Abstract
We show that LPA1 (lysophosphatidic acid receptor-1) is constitutively localized in the nucleus of mammalian cells. LPA1 also traffics from cell membranes to the nucleus in response to LPA (lysophosphatidic acid). Several lines of evidence suggest an important role for cell-matrix interaction in regulating the constitutive nuclear localization of LPA1. First, the RGDS peptide, which blocks cell matrix-induced integrin clustering and cytoskeletal rearrangement, reduced the number of cells containing LPA1 in the nucleus. Secondly, a higher proportion of cells contained nuclear LPA1 when adhesion on fibronectin-coated glass was compared with adherence to polylysine-coated glass. Thirdly, pre-treatment of cells with the Rho kinase inhibitor (Y27632) or the myosin light chain kinase inhibitor (ML9) reduced the number of cells containing nuclear LPA1. The addition of LPA and/or Ki16425 (which binds to LPA1) to isolated nuclei containing LPA1 induced the phosphorylation of several proteins with molecular masses of 34, 32, 14 and 11 kDa. These findings demonstrate that trafficking of LPA1 to the nucleus is influenced by cell-matrix interactions and that nuclear LPA1 may be involved in regulating intranuclear protein phosphorylation and signalling.
Keywords: cell matrix, integrin, lysophosphatidic acid receptor-1 (LPA1), nuclear protein phosphorylation, protean agonism
Abbreviations: BEGM, bronchial epithelium growth medium; CHO, Chinese hamster ovary; COX2, cyclo-oxgenase 2; DAPI, 4′,6-diamidino-2-phenylindole; DMEM, Dulbecco's modified Eagle's medium; FCS, foetal calf serum; GPCR, G-protein-coupled receptors; HBEC, human bronchial epithelial cell; iNOS, inducible nitric oxide synthase; LPA, lysophosphatidic acid; LPA1, lysophosphatidic acid receptor-1; MLCK, myosin light chain kinase; NGF, nerve growth factor; p42/p44 MAPK, p42/p44 mitogen-activated protein kinase; PLA2, phospholipase A2; PTX, pertussis toxin; siRNA, small interfering RNA
INTRODUCTION
The bioactive lipid LPA (lysophosphatidic acid) is produced by a lysophospholipase D (autotaxin) and can be dephosphorylated by lipid phosphate phosphatases [1,2]. LPA binds to a family of GPCR (G-protein-coupled receptors). Three of these are members of the endothelial differentiation gene family of receptors, which have been renamed LPA1–3 (lysophosphatidic acid receptor-1–3), whereas GPR23 (also known as LPA4) is a member of the purinergic GPCR cluster [1,3]. The LPA1 gene is located on chromosome 9p31.3-32 and encodes a 364-amino-acid protein [3,4]. LPA1 is coupled to effectors via the heterotrimeric G-protein Gi to inhibit adenylyl cyclase and to stimulate activation of the p42/p44 MAPK (p42/p44 mitogen-activated protein kinase) pathway linked to mitogenesis [1]. This receptor can also activate Rho-dependent signalling to modulate actin stress fibre formation [5]. LPA1 is widely expressed in embryonic neural tissue, where it may be involved in regulating neurogenesis. Targeted deletion of LPA1 caused neonatal lethality due to decreased suckling, possibly due to olfaction defects, and also caused craniofacial abnormalities [6].
Gobeil et al. [7] have shown that LPA1 is constitutively localized in the nucleus of porcine cerebral microvascular endothelial cells, and in rat hepatoma HTC4 cells and rat liver cells stably transfected with LPA1. Nuclear LPA1 may function in an intracrine manner to regulate iNOS (inducible nitric oxide synthase) and COX2 (cyclo-oxgenase 2) expression. These LPA-stimulated responses are inhibited by PLA2 (phospholipase A2) inhibitors, suggesting a requirement for arachidonic acid metabolites and/or LPA formed by the action of PLA2 [7]. In addition, Gobeil et al. [7] have shown that LPA1 co-localizes with caveolin 1 in a nuclear cell-free system. We have shown previously significant constitutive localization of LPA1 in the nucleus of PC12 cells, and that LPA stimulates the translocation of LPA1 to the nucleus of these cells [8].
In the present report, we have defined the mechanisms regulating the constitutive and LPA-induced nuclear localization of the LPA1 and investigated whether the receptor has an intranuclear signalling function.
MATERIALS AND METHODS
Materials
All biochemicals including LPA were from Sigma Chemical Co. CHO (Chinese hamster ovary) and PC12 cells and culture supplies were from Invitrogen. Anti-LPA1328–344, an antibody raised against the C-terminus of LPA1 corresponding to amino acids C328QRSENPTGPTESSDRS344 was from Upstate Biotechnology. Anti-(phosphoserine/threonine) and anti-phosphotyrosine antibodies were from Cell Signalling Technologies. Polyclonal anti-(phospho-p42/p44 MAPK) and anti-(p42 MAPK) antibodies were from New England Biolabs. Anti-(N-terminal LPA1) antibody was from MBL Int. Corporation. Ki16425 was a gift from Kirin Brewery (Japan).
Cell culture
PC12 and CHO cells were maintained in DMEM (Dulbecco's modified Eagle's medium) with 10% (v/v) FCS (foetal calf serum) and 10% (v/v) horse serum. Unless otherwise stated, cells were maintained in serum free DMEM for 24 h before experimentation. Primary HBECs (human bronchial epithelial cells) were isolated following typical procedures, as described previously [9,10]. Following overnight digestion of the tissues at 4 °C with 0.1% Sigma Type XIV protease (Sigma) in Ham's F-12 medium containing 100 units/ml penicillin, 100 μg/ml streptomycin, 2.5 μg/ml amphotericin B and 50 μg/ml gentamicin (all antibiotics were purchased from GIBCO®), the protease was neutralized by the addition of 10% foetal calf serum (Invitrogen) and the epithelial cells were freed from the tissue by agitation and isolated by centrifugation at 500 g for 10 min at 4 °C. The washed primary HBECs were then seeded on Vitrogen 100/sterile water-coated (1:75, v/v; Cohesion, Palo Alto, CA, U.S.A.) P-100 dishes in BEGM (bronchial epithelium growth medium), as described by Bernacki et al. [11]. Upon reaching confluence, HBECs were transferred to glass coverslips and grown to ∼60% confluence in BEGM.
Nuclear fractionation
Serum-deprived PC12 cell pellets were resuspended in buffer containing 10 mM Tris/HCl, pH 7.4, 10 mM NaCl, 3 mM MgCl2, 100 μg/ml soybean trypsin inhibitor and 1 mM PMSF, and homogenized with 50 strokes with a Dounce tissue grinder. The cell homogenate was then centrifuged at 880 g for 10 min at 4 °C. The ‘low-speed’ nuclear pellet obtained from the centrifugation was resuspended in buffer containing 10 mM Tris/HCl, pH 7.4, 10 mM NaCl, 3 mM MgCl2, 100 μg/ml soybean trypsin inhibitor, 1 mM PMSF and 0.1% (v/v) Nonidet P-40 for 5–10 min at 4 °C. After incubation the nuclei were sedimented by centrifugation at 880 g for 10 min at 4 °C. The pellet was washed a further three times in 10 mM Tris/HCl, pH 7.4 containing 10 mM NaCl, 3 mM MgCl2, 100 μg/ml soybean trypsin inhibitor and 1 mM PMSF before use. The low-speed supernatant from the initial centrifugation was centrifuged at 15000 g for 15 min at 4 °C. The resultant supernatant was removed and centrifuged again at 36000 rev./min in a 50.2Ti rotor for 60 min at 4 °C. The subsequent ‘high-speed’ pellet and supernatant were then taken for analysis.
HBECs were cultured on P-100 dishes and grown to ∼90% confluence in BEGM medium. The PBS/phosphatase inhibitors, 1× hypotonic buffer and complete lysis buffer (10 mM dithiothreitol, Lysis Buffer AM1, protease inhibitor cocktail) were provided in the Active Motif Nuclear Extract kit and prepared according to the manufacturer's instructions. Cells were washed with PBS/phosphatase inhibitor and collected by gentle scraping with a cell lifter. The cell homogenate was centrifuged at 40 g for 5 min at 4 °C and the supernatant discarded. The cell pellet was resuspended in 1× hypotonic buffer, incubated on ice for 15 min and then detergent (provided in the Active Motif Nuclear Extract kit) was added. The suspension was vortexed for 10 s at the highest setting before centrifugation at 14000 g for 30 s at 4 °C. The supernatant fraction from the nuclear extract was transferred to a pre-chilled tube and stored at −80 °C until use. The nuclear pellet was resuspended in complete lysis buffer, vortexed for 10 s and then incubated on ice for 30 min on a rocking platform at 150 rev./min. The nuclei were again vortexed for 30 s and finally centrifuged at 14000 g for 10 min at 4 °C. The nuclear pellet was transferred to a new tube and stored at −80 °C until use. Purity of the nuclear pellets was confirmed by detection of lamin B and the absence of lactate dehydrogenase (results not shown).
Transfection
Smartpool human LPA1 RNA duplexes were purchased from Dharmacon Research Inc. HBECs (P1) were cultured either in T-75 flasks or on 12 mm glass coverslips. At ∼60% confluence, transient transfection of LPA1 siRNA (small interfering RNA) was carried out using Transmessenger Transfection Reagent® (Qiagen). LPA1 siRNA (200 nM) was condensed with Enhancer R and formulated with Transmessenger reagent, according to the manufacturer's instructions. The transfection complex was diluted in 450 μl of BEGM medium and added directly to the cells. After an incubation of 3 h at 37 °C the transfection mixture was replaced with fresh DMEM. Cells were taken 72 h after transfection for preparation of nuclear pellets or fixed for immunofluorescence staining.
Western blotting
Immunoblotting was performed as described previously [8]. Immunoreactive proteins were visualized using enhanced chemilu-minescence detection.
Immunofluorescence
Cells were grown on 12 mm glass coverslips to a confluence of 50 or 100%. Cells were fixed in 3.7% (v/v) formaldehyde in PBS for 10 min, then permeabilized with 0.1% Triton X-100 in PBS for 1 min. Non-specific binding was reduced by pre-incubating cells in blocking solution containing 5% (v/v) FCS and 1% (v/v) BSA in PBS for 1 h. Cells were incubated with primary antibodies (1:100 dilution in blocking solution) for 1 h at room temperature (20 °C; or overnight at 4 °C) and then incubated with FITC-conjugated secondary antibodies (1:100) for 1 h. Cells were mounted on glass slides using Vectashield mounting medium with DAPI (4′,6-diamidino-2-phenylindole) and visualized using a Nikon E600 epi-fluorescence microscope.
Flow cytometry
HBECs grown to 60–70% confluence were challenged with 1 μM LPA for 30 min and then suspended using Accutase (Innovative Cell Technologies Inc.). Suspended cells were washed with PBS and incubated with anti-(N-terminal LPA1) antibody (LifeSpan Inc.) in PBS containing 1% (v/v) FCS and 0.04% azide at 4 °C for 30 min. Cells were washed and stained with allophycyanin-conjugated goat anti-rabbit Fab fragments (Molecular Probes) at 4 °C for 30 min. Analysis was performed on a FACScan (Becton Dickinson) using CellQuest software.
RESULTS
Nuclear localization of LPA1
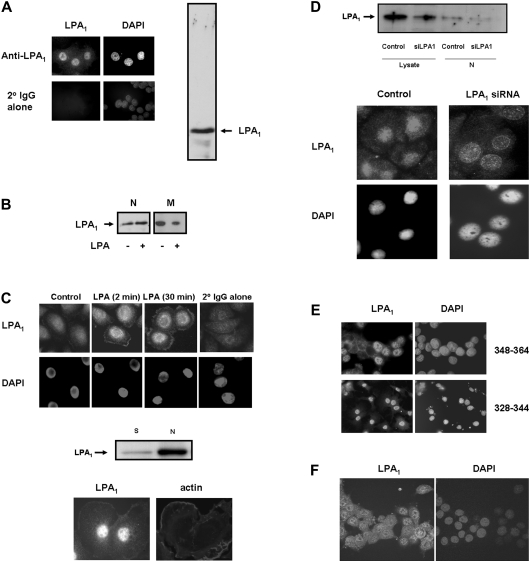
We have shown previously that PC12 cells express endogenous LPA1, but not LPA2, and also demonstrated that anti-sense-mediated down-regulation of the endogenous cell surface LPA1 reduced LPA-stimulated activation of p42/p44 MAPK [8]. Therefore these cells express functionally active LPA1. Immunofluorescent staining of LPA1with anti-LPA1328–344 antibody (Upstate Biotechnology) revealed that the receptor antigen is constitutively present in the nucleus of PC12 cells (Figure 1A, left hand panels). The staining is nuclear diffuse throughout the nucleoplasm and excluded from nucleoli. This is the most common type of localization of nuclear gene-trap proteins [12]. Moreover, many transcription factors have a nuclear diffuse localization, the largest class of which are the C2H2 zinc finger proteins, which account for more than 12% of all nuclear diffuse gene-trap proteins [12]. These findings suggest that nuclear LPA1 may be localized in zones of high transcriptional activity. Immunofluorescent staining of LPA1 was not detected when cells were incubated with secondary antibody alone (Figure 1A, left hand panels). The constitutive localization of LPA1 in the nucleus of PC12 cells was confirmed by Western blotting of the low-speed nuclear pellets probed with anti-LPA1328–344 antibody, which showed a single major immunoreactive protein band corresponding to native LPA1 (Figure 1A, right hand panel). Nuclear LPA1 migrated with a molecular mass of 36–38 kDa. The electrophoretic mobility of LPA1 in PC12 cells was slightly faster than the predicted molecular mass of LPA1 (42 kDa), suggesting that LPA1 in these cells might be subject to post-translational modification.
Figure 1. Detection of nuclear LPA1.
(A and B) Serum-deprived PC12 cells or (C) HBECs were immunostained with anti-LPA1328–344 antibody (A and B) and anti-(N-terminal LPA1) antibody (C), and detected using anti-(rabbit FITC-conjugated) secondary antibody. DAPI staining was used as a nuclear marker. (A) Western blot (right hand panel) was probed with anti-LPA1328–344 antibody, which showed a single immunoreactive band corresponding to LPA1 in the low-speed nuclear pellet from PC12 cells. (B) Western blot probed with anti-LPA1328–344 antibody showing the treatment of serum-deprived PC12 cells with 5 μM LPA for 10 min results in an increase in the amount of LPA1 in the low-speed nuclear pellet (N) and in a commensurate decrease in LPA1 in the high-speed membrane pellet fraction (M). (C) (Top panels) HBECs were treated with 1 μM LPA or untreated for the indicated times. (Bottom panels) PC12 cells were treated with 10 ng/ml NGF for 5 min to demonstrate plasma membrane localization of LPA1. PC12 cells and HBECs were immunostained with anti-LPA1328–344 antibody and anti-(N-terminal LPA1) antibody respectively. The middle panel shows a Western blot of high-speed hypotonic-treated nuclear pellets (N) and hypotonic/detergent high-speed supernatant fraction isolated from a nuclear pellet treated with this buffer mix (S) from HBECs, which was probed with anti-(N-terminal LPA1) antibody. (D) The upper panel shows a Western blot probed with anti-(N-terminal LPA1) antibody looking at the effect of siRNA-mediated elimination of the LPA1 in lysates and high-speed hypotonic-treated nuclear pellets (N) from serum-deprived HBECs. The lower panel shows immunofluorescence of control and siRNA LPA1-treated HBECs stained with anti-(N-terminal LPA1) antibody. (E) PC12 cells were immunostained with anti-LPA1 antibodies [and detected using anti-(rabbit FITC-conjugated) secondary antibody] raised against different C-terminal regions of LPA1 (see the Results section for details). DAPI staining was used as a nuclear marker. (F) Confocal immunofluorescent images of LPA1 nuclear localization in serum-deprived PC12 cells, using anti-LPA1348–364 antibody and detection with anti-(rabbit FITC-conjugated) secondary antibody.
Stimulation of PC12 cells with LPA resulted in an increase in the amount of LPA1 in the low-speed nuclear pellet (Figure 1B). A commensurate reduction in the amount of LPA1 was detected in the high-speed membrane pellet containing plasma membranes (Figure 1B). These findings suggest that LPA1 may traffic to the nucleus from cell membranes upon ligand activation.
Endogenous LPA1 is also constitutively localized in the nucleus of HBECs detected by immunofluorescent staining of cells (Figure 1C, top panels) and Western blotting analysis of hypotonic-treated nuclear pellets with anti-(N-terminal LPA1) antibody (Figure 1C, middle panel). Identification of nuclear LPA1 in HBECs was confirmed by transfection of HBECs with siRNA against LPA1, which substantially reduced the amount of LPA1 in cell lysates and hypotonic-treated nuclear pellets (Figure 1D, top panel). The reduction in nuclear LPA1 was also shown by immunofluorescence with anti-(N-terminal LPA1) antibody. siRNA against LPA1 does not completely abolish the expression of LPA1, but there was a visible decrease in the intensity of anti-(N-terminal LPA1) immunoreactivity in the nucleus of HBECs treated with siRNA against LPA1 compared with control cells (Figure 1D, bottom panels).
To eliminate the possibility of epitope cross-reactivity, two additional anti-LPA1 antibodies were used. These included another antibody raised against amino acids 328–344 of human LPA1 (purchased from Merck Biosciences) and an antibody raised against amino acids 348–364 of LPA1 (anti-LPA1348–364; L348NHTILAGVHSNDHSVV364; Affiniti Bioreagents). Immunofluorescent staining with these antibodies demonstrated the presence of LPA1 in the nucleus of PC12 cells (Figure 1E). Localization of LPA1 is again nuclear diffuse and excluded from nucleoli. Thus antibodies that were raised against different C-terminal regions of LPA1 produced similar results. We also detected nuclear localization of LPA1 by confocal analysis of cells immunostained with anti-LPA1348–364 antibody (Figure 1F). Using both confocal and epifluorescent microscopy, we did not observe any immunostaining of the plasma membrane of PC12 cells or HBECs with the anti-LPA1328–344 antibody. However, significant plasma membrane localization of LPA1 was detected in HBECs treated with LPA or in PC12 cells treated with NGF (nerve growth factor) to induce cortical actin localization and membrane ruffling (Figure 1C, bottom panel). The increased LPA1 immunoreactivity detected in the plasma membrane of HBECs was not due to increased LPA1 number, determined by FACS analysis using anti-(N-terminal LPA1) antibody. Indeed, treatment with 1 μM LPA for 30 min induced a decrease in cell-surface LPA1 number from 42.4 and 54% in control cells to 22.6 and 36.5% in LPA-treated cells, in two separate experiments. These results therefore suggest that significant LPA- or NGF-induced changes in plasma membrane organization may concentrate LPA1, possibly in micro-domains, which may allow improved access of the antibody to LPA1 antigen.
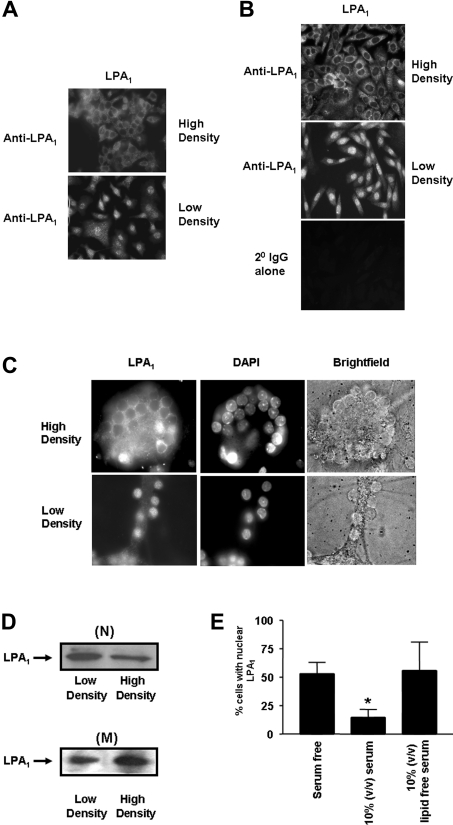
The localization of LPA1 in the nucleus depends on cell density
Anti-LPA1328–344 immunoreactivity was not detectable in the nuclei of PC12 cells grown at high density, whereas it was present in the nuclei from cells grown at low density (Figure 2A). An identical relationship between cell density and the nuclear localization of endogenous LPA1 was also observed in CHO cells (Figure 2B) and rat cerebellar granule neurons (Figure 2C). These results were confirmed by Western blotting using the anti-LPA1328–344 antibody to detect LPA1 from low-speed nuclear and high-speed membrane pellets from PC12 cells (Figure 2D). The amount of LPA1 was lower in the high-speed membrane pellet isolated from cells grown at low density compared with high density. This correlated with an increase in the amount of LPA1 in the low-speed nuclear pellet from cells grown at low density compared with high density (Figure 2D). These findings are therefore consistent with the possibility that LPA1 traffics to the nucleus in a cell density-dependent manner.
Figure 2. Effect of cell density and serum on the nuclear localization of LPA1.
PC12 cells, CHO cells and cerebellar granule neurons were grown to either low or high density and immunostained with anti-LPA1328–344 antibody and detected with anti-(rabbit FITC-conjugated) secondary antibody to show the effect of cell density on the nuclear localization of LPA1 in: (A) serum-deprived PC12 cells; (B) serum-deprived CHO cells, and; (C) cerebellar granule neurones. DAPI staining was used as a nuclear marker. (D) Low-speed nuclear pellets (N) and high-speed membrane pellets (M) from serum-deprived PC12 cells grown to low and high density were subjected to Western blotting probed with anti-LPA1328–344 antibody. (E) Histogram showing the effect of serum and delipidation of serum on the number of PC12 cells containing LPA1 in the nucleus. Cells were maintained in serum or delipidated serum or serum-free conditions for 24 h. Results are means±S.D. for three separate experiments (*P<0.05 for quiescent versus serum).
Figure 2(E) shows that the number of PC12 cells LPA1-localized in the nucleus was markedly decreased when the cells were maintained in serum for 24 h. Delipidation of the serum or removal of serum prevented the reduction in the number of cells containing nuclear LPA1.
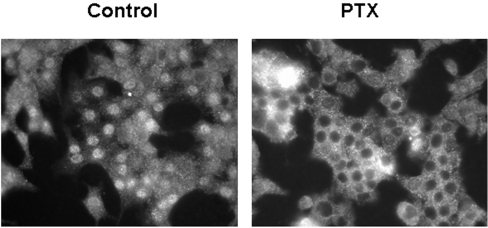
Constitutive nuclear localization of LPA1 is PTX (pertussis toxin)-sensitive
Constitutive trafficking of LPA1 from cell membranes to the nucleus appears to be dependent upon G-protein activation. This is evident from the finding that the pre-treatment of serum-deprived PC12 cells with PTX (which uncouples LPA1 from Gi/o-protein) reduced the number of cells containing LPA1 in the nucleus from ∼90% in control cells to ∼20% in PTX-treated cells, in three separate experiments (Figure 3).
Figure 3. Constitutive nuclear localization of LPA1.
Serum-deprived PC12 cells were immunostained with anti-LPA1328–344 antibody and detected using anti-(rabbit FITC-conjugated) secondary antibody. Nuclear localization of LPA1 in serum-deprived PC12 cells was reduced by pre-treating cells with 100 ng/ml PTX for 24 h, which was added at the same time as serum was removed from PC12 cells.
Role of Rho kinase signalling in regulating nuclear localization of LPA1 in PC12 cells
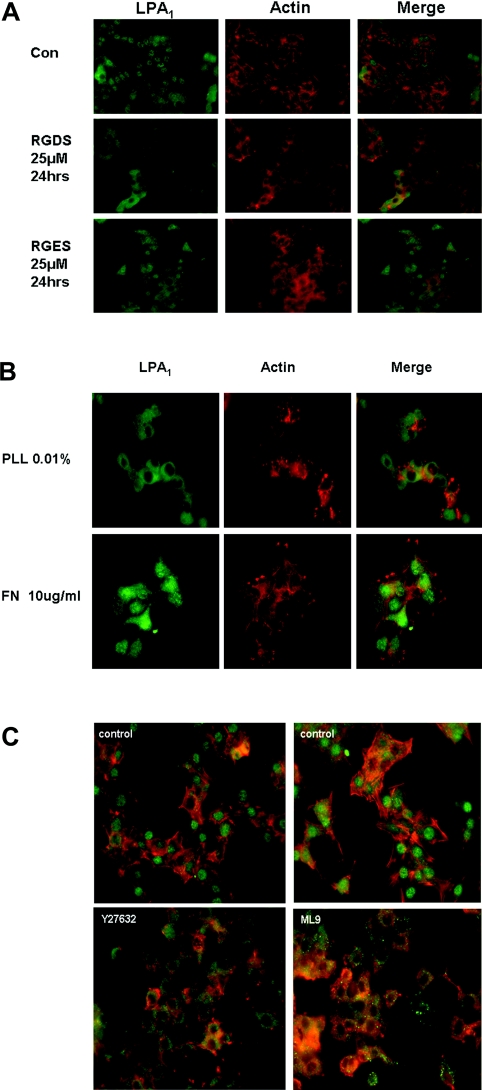
On the basis of cell density-dependent nuclear localization of LPA1, we considered whether the interaction of cells with the cell matrix might have a significant role in regulating the nuclear localization of LPA1. Therefore, the possible involvement of an integrin-dependent cytoskeletal mechanism regulating nuclear localization of LPA1 in PC12 cells was investigated. We first used the RGDS peptide, which disrupts the interaction between integrins and secreted extracellular matrix proteins that contain RGD motifs (e.g. fibronectin). The RGDS peptide prevents focal adhesion assembly and disrupts the integrity of the cytoskeleton induced by fibronectin engagement with integrin [13]. Using this peptide, we found that the number of cells containing nuclear LPA1 was reduced from ∼90% in control cells to ∼5% in RGDS-treated cells, whereas it remained at 75–85% in RGES-treated cells [results shown in Figure 4(A), n=3]. These findings are consistent with the observation that RGDS disrupted actin polymerization in PC12 cells (Figure 4A), evidenced by marked changes in the organized structure of actin (red phallodin staining in Figure 4A). Furthermore, a higher proportion of cells contained nuclear LPA1 when adhesion on fibronectin-coated glass (75%) was compared with adherence to polylysine-coated glass (15%) (Figure 4B). Fibronectin engages integrins to induce integrin clustering, focal adhesion formation and actin polymerization. In contrast, polylysine does not engage integrins and has no effect on the actin cytoskeleton. These properties are consistent with results presented in Figure 4(B), which show that fibronectin, but not polylysine, stimulates actin polymerization in PC12 cells, evident by phallodin red staining of the cells (see marked rearrangement of actin fibres with fibronectin compared with polylysine in Figure 4B).
Figure 4. The effect of cell matrix and Rho-dependent signalling on nuclear LPA1 in PC12 cells.
Serum-deprived PC12 cells were immunostained using anti-LPA1328–344 antibody and detected with anti-(rabbit FITC-conjugated) secondary antibody (green). Actin was stained using phallodin red (red). (A) Serum-deprived PC12 cells were treated with 25 μM RGDS or 25 μM RGES for 24 h; or were grown on (B) 10 μg/ml fibronectin (FN) or 0.01% polylysine (PLL)-coated plates; or (C) were treated with 10 μM Y27632 or 10 μM ML9 for 4 h or untreated. Results shown are a single representative of three experiments.
To further substantiate a role for integrin-mediated Rho activation and the ability of actomyosin to contract, we found that the pre-treatment of PC12 cells with the Rho kinase inhibitor (10 μM Y27632 for 4 h) or the MLCK (myosin light chain kinase) inhibitor (10 μM ML9 for 4 h) reduced the number of cells containing nuclear LPA1 and disrupted actin polymerization in PC12 cells (Figure 4C; proportions of cells containing nuclear LPA1: control, 85–95%; Y27632-treated, 3–5%; ML9-treated, 2%; n=3).
Functional role of nuclear LPA1
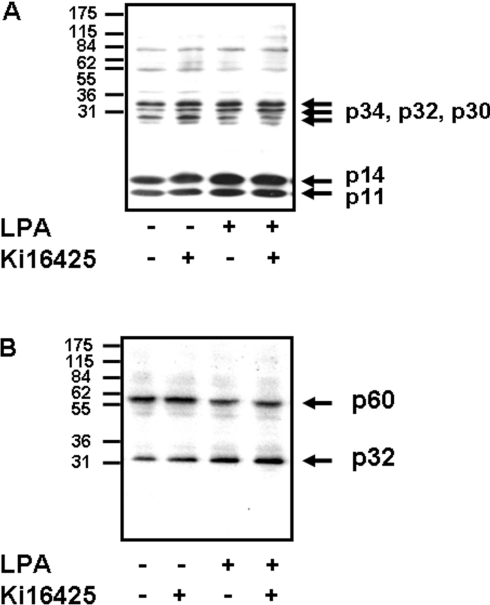
To evaluate the function of nuclear LPA1, we assessed the effect of LPA on the phosphorylation of nuclear proteins in PC12 cells. To designate effects to LPA1 we used Ki16425, which selectively binds to LPA1 [14]. The addition of LPA and/or Ki16425 to isolated nuclei present in the low-speed nuclear pellet induced the serine/threonine phosphorylation of several proteins with molecular masses of 34 kDa, 32 kDa, 14 kDa and 11 kDa (labelled p34, p32, p14 and p11 respectively in Figure 5A). Ki16425 also stimulated the serine/threonine phosphorylation of an additional protein with a molecular mass of 30 kDa (labelled p30 in Figure 5A), which was blocked by LPA. The addition of LPA and/or Ki16425 to isolated nuclei also stimulated the tyrosine phosphorylation of a 32 kDa protein (labelled p32 in Figure 5B). Finally, LPA stimulated the dephosphorylation of a 60 kDa phosphotyrosine containing protein (labelled p60 in Figure 5B), which was not mimicked or reduced by Ki16425.
Figure 5. Effect of Ki16425 and LPA on protein phosphorylation.
Freshly prepared nuclei in low-speed nuclear pellets from serum-deprived PC12 cells were untreated or treated with 10 μM Ki16425 for 20 min, or with 5 μM LPA for 5 min, or with 10 μM Ki16425 for 20 min followed by 5μM LPA for 5 min. The Western blots show the effect of Ki16425 and/or LPA on (A) nuclear protein serine/threonine phosphorylation and (B) nuclear protein tyrosine phosphorylation.
DISCUSSION
The results presented in this report demonstrate that LPA1 is constitutively localized in the nucleus of HBEC, PC12 and CHO cells. LPA stimulates an acute increase in the amount of LPA1 in the nuclear fractions of the cells and this may be a consequence of increased trafficking of LPA1 from cell membranes. This finding extends our previous studies [8] and is in agreement with those of Gobeil et al. [7] concerning constitutive localization of LPA1 in the nucleus of liver cells. The results are also consistent with the growing body of evidence demonstrating that a number of GPCR (e.g. endothelin, angiotensin 1, apelin R1 and bradykinin 2 receptors [15,16]) are localized in the nucleus along with attendant signalling molecules, such as GRK5 (G-protein-coupled-receptor kinase-5) and regulators of G-protein signalling [17,18]. The trafficking of LPA1 to the nucleus of PC12 cells appears to be dependent, in part, upon G-protein coupling, on the basis of results showing that the pre-treatment of these cells with PTX (which uncouples LPA1 from Gi/o-protein) dramatically reduced the number of cells containing LPA1 in the nucleus (Figure 3).
The nuclear localization of LPA1 is also dependent upon integrin clustering. Integrin clustering induces activation of Rho and Rho kinase [19], leading to calcium sensitization of actomyosin contraction via inhibition of a myosin light chain phosphatase. MLCK plays a critical role in initiating phosphorylation of myosin and subsequent cross-bridge formation with polymerized actin. Inhibitors of Rho kinase and MLCK reduce the number of cells containing LPA1 in the nucleus. Moreover, fibronectin, which induced actin polymerization, increased the number of cells containing LPA1 in the nucleus, while RGDS (which disrupts actin polymerization) was inhibitory. These findings also raise the possibility that LPA may acutely regulate the trafficking of LPA1 to the nucleus via Rho-mediated signalling. We have also shown that serum treatment of cells for 24 h decreased the number of cells containing LPA1 in the nucleus and this was reversed by delipidation or removal of serum. One possible explanation might be that the LPA present in serum induces a chronic desensitization of LPA1 trafficking to the nucleus and that delipidation removes LPA, thereby relieving this inhibitory effect.
LPA1 trafficking from cell membranes to the nucleus may involve lipid rafts. Indeed, lipid rafts represent a major route for nuclear entry by viruses such as HIV (for a review, see [20]), and also for fibroblast growth factor- and vascular endothelial growth factor-receptor traffic from the plasma membrane to the nucleus in an agonist-dependent and -independent manner [21,22]. LPA1 may also be maintained in a hydrophobic environment within the nucleus, as this organelle is rich in lipid environments that are localized in areas of transcriptional activity. These lipid microenvironments contain the lipid raft components caveolin-1, cholesterol and phospholipids [23]. Indeed, Gobeil et al. [7] have demonstrated co-localization of LPA1 with caveolin-1 in the nucleus. Thus caveolin-mediated endocytosis may be involved in nuclear trafficking of LPA1. In ML9-treated cells, LPA1 appears to ‘back up’ in cytoplasmic vesicular structures. These findings are consistent with the possibility that ML9 traps endocytic vesicles containing LPA1 in the cytoplasm. This is entirely consistent with the mode of action of ML9, which does not disrupt the formation of endosomes containing LPA1, but instead inhibits actomyosin formation and thus affects the contraction required to transport endosomes to intracellular compartments.
We have also shown that the addition of LPA to isolated nuclei induced the serine/threonine phosphorylation of four proteins with molecular masses of 11, 14, 32 and 34 kDa. These phosphorylation events were insensitive to inhibition by Ki16425, which itself stimulated phosphorylation of the same proteins. The fact that both LPA and Ki16425 bind to LPA1 and that both induce nuclear phosphorylation of the same substrates provides evidence that these events are probably mediated by LPA1. Ki16425 also stimulated phosphorylation of a 30 kDa protein, which was reduced by LPA. Further studies are required to identify the mechanisms by which LPA and Ki16425 stimulate nuclear protein phosphorylation. However, Ki16425 is unusual as it functions as a protean agonist of cell-surface LPA1 receptor, being able to induce activation of p42/p44 MAPK on its own, while reducing the LPA-stimulated activation of this protein kinase pathway in PC12 cells [24]. In the present report, we also show that LPA stimulated the dephosphorylation of a 60 kDa nuclear tyrosine-phosphorylated protein. As this was not mimicked or reduced by Ki16425, there is a possibility that this protein is regulated by a distinct nuclear LPA receptor that does not bind Ki16425.
Additional evidence to support the concept that nuclear LPA1 regulates protein phosphorylation has been reported by Gobeil et al. [7], who showed that LPA induced the phosphorylation of Akt in isolated liver nuclei. Moreover, LPA-induced phosphorylation of Akt was blocked by the phosphoinositide 3-kinase inhibitor, wortmannin, which also reduced LPA-stimulated iNOS expression [7]. However, it will be important to identify the kinase(s) responsible for the phosphorylation in the present report to exclude direct effects of LPA and Ki16425 on this kinase.
An intranuclear role for LPA has been suggested previously by others showing that the induction of COX2 and iNOS is inhibited by PLA2 inhibitors, suggesting that LPA might be formed by the action of PLA2 on nuclear phosphatidic acid [7]. Indeed, it has been reported that using a human endothelial cell line EA.hy.926 (in which cPLA2 is present in the cytosol and nucleus, and moves to membranes via its calcium-dependent lipid-binding domain following agonist stimulation) nuclear localization of cPLA2 is dependent on cell density, with cells grown at low density containing higher levels of nuclear cPLA2 compared with contact-inhibited high-density or serum-deprived cells [25]. Therefore the effect of cell density on the nuclear localization of cPLA2 is very similar to that regulating LPA1 in PC12 cells.
In summary, the prevailing view is that LPA1 is localized at the cell surface where it functions to transduce extracellular LPA signals into biological responses. Our findings suggest that LPA1 is also present in the nucleus of cells where it may participate in intranuclear LPA signalling.
Acknowledgments
This work was supported by grants from the Biotechnology and Biological Sciences Research Council to N.J.P. and S.P. and NIH (National Institutes of Health) RO1 grants HL 71152 to V.N. and HL92160 to G.T.
References
- 1.Fukushima N., Chun J. The LPA receptors. Prostaglandins. 2001;64:21–32. doi: 10.1016/s0090-6980(01)00105-8. [DOI] [PubMed] [Google Scholar]
- 2.Pyne S., Kong K. C., Darroch P. I. Lysophosphatidic acid and sphingosine 1-phosphate biology: the role of lipid phosphate phosphatases. Semin. Cell. Dev. Biol. 2004;15:491–501. doi: 10.1016/j.semcdb.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 3.An S., Dickens M. A., Bleu T., Hallmark O. G., Goetzl E. J. Molecular cloning of the human Edg2 protein and its identification as a functional cellular receptor for lysophosphatidic acid. Biochem. Biophys. Res. Commun. 1997;231:619–622. doi: 10.1006/bbrc.1997.6150. [DOI] [PubMed] [Google Scholar]
- 4.Contos J. J., Chun J. Complete cDNA sequence, genomic structure, and chromosomal localization of the LPA receptor gene, lpA1/vzg-1/Gpcr26. Genomics. 1998;51:364–378. doi: 10.1006/geno.1998.5400. [DOI] [PubMed] [Google Scholar]
- 5.Ye X., Ishii I., Kingsbury M. A., Chun J. Lysophosphatidic acid as a novel cell survival/apoptotic factor. Biochim. Biophys. Acta. 2002;1585:108–113. doi: 10.1016/s1388-1981(02)00330-x. [DOI] [PubMed] [Google Scholar]
- 6.Contos J. J., Fukushima N., Weiner J. A., Kaushal D., Chun J. Requirement for the lpA1 lysophosphatidic acid receptor gene in normal suckling behavior. Proc. Natl. Acad. Sci. U.S.A. 2000;97:13384–13389. doi: 10.1073/pnas.97.24.13384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gobeil F., Bernier S. G., Vasquez-Tello A., Brault S., Beauchamp M. H., Quiniou C., Marrache A. M., Checchin D., Sennlaub F., Hou X., et al. Modulation of pro-inflammatory gene expression by nuclear lysophosphatidic acid receptor type-1. J. Biol. Chem. 2003;278:38875–38883. doi: 10.1074/jbc.M212481200. [DOI] [PubMed] [Google Scholar]
- 8.Moughal N., Waters C., Sambi B., Pyne S., Pyne N. J. Nerve growth factor signalling involves interaction between the Trk A receptor and receptor 1 systems: Nuclear translocation of the receptor 1 and Trk A receptors in pheochromocytoma 12 cells. Cell Signal. 2004;16:127–136. doi: 10.1016/j.cellsig.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 9.Spannhake E. W., Reddy S. P. M., Jacoby D. B., Yu X. Y., Saatian B., Tian J. Synergism between rhinovirus infection and oxidant pollutant exposure enhances airway epithelial cell cytokine production. Environ. Health Perspect. 2002;110:665–670. doi: 10.1289/ehp.02110665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saatian B., Yu X. Y., Lane A. P., Doyle T., Casolaro V., Spannhake E. W. Expression of genes for B7-H3 and other T cell ligands by nasal epithelial cells during differentiation and activation. Am. J. Physiol. Lung Cell Mol. Physiol. 2004;287:L217–L225. doi: 10.1152/ajplung.00132.2003. [DOI] [PubMed] [Google Scholar]
- 11.Bernacki S. H., Nelson A. L., Abdullah L., Sheehan J. K., Harris A., Davis C. W., Randell S. H. Mucin gene expression during differentiation of human airway epithelia in vitro. Muc4 and muc5b are strongly induced. Am. J. Respir. Cell Mol. Biol. 1999;20:595–604. doi: 10.1165/ajrcmb.20.4.3442. [DOI] [PubMed] [Google Scholar]
- 12.Sutherland H. G., Mumford G. K., Newton K., Ford L. V., Farrall R., Dellaire G., Caceres J. F., Bickmore W. A. Large-scale identification of mammalian proteins localized to nuclear sub-compartments. Hum. Mol. Genet. 2001;10:1995–2011. doi: 10.1093/hmg/10.18.1995. [DOI] [PubMed] [Google Scholar]
- 13.Sanchez-Aparicio P., Dominguez-Jimenez C., Garcia-Pardo A. Activation of the alpha 4 beta 1 integrin through the beta 1 subunit induces recognition of the RGDS sequence in fibronectin. J. Cell Biol. 1994;126:271–279. doi: 10.1083/jcb.126.1.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ohta H., Sato K., Murata N., Damirin A., Malchinkhuu E., Kon J., Kimura T., Tobo M., Yamazaki Y., Watanabe T., et al. Ki16425, a subtype-selective antagonist for EDG-family lysophosphatidic acid receptors. Mol. Pharmacol. 2003;64:994–1005. doi: 10.1124/mol.64.4.994. [DOI] [PubMed] [Google Scholar]
- 15.Boivin B., Chevalier D., Villeneuve L. R., Rousseau E., Allen B. G. Functional endothelin receptors are present on nuclei in cardiac ventricular myocytes. J. Biol. Chem. 2003;278:29153–29163. doi: 10.1074/jbc.M301738200. [DOI] [PubMed] [Google Scholar]
- 16.Lee D. K., Lanca A. J., Cheng R., Nguyen T., Ji X. D., Gobeil F., Jr, Chemtob S., George S. R., O'Dowd B. F. Agonist-independent nuclear localization of the Apelin, angiotensin AT1, and bradykinin B2 receptors. J. Biol. Chem. 2004;279:7901–7908. doi: 10.1074/jbc.M306377200. [DOI] [PubMed] [Google Scholar]
- 17.Yi X. P., Gerdes A. M., Li F. Myocyte redistribution of GRK2 and GRK5 in hypertensive, heart-failure-prone rats. Hypertension. 2002;39:1058–1063. doi: 10.1161/01.hyp.0000019130.09167.3b. [DOI] [PubMed] [Google Scholar]
- 18.Chatterjee T. K., Fisher R. A. Cytoplasmic, nuclear, and Golgi localization of RGS proteins. Evidence for N-terminal and RGS domain sequences as intracellular targeting motifs. J. Biol. Chem. 2000;275:24013–24021. doi: 10.1074/jbc.M002082200. [DOI] [PubMed] [Google Scholar]
- 19.Bourdoulous S., Orend G., MacKenna D. A., Pasqualini R., Ruoslahti E. Fibronectin matrix regulates activation of RHO and CDC42 GTPases and cell cycle progression. J. Cell. Biol. 1998;143:267–276. doi: 10.1083/jcb.143.1.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ferrari A., Pellegrini V., Arcangeli C., Fittipaldi A., Giacca M., Beltram F. Caveolae-mediated internalization of extracellular HIV-1 tat fusion proteins visualized in real time. Mol. Ther. 2003;8:284–294. doi: 10.1016/s1525-0016(03)00122-9. [DOI] [PubMed] [Google Scholar]
- 21.Reilly J. F., Mizukoshi E., Maher P. A. Ligand dependent and independent internalization and nuclear translocation of fibroblast growth factor (FGF) receptor 1. DNA Cell Biol. 2004;23:538–548. doi: 10.1089/dna.2004.23.538. [DOI] [PubMed] [Google Scholar]
- 22.Feng Y., Venema V. J., Venema R. C., Tsai N., Caldwell R. B. VEGF induces nuclear translocation of Flk-1/KDR, endothelial nitric oxide synthase, and caveolin-1 in vascular endothelial cells. Biochem. Biophys. Res. Commun. 1999;256:192–197. doi: 10.1006/bbrc.1998.9790. [DOI] [PubMed] [Google Scholar]
- 23.Albi E. E., Viola Magni M. P. The role of intranuclear lipids. Biol. Cell. 2004;96:557–567. doi: 10.1016/j.biolcel.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 24.Moughal N. A., Waters C. M., Valentine W. J., Connell M., Richardson J. C., Tigyi G., Pyne S., Pyne N. J. Protean agonism of the lysophosphatidic acid receptor-1 with Ki16425 reduces nerve growth factor-induced neurite outgrowth in PC12 cells. J. Neurochem. 2006. In Press. [DOI] [PubMed]
- 25.Grewal S., Morrison E. E., Ponnambalam S., Walker J. H. Nuclear localization of cytosolic phospholipase A2-α in the EA.hy.926 human endothelial cell line is proliferation dependent and modulated by phosphorylation. J. Cell Sci. 2002;115:4533–4543. doi: 10.1242/jcs.00146. [DOI] [PubMed] [Google Scholar]