., Shirakawa R., Kita T. Small GTPase Rho regulates thrombin-induced platelet aggregation. Biochem. Biophys. Res. Commun. 2001;280:970–975. doi: 10.1006/bbrc.2001.4237. [DOI] [PubMed] [Google Scholar]
- 25.Jenkins G. H., Fisette P. L., Anderson R. A. Type I phosphatidylinositol 4-phosphate 5-kinase isoforms are specifically stimulated by phosphatidic acid. J. Biol. Chem. 1994;269:11547–11554. [PubMed] [Google Scholar]
- 26.Jones D. R., Sanjuan M. A., Merida I. Type Iα phosphatidylinositol 4-phosphate 5-kinase is a putative target for increased intracellular phosphatidic acid. FEBS Lett. 2000;476:160–165. doi: 10.1016/s0014-5793(00)01702-6. [DOI] [PubMed] [Google Scholar]
- 27.Divecha N., Roefs M., Halstead J. R., D'Andrea S., Fernandez-Borga M., Oomen L., Saqib K. M., Wakelam M. J., D'Santos C. Interaction of the type Iα PIPkinase with phospholipase D: a role for the local generation of phosphatidylinositol 4,5-bisphosphate in the regulation of PLD2 activity. EMBO J. 2000;19:5440–5449. doi: 10.1093/emboj/19.20.5440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Powner D. J., Wakelam M. J. The regulation of phospholipase D by inositol phospholipids and small GTPases. FEBS Lett. 2002;531:62–64. doi: 10.1016/s0014-5793(02)03410-5. [DOI] [PubMed] [Google Scholar]
- 29.Powner D. J., Payne R. M., Pettitt T. R., Giudici M. L., Irvine R. F., Wakelam M. J. Phospholipase D2 stimulates integrin-mediated adhesion via phosphatidylinositol 4-phosphate 5-kinase Iγb. J. Cell Sci. 2005;118:2975–2986. doi: 10.1242/jcs.02432. [DOI] [PubMed] [Google Scholar]
- 30.Ling K., Doughman R. L., Firestone A. J., Bunce M. W., Anderson R. A. Type Iγ phosphatidylinositol phosphate kinase targets and regulates focal adhesions. Nature (London) 2002;420:89–93. doi: 10.1038/nature01082. [DOI] [PubMed] [Google Scholar]
- 31.Takai Y., Sasaki T., Matozaki T. Small GTP-binding proteins. Physiol. Rev. 2001;81:153–208. doi: 10.1152/physrev.2001.81.1.153. [DOI] [PubMed] [Google Scholar]
- 32.McTaggart S. J. Isoprenylated proteins. Cell. Mol. Life Sci. 2006;63:255–267. doi: 10.1007/s00018-005-5298-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang F. L., Casey P. J. Protein prenylation: molecular mechanisms and functional consequences. Annu. Rev. Biochem. 1996;65:241–269. doi: 10.1146/annurev.bi.65.070196.001325. [DOI] [PubMed] [Google Scholar]
- 34.Meller N., Merlot S., Guda C. CZH proteins: a new family of Rho-GEFs. J. Cell Sci. 2005;118:4937–4946. doi: 10.1242/jcs.02671. [DOI] [PubMed] [Google Scholar]
- 35.Erickson J. W., Cerione R. A. Structural elements, mechanism, and evolutionary convergence of Rho protein-guanine nucleotide exchange factor complexes. Biochemistry. 2004;43:837–842. doi: 10.1021/bi036026v. [DOI] [PubMed] [Google Scholar]
- 36.Moon S. Y., Zheng Y. Rho GTPase-activating proteins in cell regulation. Trends Cell Biol. 2003;13:13–22. doi: 10.1016/s0962-8924(02)00004-1. [DOI] [PubMed] [Google Scholar]
- 37.Dovas A., Couchman J. R. RhoGDI: multiple functions in the regulation of Rho family GTPase activities. Biochem. J. 2005;390:1–9. doi: 10.1042/BJ20050104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Burridge K., Wennerberg K. Rho and Rac take center stage. Cell. 2004;116:167–179. doi: 10.1016/s0092-8674(04)00003-0. [DOI] [PubMed] [Google Scholar]
- 39.Schmidt A., Hall A. Guanine nucleotide exchange factors for Rho GTPases: turning on the switch. Genes Dev. 2002;16:1587–1609. doi: 10.1101/gad.1003302. [DOI] [PubMed] [Google Scholar]
- 40.Mertens A. E., Roovers R. C., Collard J. G. Regulation of Tiam1-Rac signalling. FEBS Lett. 2003;546:11–16. doi: 10.1016/s0014-5793(03)00435-6. [DOI] [PubMed] [Google Scholar]
- 41.Cote J. F., Motoyama A. B., Bush J. A., Vuori K. A novel and evolutionarily conserved PtdIns(3,4,5)P3-binding domain is necessary for DOCK180 signalling. Nat. Cell Biol. 2005;7:797–807. doi: 10.1038/ncb1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tolias K. F., Cantley L. C., Carpenter C. L. Rho family GTPases bind to phosphoinositide kinases. J. Biol. Chem. 1995;270:17656–17659. doi: 10.1074/jbc.270.30.17656. [DOI] [PubMed] [Google Scholar]
- 43.Randazzo P. A. Functional interaction of ADP-ribosylation factor 1 with phosphatidylinositol 4,5-bisphosphate. J. Biol. Chem. 1997;272:7688–7692. [PubMed] [Google Scholar]
- 44.Macia E., Paris S., Chabre M. Binding of the PH and polybasic C-terminal domains of ARNO to phosphoinositides and to acidic lipids. Biochemistry. 2000;39:5893–5901. doi: 10.1021/bi992795w. [DOI] [PubMed] [Google Scholar]
- 45.Paris S., Beraud-Dufour S., Robineau S., Bigay J., Antonny B., Chabre M., Chardin P. Role of protein–phospholipid interactions in the activation of ARF1 by the guanine nucleotide exchange factor Arno. J. Biol. Chem. 1997;272:22221–22226. doi: 10.1074/jbc.272.35.22221. [DOI] [PubMed] [Google Scholar]
- 46.Ridley A. J., Hall A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell. 1992;70:389–399. doi: 10.1016/0092-8674(92)90163-7. [DOI] [PubMed] [Google Scholar]
- 47.Chong L. D., Traynor-Kaplan A., Bokoch G. M., Schwartz M. A. The small GTP-binding protein Rho regulates a phosphatidylinositol 4-phosphate 5-kinase in mammalian cells. Cell. 1994;79:507–513. doi: 10.1016/0092-8674(94)90259-3. [DOI] [PubMed] [Google Scholar]
- 48.Oude Weernink P. A., Schulte P., Guo Y., Wetzel J., Amano M., Kaibuchi K., Haverland S., Voss M., Schmidt M., Mayr G. W., Jakobs K. H. Stimulation of phosphatidylinositol-4-phosphate 5-kinase by Rho-kinase. J. Biol. Chem. 2000;275:10168–10174. doi: 10.1074/jbc.275.14.10168. [DOI] [PubMed] [Google Scholar]
- 49.Schmidt M., Bienek C., Rumenapp U., Zhang C., Lummen G., Jakobs K. H., Just I., Aktories K., Moos M., von Eichel-Streiber C. A role for Rho in receptor- and G protein-stimulated phospholipase C: reduction in phosphatidylinositol 4,5-bisphosphate by Clostridium difficile toxin B. Naunyn Schmiedebergs Arch. Pharmacol. 1996;354:87–94. doi: 10.1007/BF00178707. [DOI] [PubMed] [Google Scholar]
- 50.Schmidt M., Rumenapp U., Bienek C., Keller J., von Eichel-Streiber C., Jakobs K. H. Inhibition of receptor signaling to phospholipase D by Clostridium difficile toxin B: role of Rho proteins. J. Biol. Chem. 1996;271:2422–2426. doi: 10.1074/jbc.271.5.2422. [DOI] [PubMed] [Google Scholar]
- 51.Ren X. D., Bokoch G. M., Traynor-Kaplan A., Jenkins G. H., Anderson R. A., Schwartz M. A. Physical association of the small GTPase Rho with a 68-kDa phosphatidylinositol 4-phosphate 5-kinase in Swiss 3T3 cells. Mol. Biol. Cell. 1996;7:435–442. doi: 10.1091/mbc.7.3.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Amano M., Chihara K., Kimura K., Fukata Y., Nakamura N., Matsuura Y., Kaibuchi K. Formation of actin stress fibers and focal adhesions enhanced by Rho-kinase. Science. 1997;275:1308–1311. doi: 10.1126/science.275.5304.1308. [DOI] [PubMed] [Google Scholar]
- 53.Yamamoto M., Hilgemann D. H., Feng S., Bito H., Ishihara H., Shibasaki Y., Yin H. L. Phosphatidylinositol 4,5-bisphosphate induces actin stress-fiber formation and inhibits membrane ruffling in CV1 cells. J. Cell Biol. 2001;152:867–876. doi: 10.1083/jcb.152.5.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gratacap M. P., Payrastre B., Nieswandt B., Offermanns S. Differential regulation of Rho and Rac through heterotrimeric G-proteins and cyclic nucleotides. J. Biol. Chem. 2001;276:47906–47913. doi: 10.1074/jbc.M104442200. [DOI] [PubMed] [Google Scholar]
- 55.Ling K., Doughman R. L., Iyer V. V., Firestone A. J., Bairstow S. F., Mosher D. F., Schaller M. D., Anderson R. A. Tyrosine phosphorylation of type Iγ phosphatidylinositol phosphate kinase by Src regulates an integrin–talin switch. J. Cell Biol. 2003;163:1339–1349. doi: 10.1083/jcb.200310067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yamazaki M., Miyazaki H., Watanabe H., Sasaki T., Maehama T., Frohman M. A., Kanaho Y. Phosphatidylinositol 4-phosphate 5-kinase is essential for ROCK-mediated neurite remodeling. J. Biol. Chem. 2002;277:17226–17230. doi: 10.1074/jbc.M109795200. [DOI] [PubMed] [Google Scholar]
- 57.Matsui T., Yonemura S., Tsukita S., Tsukita S. Activation of ERM proteins in vivo by Rho involves phosphatidyl-inositol 4-phosphate 5-kinase and not ROCK kinases. Curr. Biol. 1999;9:1259–1262. doi: 10.1016/s0960-9822(99)80508-9. [DOI] [PubMed] [Google Scholar]
- 58.Bretscher A., Edwards K., Fehon R. G. ERM proteins and merlin: integrators at the cell cortex. Nat. Rev. Mol. Cell Biol. 2002;3:586–599. doi: 10.1038/nrm882. [DOI] [PubMed] [Google Scholar]
- 59.Ridley A. J., Paterson H. F., Johnston C. L., Diekmann D., Hall A. The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell. 1992;70:401–410. doi: 10.1016/0092-8674(92)90164-8. [DOI] [PubMed] [Google Scholar]
- 60.Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279:509–514. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- 61.Tolias K. F., Couvillon A. D., Cantley L. C., Carpenter C. L. Characterization of a Rac1- and RhoGDI-associated lipid kinase signaling complex. Mol. Cell. Biol. 1998;18:762–770. doi: 10.1128/mcb.18.2.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tolias K. F., Hartwig J. H., Ishihara H., Shibasaki Y., Cantley L. C., Carpenter C. L. Type Iα phosphatidylinositol-4-phosphate 5-kinase mediates Rac-dependent actin assembly. Curr. Biol. 2000;10:153–156. doi: 10.1016/s0960-9822(00)00315-8. [DOI] [PubMed] [Google Scholar]
- 63.van Hennik P. B., ten Klooster J. P., Halstead J. R., Voermans C., Anthony E. C., Divecha N., Hordijk P. L. The C-terminal domain of Rac1 contains two motifs that control targeting and signaling specificity. J. Biol. Chem. 2003;278:39166–39175. doi: 10.1074/jbc.M307001200. [DOI] [PubMed] [Google Scholar]
- 64.Weernink P. A., Meletiadis K., Hommeltenberg S., Hinz M., Ishihara H., Schmidt M., Jakobs K. H. Activation of type I phosphatidylinositol 4-phosphate 5-kinase isoforms by the Rho GTPases, RhoA, Rac1, and Cdc42. J. Biol. Chem. 2004;279:7840–7849. doi: 10.1074/jbc.M312737200. [DOI] [PubMed] [Google Scholar]
- 65.Hartwig J. H., Bokoch G. M., Carpenter C. L., Janmey P. A., Taylor L. A., Toker A., Stossel T. P. Thrombin receptor ligation and activated Rac uncap actin filament barbed ends through phosphoinositide synthesis in permeabilized human platelets. Cell. 1995;82:643–653. doi: 10.1016/0092-8674(95)90036-5. [DOI] [PubMed] [Google Scholar]
- 66.Doughman R. L., Firestone A. J., Anderson R. A. Phosphatidylinositol phosphate kinases put PI4,5P2 in its place. J. Membr. Biol. 2003;194:77–89. doi: 10.1007/s00232-003-2027-7. [DOI] [PubMed] [Google Scholar]
- 67.Chatah N. E., Abrams C. S. G-protein-coupled receptor activation induces the membrane translocation and activation of phosphatidylinositol-4-phosphate 5-kinase Iα by a Rac- and Rho-dependent pathway. J. Biol. Chem. 2001;276:34059–34065. doi: 10.1074/jbc.M104917200. [DOI] [PubMed] [Google Scholar]
- 68.Kozma R., Ahmed S., Best A., Lim L. The Ras-related protein Cdc42Hs and bradykinin promote formation of peripheral actin microspikes and filopodia in Swiss 3T3 fibroblasts. Mol. Cell. Biol. 1995;15:1942–1952. doi: 10.1128/mcb.15.4.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nobes C. D., Hall A. Rho, rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell. 1995;81:53–62. doi: 10.1016/0092-8674(95)90370-4. [DOI] [PubMed] [Google Scholar]
- 70.Rohatgi R., Ma L., Miki H., Lopez M., Kirchhausen T., Takenawa T., Kirschner M. W. The interaction between N-WASP and the Arp2/3 complex links Cdc42-dependent signals to actin assembly. Cell. 1999;97:221–231. doi: 10.1016/s0092-8674(00)80732-1. [DOI] [PubMed] [Google Scholar]
- 71.Kahn R. A., Volpicelli-Daley L., Bowzard B., Shrivastava-Ranjan P., Li Y., Zhou C., Cunningham L. Arf family GTPases: roles in membrane traffic and microtubule dynamics. Biochem. Soc. Trans. 2005;33:1269–1272. doi: 10.1042/BST0331269. [DOI] [PubMed] [Google Scholar]
- 72.Brown F. D., Rozelle A. L., Yin H. L., Balla T., Donaldson J. G. Phosphatidylinositol 4,5-bisphosphate and Arf6-regulated membrane traffic. J. Cell Biol. 2001;154:1007–1017. doi: 10.1083/jcb.200103107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Donaldson J. G. Multiple roles for Arf6: sorting, structuring, and signaling at the plasma membrane. J. Biol. Chem. 2003;278:41573–41576. doi: 10.1074/jbc.R300026200. [DOI] [PubMed] [Google Scholar]
- 74.Donaldson J. G., Honda A. Localization and function of Arf family GTPases. Biochem. Soc. Trans. 2005;33:639–642. doi: 10.1042/BST0330639. [DOI] [PubMed] [Google Scholar]
- 75.Takenawa T., Itoh T. Phosphoinositides, key molecules for regulation of actin cytoskeletal organization and membrane traffic from the plasma membrane. Biochim. Biophys. Acta. 2001;1533:190–206. doi: 10.1016/s1388-1981(01)00165-2. [DOI] [PubMed] [Google Scholar]
- 76.Brown H. A., Gutowski S., Moomaw C. R., Slaughter C., Sternweis P. C. ADP-ribosylation factor, a small GTP-dependent regulatory protein, stimulates phospholipase D activity. Cell. 1993;75:1137–1144. doi: 10.1016/0092-8674(93)90323-i. [DOI] [PubMed] [Google Scholar]
- 77.Cockcroft S., Thomas G. M., Fensome A., Geny B., Cunningham E., Gout I., Hiles I., Totty N. F., Truong O., Hsuan J. J. Phospholipase D: a downstream effector of ARF in granulocytes. Science. 1994;263:523–526. doi: 10.1126/science.8290961. [DOI] [PubMed] [Google Scholar]
- 78.Donaldson J. G., Jackson C. L. Regulators and effectors of the ARF GTPases. Curr. Opin. Cell Biol. 2000;12:475–482. doi: 10.1016/s0955-0674(00)00119-8. [DOI] [PubMed] [Google Scholar]
- 79.Jones D. H., Morris J. B., Morgan C. P., Kondo H., Irvine R. F., Cockcroft S. Type I phosphatidylinositol 4-phosphate 5-kinase directly interacts with ADP-ribosylation factor 1 and is responsible for phosphatidylinositol 4,5-bisphosphate synthesis in the Golgi compartment. J. Biol. Chem. 2000;275:13962–13966. doi: 10.1074/jbc.c901019199. [DOI] [PubMed] [Google Scholar]
- 80.Godi A., Pertile P., Meyers R., Marra P., Di Tullio G., Iurisci C., Luini A., Corda D., De Matteis M. A. ARF mediates recruitment of PtdIns-4-OH kinase-β and stimulates synthesis of PtdIns(4,5)P2 on the Golgi complex. Nat. Cell Biol. 1999;1:280–287. doi: 10.1038/12993. [DOI] [PubMed] [Google Scholar]
- 81.Skippen A., Jones D. H., Morgan C. P., Li M., Cockcroft S. Mechanism of ADP ribosylation factor-stimulated phosphatidylinositol 4,5-bisphosphate synthesis in HL60 cells. J. Biol. Chem. 2002;277:5823–5831. doi: 10.1074/jbc.M110274200. [DOI] [PubMed] [Google Scholar]
- 82.Lawrence J. T., Birnbaum M. J. ADP-ribosylation factor 6 regulates insulin secretion through plasma membrane phosphatidylinositol 4,5-bisphosphate. Proc. Natl. Acad. Sci. U.S.A. 2003;100:13320–13325. doi: 10.1073/pnas.2232129100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Radhakrishna H., Al-Awar O., Khachikian Z., Donaldson J. G. ARF6 requirement for Rac ruffling suggests a role for membrane trafficking in cortical actin rearrangements. J. Cell Sci. 1999;112:855–866. doi: 10.1242/jcs.112.6.855. [DOI] [PubMed] [Google Scholar]
- 84.Santy L. C., Casanova J. E. Activation of ARF6 by ARNO stimulates epithelial cell migration through downstream activation of both Rac1 and phospholipase D. J. Cell Biol. 2001;154:599–610. doi: 10.1083/jcb.200104019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Franco M., Peters P. J., Boretto J., van Donselaar E., Neri A., D'Souza-Schorey C., Chavrier P. EFA6, a sec7 domain-containing exchange factor for ARF6, coordinates membrane recycling and actin cytoskeleton organization. EMBO J. 1999;18:1480–1491. doi: 10.1093/emboj/18.6.1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Krauss M., Kinuta M., Wenk M. R., De Camilli P., Takei K., Haucke V. ARF6 stimulates clathrin/AP-2 recruitment to synaptic membranes by activating phosphatidylinositol phosphate kinase type Iγ. J. Cell Biol. 2003;162:113–124. doi: 10.1083/jcb.200301006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yin H. L., Janmey P. A. Phosphoinositide regulation of the actin cytoskeleton. Annu. Rev. Physiol. 2003;65:761–789. doi: 10.1146/annurev.physiol.65.092101.142517. [DOI] [PubMed] [Google Scholar]
- 88.De Matteis M. A., Godi A. PI-loting membrane traffic. Nat. Cell Biol. 2004;6:487–492. doi: 10.1038/ncb0604-487. [DOI] [PubMed] [Google Scholar]
- 89.Wenk M. R., De Camilli P. Protein–lipid interactions and phosphoinositide metabolism in membrane traffic: insights from vesicle recycling in nerve terminals. Proc. Natl. Acad. Sci. U.S.A. 2004;101:8262–8269. doi: 10.1073/pnas.0401874101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hernandez-Deviez D. J., Roth M. G., Casanova J. E., Wilson J. M. ARNO and ARF6 regulate axonal elongation and branching through downstream activation of phosphatidylinositol 4-phosphate 5-kinase α. Mol. Biol. Cell. 2004;15:111–120. doi: 10.1091/mbc.E03-06-0410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wong K. W., Isberg R. R. Arf6 and phosphoinositol-4-phosphate-5-kinase activities permit bypass of the Rac1 requirement for β1 integrin-mediated bacterial uptake. J. Exp. Med. 2003;198:603–614. doi: 10.1084/jem.20021363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Balana M. E., Niedergang F., Subtil A., Alcover A., Chavrier P., Dautry-Varsat A. ARF6 GTPase controls bacterial invasion by actin remodelling. J. Cell Sci. 2005;118:2201–2210. doi: 10.1242/jcs.02351. [DOI] [PubMed] [Google Scholar]
- 93.Galandrini R., Micucci F., Tassi I., Cifone M. G., Cinque B., Piccoli M., Frati L., Santoni A. Arf6: a new player in FcγRIIIA lymphocyte-mediated cytotoxicity. Blood. 2005;106:577–583. doi: 10.1182/blood-2004-10-4100. [DOI] [PubMed] [Google Scholar]
- 94.Caroni P. New EMBO members' review: actin cytoskeleton regulation through modulation of PI(4,5)P2 rafts. EMBO J. 2001;20:4332–4336. doi: 10.1093/emboj/20.16.4332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wang Y. J., Li W. H., Wang J., Xu K., Dong P., Luo X., Yin H. L. Critical role of PIP5KIγ87 in InsP3-mediated Ca2+ signaling. J. Cell Biol. 2004;167:1005–1010. doi: 10.1083/jcb.200408008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lassing I., Lindberg U. Polyphosphoinositide synthesis in platelets stimulated with low concentrations of thrombin is enhanced before the activation of phospholipase C. FEBS Lett. 1990;262:231–233. doi: 10.1016/0014-5793(90)80197-q. [DOI] [PubMed] [Google Scholar]
- 97.Grondin P., Plantavid M., Sultan C., Breton M., Mauco G., Chap H. Interaction of pp60c-src, phospholipase C, inositol-lipid, and diacyglycerol kinases with the cytoskeletons of thrombin-stimulated platelets. J. Biol. Chem. 1991;266:15705–15709. [PubMed] [Google Scholar]
- 98.Hinchliffe K. A., Irvine R. F., Divecha N. Aggregation-dependent, integrin-mediated increases in cytoskeletally associated PtdInsP2 (4,5) levels in human platelets are controlled by translocation of PtdIns 4-P 5-kinase C to the cytoskeleton. EMBO J. 1996;15:6516–6524. [PMC free article] [PubMed] [Google Scholar]
- 99.Higgs H. N., Pollard T. D. Regulation of actin filament network formation through ARP2/3 complex: activation by a diverse array of proteins. Annu. Rev. Biochem. 2001;70:649–676. doi: 10.1146/annurev.biochem.70.1.649. [DOI] [PubMed] [Google Scholar]
- 100.Takenawa T., Miki H. WASP and WAVE family proteins: key molecules for rapid rearrangement of cortical actin filaments and cell movement. J. Cell Sci. 2001;114:1801–1809. doi: 10.1242/jcs.114.10.1801. [DOI] [PubMed] [Google Scholar]
- 101.Azim A. C., Barkalow K., Chou J., Hartwig J. H. Activation of the small GTPases, rac and cdc42, after ligation of the platelet PAR-1 receptor. Blood. 2000;95:959–964. [PubMed] [Google Scholar]
- 102.Hoffman G. R., Nassar N., Cerione R. A. Structure of the Rho family GTP-binding protein Cdc42 in complex with the multifunctional regulator RhoGDI. Cell. 2000;100:345–356. doi: 10.1016/s0092-8674(00)80670-4. [DOI] [PubMed] [Google Scholar]
- 103.DerMardirossian C., Bokoch G. M. GDIs: central regulatory molecules in Rho GTPase activation. Trends Cell Biol. 2005;15:356–363. doi: 10.1016/j.tcb.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 104.Dransart E., Olofsson B., Cherfils J. RhoGDIs revisited: novel roles in Rho regulation. Traffic. 2005;6:957–966. doi: 10.1111/j.1600-0854.2005.00335.x. [DOI] [PubMed] [Google Scholar]
- 105.Robbe K., Otto-Bruc A., Chardin P., Antonny B. Dissociation of GDP dissociation inhibitor and membrane translocation are required for efficient activation of Rac by the Dbl homology-pleckstrin homology region of Tiam. J. Biol. Chem. 2003;278:4756–4762. doi: 10.1074/jbc.M210412200. [DOI] [PubMed] [Google Scholar]
- 106.Del Pozo M. A., Kiosses W. B., Alderson N. B., Meller N., Hahn K. M., Schwartz M. A. Integrins regulate GTP-Rac localized effector interactions through dissociation of Rho-GDI. Nat. Cell Biol. 2002;4:232–239. doi: 10.1038/ncb759. [DOI] [PubMed] [Google Scholar]
- 107.Hancock J. F., Paterson H., Marshall C. J. A polybasic domain or palmitoylation is required in addition to the CAAX motif to localize p21ras to the plasma membrane. Cell. 1990;63:133–139. doi: 10.1016/0092-8674(90)90294-o. [DOI] [PubMed] [Google Scholar]
- 108.Colley W. C., Sung T. C., Roll R., Jenco J., Hammond S. M., Altshuller Y., Bar-Sagi D., Morris A. J., Frohman M. A. Phospholipase D2, a distinct phospholipase D isoform with novel regulatory properties that provokes cytoskeletal reorganization. Curr. Biol. 1997;7:191–201. doi: 10.1016/s0960-9822(97)70090-3. [DOI] [PubMed] [Google Scholar]
- 109.Hammond S. M., Jenco J. M., Nakashima S., Cadwallader K., Gu Q., Cook S., Nozawa Y., Prestwich G. D., Frohman M. A., Morris A. J. Characterization of two alternately spliced forms of phospholipase D1: activation of the purified enzymes by phosphatidylinositol 4,5-bisphosphate, ADP-ribosylation factor, and Rho family monomeric GTP-binding proteins and protein kinase C-α. J. Biol. Chem. 1997;272:3860–3868. doi: 10.1074/jbc.272.6.3860. [DOI] [PubMed] [Google Scholar]
- 110.Hodgkin M. N., Masson M. R., Powner D., Saqib K. M., Ponting C. P., Wakelam M. J. Phospholipase D regulation and localisation is dependent upon a phosphatidylinositol 4,5-biphosphate-specific PH domain. Curr. Biol. 2000;10:43–46. doi: 10.1016/s0960-9822(99)00264-x. [DOI] [PubMed] [Google Scholar]
- 111.Kooijman E. E., Chupin V., Fuller N. L., Kozlov M. M., de Kruijff B., Burger K. N., Rand P. R. Spontaneous curvature of phosphatidic acid and lysophosphatidic acid. Biochemistry. 2005;44:2097–2102. doi: 10.1021/bi0478502. [DOI] [PubMed] [Google Scholar]
- 112.Le Roy C., Wrana J. L. Clathrin- and non-clathrin-mediated endocytic regulation of cell signalling. Nat. Rev. Mol. Cell Biol. 2005;6:112–126. doi: 10.1038/nrm1571. [DOI] [PubMed] [Google Scholar]
- 113.Liscovitch M., Cantley L. C. Signal transduction and membrane traffic: the PITP/phosphoinositide connection. Cell. 1995;81:659–662. doi: 10.1016/0092-8674(95)90525-1. [DOI] [PubMed] [Google Scholar]
- 114.Haucke V. Phosphoinositide regulation of clathrin-mediated endocytosis. Biochem. Soc. Trans. 2005;33:1285–1289. doi: 10.1042/BST0331285. [DOI] [PubMed] [Google Scholar]
- 115.Homma K., Terui S., Minemura M., Qadota H., Anraku Y., Kanaho Y., Ohya Y. Phosphatidylinositol-4-phosphate 5-kinase localized on the plasma membrane is essential for yeast cell morphogenesis. J. Biol. Chem. 1998;273:15779–15786. doi: 10.1074/jbc.273.25.15779. [DOI] [PubMed] [Google Scholar]
- 116.Desrivieres S., Cooke F. T., Parker P. J., Hall M. N. MSS4, a phosphatidylinositol-4-phosphate 5-kinase required for organization of the actin cytoskeleton in Saccharomyces cerevisiae. J. Biol. Chem. 1998;273:15787–15793. doi: 10.1074/jbc.273.25.15787. [DOI] [PubMed] [Google Scholar]
- 117.Kobayashi T., Takematsu H., Yamaji T., Hiramoto S., Kozutsumi Y. Disturbance of sphingolipid biosynthesis abrogates the signaling of Mss4, phosphatidylinositol-4-phosphate 5-kinase, in yeast. J. Biol. Chem. 2005;280:18087–18094. doi: 10.1074/jbc.M414138200. [DOI] [PubMed] [Google Scholar]
- 118.Lorberg A., Jacoby J. J., Schmitz H. P., Heinisch J. J. The PH domain of the yeast GEF Rom2p serves an essential function in vivo. Mol. Genet. Genomics. 2001;266:505–513. doi: 10.1007/s004380100579. [DOI] [PubMed] [Google Scholar]
- 119.Levin D. E. Cell wall integrity signaling in Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 2005;69:262–291. doi: 10.1128/MMBR.69.2.262-291.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Calonge T. M., Nakano K., Arellano M., Arai R., Katayama S., Toda T., Mabuchi I., Perez P. Schizosaccharomyces pombe rho2p GTPase regulates cell wall α-glucan biosynthesis through the protein kinase pck2p. Mol. Biol. Cell. 2000;11:4393–4401. doi: 10.1091/mbc.11.12.4393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Arellano M., Duran A., Perez P. Localisation of the Schizosaccharomyces pombe rho1p GTPase and its involvement in the organisation of the actin cytoskeleton. J. Cell Sci. 1997;110:2547–2555. doi: 10.1242/jcs.110.20.2547. [DOI] [PubMed] [Google Scholar]
- 122.Zhang Y., Sugiura R., Lu Y., Asami M., Maeda T., Itoh T., Takenawa T., Shuntoh H., Kuno T. Phosphatidylinositol 4-phosphate 5-kinase Its3 and calcineurin Ppb1 coordinately regulate cytokinesis in fission yeast. J. Biol. Chem. 2000;275:35600–35606. doi: 10.1074/jbc.M005575200. [DOI] [PubMed] [Google Scholar]
- 123.Deng L., Sugiura R., Ohta K., Tada K., Suzuki M., Hirata M., Nakamura S., Shuntoh H., Kuno T. Phosphatidylinositol-4-phosphate 5-kinase regulates fission yeast cell integrity through a phospholipase C-mediated protein kinase C-independent pathway. J. Biol. Chem. 2005;280:27561–27568. doi: 10.1074/jbc.M502660200. [DOI] [PubMed] [Google Scholar]
- 124.Mutoh T., Nakano K., Mabuchi I. Rho1-GEFs Rgf1 and Rgf2 are involved in formation of cell wall and septum, while Rgf3 is involved in cytokinesis in fission yeast. Genes Cells. 2005;10:1189–1202. doi: 10.1111/j.1365-2443.2005.00908.x. [DOI] [PubMed] [Google Scholar]
- 125.Garcia P., Tajadura V., Garcia I., Sanchez Y. Rgf1p is a specific Rho1-GEF that coordinates cell polarization with cell wall biogenesis in fission yeast. Mol. Biol. Cell. 2006;17:1620–1631. doi: 10.1091/mbc.E05-10-0933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Yamamoto A., DeWald D. B., Boronenkov I. V., Anderson R. A., Emr S. D., Koshland D. Novel PI(4)P 5-kinase homologue, Fab1p, essential for normal vacuole function and morphology in yeast. Mol. Biol. Cell. 1995;6:525–539. doi: 10.1091/mbc.6.5.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Gary J. D., Wurmser A. E., Bonangelino C. J., Weisman L. S., Emr S. D. Fab1p is essential for PtdIns(3)P 5-kinase activity and the maintenance of vacuolar size and membrane homeostasis. J. Cell Biol. 1998;143:65–79. doi: 10.1083/jcb.143.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.McEwen R. K., Dove S. K., Cooke F. T., Painter G. F., Holmes A. B., Shisheva A., Ohya Y., Parker P. J., Michell R. H. Complementation analysis in PtdInsP kinase-deficient yeast mutants demonstrates that Schizosaccharomyces pombe and murine Fab1p homologues are phosphatidylinositol 3-phosphate 5-kinases. J. Biol. Chem. 1999;274:33905–33912. doi: 10.1074/jbc.274.48.33905. [DOI] [PubMed] [Google Scholar]
- 129.Cooke F. T., Dove S. K., McEwen R. K., Painter G., Holmes A. B., Hall M. N., Michell R. H., Parker P. J. The stress-activated phosphatidylinositol 3-phosphate 5-kinase Fab1p is essential for vacuole function in S. cerevisiae. Curr. Biol. 1998;8:1219–1222. doi: 10.1016/s0960-9822(07)00513-1. [DOI] [PubMed] [Google Scholar]
- 130.DeWald D. B. Phosphoinositide signaling: vac to the future in fab1 kinase regulation. Curr. Biol. 2002;12:R491–492. doi: 10.1016/s0960-9822(02)00966-1. [DOI] [PubMed] [Google Scholar]
- 131.Bonangelino C. J., Nau J. J., Duex J. E., Brinkman M., Wurmser A. E., Gary J. D., Emr S. D., Weisman L. S. Osmotic stress-induced increase of phosphatidylinositol 3,5-bisphosphate requires Vac14p, an activator of the lipid kinase Fab1p. J. Cell Biol. 2002;156:1015–1028. doi: 10.1083/jcb.200201002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Odorizzi G., Babst M., Emr S. D. Fab1p PtdIns(3)P 5-kinase function essential for protein sorting in the multivesicular body. Cell. 1998;95:847–858. doi: 10.1016/s0092-8674(00)81707-9. [DOI] [PubMed] [Google Scholar]
- 133.Dove S. K., Piper R. C., McEwen R. K., Yu J. W., King M. C., Hughes D. C., Thuring J., Holmes A. B., Cooke F. T., Michell R. H., et al. Svp1p defines a family of phosphatidylinositol 3,5-bisphosphate effectors. EMBO J. 2004;23:1922–1933. doi: 10.1038/sj.emboj.7600203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Michell R. H., Heath V. L., Lemmon M. A., Dove S. K. Phosphatidylinositol 3,5-bisphosphate: metabolism and cellular functions. Trends Biochem. Sci. 2006;31:52–63. doi: 10.1016/j.tibs.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 135.Mollapour M., Phelan J. P., Millson S. H., Piper P. W., Cooke F. T. Weak acid and alkali stress regulate phosphatidylinositol bisphosphate synthesis in Saccharomyces cerevisiae. Biochem. J. 2006;395:73–80. doi: 10.1042/BJ20051765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Tong Q., Stockand J. D. Receptor tyrosine kinases mediate epithelial Na+ channel inhibition by epidermal growth factor. Am. J. Physiol. Renal Physiol. 2005;288:F150–F161. doi: 10.1152/ajprenal.00261.2004. [DOI] [PubMed] [Google Scholar]
- 137.Ma H. P., Saxena S., Warnock D. G. Anionic phospholipids regulate native and expressed epithelial sodium channel (ENaC) J. Biol. Chem. 2002;277:7641–7644. doi: 10.1074/jbc.C100737200. [DOI] [PubMed] [Google Scholar]
- 138.Yue G., Malik B., Yue G., Eaton D. C. Phosphatidylinositol 4,5-bisphosphate (PIP2) stimulates epithelial sodium channel activity in A6 cells. J. Biol. Chem. 2002;277:11965–11969. doi: 10.1074/jbc.M108951200. [DOI] [PubMed] [Google Scholar]
- 139.Staruschenko A., Nichols A., Medina J. L., Camacho P., Zheleznova N. N., Stockand J. D. Rho small GTPases activate the epithelial Na+ channel. J. Biol. Chem. 2004;279:49989–49994. doi: 10.1074/jbc.M409812200. [DOI] [PubMed] [Google Scholar]
- 140.Staruschenko A., Patel P., Tong Q., Medina J. L., Stockand J. D. Ras activates the epithelial Na+ channel through phosphoinositide 3-OH kinase signaling. J. Biol. Chem. 2004;279:37771–37778. doi: 10.1074/jbc.M402176200. [DOI] [PubMed] [Google Scholar]
- 141.Pochynyuk O., Staruschenko A., Tong Q., Medina J., Stockand J. D. Identification of a functional phosphatidylinositol 3,4,5-trisphosphate binding site in the epithelial Na+ channel. J. Biol. Chem. 2005;280:37565–37571. doi: 10.1074/jbc.M509071200. [DOI] [PubMed] [Google Scholar]
- 142.Rohacs T., Lopes C. M., Jin T., Ramdya P. P., Molnar Z., Logothetis D. E. Specificity of activation by phosphoinositides determines lipid regulation of Kir channels. Proc. Natl. Acad. Sci. U.S.A. 2003;100:745–750. doi: 10.1073/pnas.0236364100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.MacGregor G. G., Dong K., Vanoye C. G., Tang L., Giebisch G., Hebert S. C. Nucleotides and phospholipids compete for binding to the C terminus of KATP channels. Proc. Natl. Acad. Sci. U.S.A. 2002;99:2726–2731. doi: 10.1073/pnas.042688899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Enkvetchakul D., Jeliazkova I., Nichols C. G. Direct modulation of Kir channel gating by membrane phosphatidylinositol 4,5-bisphosphate. J. Biol. Chem. 2005;280:35785–35788. doi: 10.1074/jbc.C500355200. [DOI] [PubMed] [Google Scholar]
- 145.Rohacs T., Chen J., Prestwich G. D., Logothetis D. E. Distinct specificities of inwardly rectifying K+ channels for phosphoinositides. J. Biol. Chem. 1999;274:36065–36072. doi: 10.1074/jbc.274.51.36065. [DOI] [PubMed] [Google Scholar]
- 146.Du X., Zhang H., Lopes C., Mirshahi T., Rohacs T., Logothetis D. E. Characteristic interactions with phosphatidylinositol 4,5-bisphosphate determine regulation of Kir channels by diverse modulators. J. Biol. Chem. 2004;279:37271–37281. doi: 10.1074/jbc.M403413200. [DOI] [PubMed] [Google Scholar]
- 146a.Jones S. V. P. Role of the small GTPase Rho in modulation of the inwardly rectifying potassium channel Kir2.1. Mol. Pharmacol. 2003;64:987–993. doi: 10.1124/mol.64.4.987. [DOI] [PubMed] [Google Scholar]
- 147.Tong Y., Brandt G. S., Li M., Shapovalov G., Slimko E., Karschin A., Dougherty D. A., Lester H. A. Tyrosine decaging leads to substantial membrane trafficking during modulation of an inward rectifier potassium channel. J. Gen. Physiol. 2001;117:103–118. doi: 10.1085/jgp.117.2.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Giovannardi S., Forlani G., Balestrini M., Bossi E., Tonini R., Sturani E., Peres A., Zippel R. Modulation of the inward rectifier potassium channel IRK1 by the Ras signaling pathway. J. Biol. Chem. 2002;277:12158–12163. doi: 10.1074/jbc.M110466200. [DOI] [PubMed] [Google Scholar]
- 149.Cachero T. G., Morielli A. D., Peralta E. G. The small GTP-binding protein RhoA regulates a delayed rectifier potassium channel. Cell. 1998;93:1077–1085. doi: 10.1016/s0092-8674(00)81212-x. [DOI] [PubMed] [Google Scholar]
- 150.Wu L., Bauer C. S., Zhen X. G., Xie C., Yang J. Dual regulation of voltage-gated calcium channels by PtdIns(4,5)P2. Nature (London) 2002;419:947–952. doi: 10.1038/nature01118. [DOI] [PubMed] [Google Scholar]
- 151.Gamper N., Reznikov V., Yamada Y., Yang J., Shapiro M. S. Phosphatidylinositol 4,5-bisphosphate signals underlie receptor-specific Gq/11-mediated modulation of N-type Ca2+ channels. J. Neurosci. 2004;24:10980–10992. doi: 10.1523/JNEUROSCI.3869-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Beguin P., Nagashima K., Gonoi T., Shibasaki T., Takahashi K., Kashima Y., Ozaki N., Geering K., Iwanaga T., Seino S. Regulation of Ca2+ channel expression at the cell surface by the small G-protein Kir/Gem. Nature (London) 2001;411:701–706. doi: 10.1038/35079621. [DOI] [PubMed] [Google Scholar]
- 153.Zhang Z., Okawa H., Wang Y., Liman E. R. Phosphatidylinositol 4,5-bisphosphate rescues TRPM4 channels from desensitization. J. Biol. Chem. 2005;280:39185–39192. doi: 10.1074/jbc.M506965200. [DOI] [PubMed] [Google Scholar]
- 154.Liu D., Liman E. R. Intracellular Ca2+ and the phospholipid PIP2 regulate the taste transduction ion channel TRPM5. Proc. Natl. Acad. Sci. U.S.A. 2003;100:15160–15165. doi: 10.1073/pnas.2334159100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Liu B., Zhang C., Qin F. Functional recovery from desensitization of vanilloid receptor TRPV1 requires resynthesis of phosphatidylinositol 4,5-bisphosphate. J. Neurosci. 2005;25:4835–4843. doi: 10.1523/JNEUROSCI.1296-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Rohacs T., Lopes C. M., Michailidis I., Logothetis D. E. PI(4,5)P2 regulates the activation and desensitization of TRPM8 channels through the TRP domain. Nat. Neurosci. 2005;8:626–634. doi: 10.1038/nn1451. [DOI] [PubMed] [Google Scholar]
- 157.Ma R., Li W. P., Rundle D., Kong J., Akbarali H. I., Tsiokas L. PKD2 functions as an epidermal growth factor-activated plasma membrane channel. Mol. Cell. Biol. 2005;25:8285–8298. doi: 10.1128/MCB.25.18.8285-8298.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Bezzerides V. J., Ramsey I. S., Kotecha S., Greka A., Clapham D. E. Rapid vesicular translocation and insertion of TRP channels. Nat. Cell Biol. 2004;6:709–720. doi: 10.1038/ncb1150. [DOI] [PubMed] [Google Scholar]