Abstract
An unusual interaction between flucytosine and fluconazole was observed when a collection of 60 Candida lusitaniae clinical isolates was screened for cross-resistance. Among eight isolates resistant to flucytosine (MIC ≥ 128 μg/ml) and susceptible to fluconazole (0.5 < MIC < 2 μg/ml), four became flucytosine-fluconazole cross resistant when both antifungals were used simultaneously. Fluconazole resistance occurred only in the presence of high flucytosine concentrations, and the higher the fluconazole concentration used, the greater the flucytosine concentration necessary to trigger the cross-resistance. When the flucytosine- and fluconazole-resistant cells were grown in the presence of fluconazole alone, the cells reversed to fluconazole susceptibility. Genetic analyses of the progeny from crosses between resistant and sensitive isolates showed that resistance to flucytosine was derived from a recessive mutation in a single gene, whereas cross-resistance to fluconazole seemed to vary like a quantitative trait. We further demonstrated that the four clinical isolates were susceptible to 5-fluorouracil and that cytosine deaminase activity was unaffected. Kinetic transport studies with [14C]flucytosine showed that flucytosine resistance was due to a defect in the purine-cytosine permease. Our hypothesis was that extracellular flucytosine would subsequently behave as a competitive inhibitor of fluconazole uptake transport. Finally, in vitro selection of spontaneous and induced mutants indicated that such a cross-resistance mechanism could also affect other Candida species, including C. albicans, C. tropicalis, and C. glabrata. This is the first report of a putative fluconazole uptake transporter in Candida species and of a possible resistance mechanism associated with a deficiency in the uptake of this drug.
Treatment of fungal infections is challenged by the emergence of antifungal resistance, partly because of the abundant prophylactic use of well-tolerated azoles in an increasing population of immunocompromised patients and partly because of the delay in the design of new antifungal agents. During the last few years, research has dealt with the underlying molecular mechanisms of antifungal resistance, which have been extensively reviewed (14, 29, 37). Unfortunately, except for the increasing use of antifungal susceptibility tests and faster changes of treatment in case of suspicion of antifungal resistance, therapeutic practices have not actually taken advantage of this knowledge. In order to minimize the risk of therapeutic failure due to resistance, empirical antifungal combinations are more often used and even recommended (30), particularly if the treatment includes flucytosine (5FC), to which the frequency of resistance precludes its use as monotherapy. However, the simultaneous use of two antifungal agents raises two issues. The first one deals with drug interactions and is still controversial. Numerous studies conducted in vitro have reported antagonism between drugs, in particular, in the case of the combination of amphotericin B (AMB) and fluconazole (FLC) (19, 22, 34) and the combination of 5FC and azoles (31). Nevertheless, these findings were poorly confirmed or were not confirmed by in vivo experiments with animal models, nor were they confirmed in clinical practice, which often resulted in successful treatment of fungal infections with combination therapy (19, 22). The second issue concerns the selection of cross-resistance. For example, cross-resistance between agents in the azole family may result from overexpression of ATP-binding cassette drug efflux transporters (27, 28) or from point mutations of the ERG11 gene, which encodes lanosterol demethylase, thus decreasing the affinity of the target for different azoles (26). Rare, but more critical, are mutations in the Δ5,6-sterol desaturase gene of the ergosterol biosynthetic pathway which confer cross-resistance to both AMB and FLC (18, 23).
Because of the increasing use of antifungal combination therapy, we investigated the occurrence of cross-resistance resulting from the simultaneous use of two antifungal agents against Candida lusitaniae. For a long time C. lusitaniae has been falsely considered intrinsically resistant to AMB because the first descriptions of the clinical occurrence of this opportunistic pathogen reported that it was AMB resistant (15, 20, 24). From our experience and as recently reported (10, 11), most C. lusitaniae isolates are primarily susceptible to AMB. Although a mechanism that allows cells to switch from the AMB-susceptible to the AMB-resistant phenotype has been reported (38), the relatively high frequency at which this yeast can readily mutate to resistance to any antifungal should first be regarded as a consequence of its haploid genome, in which both recessive and dominant mutations can be expressed to yield resistant phenotypes. This feature makes C. lusitaniae an interesting model for the study of antifungal resistance, especially as we have reported that clinical isolates had the capacity to reproduce sexually (13), thus allowing us to use formal genetics tools.
We began studying cross-resistance to 5FC because this drug is always used in combination with another antifungal when it is used for therapy and because a negative interaction between 5FC and azoles in vitro has already been described (31, 32). Additionally, a relationship between 5FC resistance and decreased susceptibility to azoles was recently established for clinical isolates of Candida albicans (9). We report here on the screening of clinical isolates of C. lusitaniae cross resistant to 5FC and FLC. Phenotypic characterization provided evidence that resistance to both 5FC and FLC does not result from independent genetic events. Further information was gained from genetic analyses and from the identification of the biochemical mechanism responsible for 5FC resistance. Overall, the data presented strongly suggest the existence of a transporter for FLC uptake which could be subjected to competitive inhibition by 5FC. Finally, by in vitro selection of mutant strains, we demonstrate that this cross-resistance mechanism can also affect other Candida species.
MATERIALS AND METHODS
Yeast strains and media.
The yeast strains used in this study are listed in Table 1. A collection of 60 isolates described previously (13) was screened for C. lusitaniae clinical isolates with a 5FC-resistant phenotype. Each of the isolates was recovered from an individual patient. Yeast strains were routinely cultivated in liquid YPD medium (1% yeast extract, 2% peptone, 2% dextrose) at 35°C under agitation (250 rpm). Yeast nitrogen base with ammonium sulfate and without amino acids (YNB medium; Difco Laboratories, Detroit, Mich.) was used at a concentration of 0.67% and was supplemented with 2% glucose. The YNB medium was used for the preparation of crude protein extracts, for the screening of auxotrophs, and for selection of the recombinant prototroph meiotic products released from crosses between auxotrophic parents. Solid media were prepared with 2% agar (Sigma Chemical Co., St. Louis, Mo.). Agar-solidified yeast carbon base (YCB; Difco) was used at a concentration of 1.17% and was used for mating-type determination and genetic crosses.
TABLE 1.
Yeast strains used in this study
| Species | Strain no. | Genotypea | Phenotype | Origin (reference)b |
|---|---|---|---|---|
| C. lusitaniae | 6936 | MATa | Susceptible | CBS |
| C. lusitaniae | 5094 | MATα | Susceptible | CBS |
| C. lusitaniae | CL26 | MATa | 5FC resistant | Clinical, blood |
| C. lusitaniae | CL29 | MATα | 5FC resistant | Clinical, urine |
| C. lusitaniae | CL31 | MATα | 5FC resistant | Clinical, feces |
| C. lusitaniae | CL32 | MATa | 5FC resistant | Clinical, bedsore |
| C. lusitaniae | CL38 | MATα | 5FC resistant | Clinical, nose (13) |
| C. lusitaniae | CL42 | MATa | 5FC resistant | Clinical, blood |
| C. lusitaniae | CL44 | MATa | 5FC and AMB resistant | Clinical, blood (25) |
| C. lusitaniae | CL60 | MATα | 5FC resistant | Clinical, urine |
| C. lusitaniae | CLE11-801 | MATa | 5FC resistant | EMS treatment of 6936 |
| C. lusitaniae | 36/4 | MATa | Leu−, susceptible | This work |
| C. lusitaniae | 5/31 | MATα | Leu−, 5FC resistant | This work and (13) |
| C. lusitaniae | 69/3 | MATa | Lys−, susceptible | This work and (13) |
| C. albicans | 2091 | Susceptible | ATCC | |
| C. tropicalis | 94 | Susceptible | CBS | |
| C. glabrata | 90030 | Susceptible | ATCC | |
| C. neoformans | 135 | Susceptible | Clinical, CSF |
The genotypes of C. lusitaniae clinical isolates were assigned as described previously (13).
CBS, Centraalbureau voor Schimmelcultures (Utrecht, The Netherlands); ATCC, American Type Culture Collection (Manassas, Va.); CSF, cerebrospinal fluid.
Antifungal agents.
FLC (ICN Biomedicals Inc., Aurora, Ohio), itraconazole (ITC) and ketoconazole (KTC) (Janssen Pharmaceutica, Beerse, Belgium), 5FC and 5-fluorouracil (Sigma), and AMB sodium deoxycholate (Bristol-Myers Squibb, Paris, France) were used in this study. Stock solutions were prepared in water at concentrations of 3.2 mg/ml for FLC, 12.8 mg/ml for 5FC and 5-fluorouracil (5FU), and 1.6 mg/ml for AMB. Stock solutions were prepared in dimethyl sulfoxide (Sigma) at a concentration of 0.8 mg/ml for ITC and KTC.
Susceptibility testing.
Susceptibilities to the antifungal agents tested were determined by slight modifications of the twofold microdilution method recommended by the National Committee for Clinical Laboratory Standards (21). Testing was performed in 96-well plates with RPMI 1640 (RPMI; Sigma) buffered to pH 7.0 with 0.165 M morpholinepropanesulfonic acid (Sigma) and supplemented with 1.8% glucose. The final inoculum size was 104 cells per ml. The final antifungal concentrations ranged from 256 to 0.5 μg/ml for 5FC, 64 to 0.12 μg/ml for FLC, 16 to 0.03 μg/ml for AMB, and 8 to 0.015 μg/ml for both ITC and KTC. After 48 h of incubation at 30°C, the cell density was measured at 450 nm with an automated microtiter reader (Thermomax; Molecular Devices, Menlo Park, Calif.). In our experiments, the MIC was defined as the lowest drug concentration that inhibited at least 95% of the growth compared to the growth of the drug-free growth control. The MIC cutoff value was voluntarily increased from 80% (recommended by the National Committee for Clinical Laboratory Standards) to 95% to avoid any confusion between residual growth, which can be as high as 20% and which can be accepted for a strain sensitive to a single antifungal, and a significant growth level for an isolate with cross-resistance to the antifungals used in combination. Each MIC determination was done at least in triplicate.
Screening for antifungal resistance.
For the screening of antifungal-resistant isolates and for segregation analysis of the resistant phenotypes of the progenies derived from crosses, we used RPMI 1640 AutoMod (modified for autoclaving; Sigma) agar plates. Antifungal agents were added to the medium at different concentrations after the medium was autoclaved, just before they were dispensed into petri dishes. A tiny yeast colony isolated on a YPD agar plate was picked up with a toothpick and resuspended in sterile water to a final concentration of approximately 106 cells per ml. A 5-μl calibrated loop was then used to streak yeast cells onto each drug-containing RPMI agar plate and onto a drug-free RPMI plate as a growth control. Up to eight strains were tested simultaneously on each plate, and their antifungal resistance phenotypes were determined after 48 h of incubation at 30°C.
EMS mutagenesis.
Ethyl methanesulfonate (EMS) treatment of the yeast cells was performed by standard genetic procedures (5), with minor modifications. The cells were cultivated overnight in liquid YPD medium at 35°C with agitation and allowed to reach the stationary phase (ca. 2 × 108 cells/ml). The cells were centrifuged (2,000 × g, 5 min) and resuspended in fresh YPD medium to a cellular density of 108 cells/ml. Two separate 2-ml samples were transferred to 50-ml disposable sterile tubes. One sample received 2% (vol/vol) EMS (40 μl; Sigma), and one sample received 40 μl of sterile distilled water. After 3 h of incubation at room temperature, two 1-ml aliquots of each sample were transferred to two 1.5-ml microtubes and centrifuged (2,000 × g, 1 min). The cell pellets were washed three times with 0.7% NaCl and were finally resuspended in 1 ml of 0.7% NaCl. For safety purposes, all manipulations were done under a hood and all solid and liquid wastes were treated with a 5% sodium thiosulfate solution to neutralize the EMS before it was discarded. Cell viability was determined by plating 100 μl of a 10−4 dilution from both samples onto solid YPD medium. All EMS-treated cells and cells not treated with EMS were then immediately inoculated into 50 ml of liquid YPD medium and were grown for 16 h (35°C, 250 rpm). The mutant enrichment factor was calculated by plating 100 μl of each culture on RPMI agar plates supplemented with 32 μg of 5FC per ml and counting the 5FC-resistant colonies that had developed from the EMS-treated cell cultures and the cell cultures not treated with EMS after 72 h of incubation at 30°C.
Selection of auxotrophic mutants.
Auxotrophic mutants were selected from strain 6936 MATa, following EMS treatment and nystatin enrichment. For this, EMS-treated cells were inoculated into liquid YNB medium (5 × 107 cells per 10 ml of medium) and incubated at 35°C under agitation for 8 h. Aliquots of 1 ml then received 350 U of nystatin (5,010 USP units per mg; Sigma), and incubation was continued for 2 h at 35°C and then for 16 h at 4°C. After centrifugation (2,000 × g, 5 min) and three successive washes with sterile distilled water, 100 μl of the cell suspension was plated onto solid YPD medium. For the screening of auxotrophs, the colonies that developed on YPD medium were replica plated onto both solid YPD medium and YNB medium. The auxotrophic mutants, unable to grow on YNB medium, represented nearly 20% of the total colonies that survived the nystatin enrichment. The requirement of each auxotrophic mutant was characterized by supplementation of the YNB medium with different amino acids and bases. The genomes of the initial mutants were then purified once by a genetic cross with strain 5094 MATα, and MATα auxotrophic progeny were then purified by three successive backcrosses with strain 6936 MATa.
Mating type tests, genetic crosses, and ascospore isolation.
Mating type determination and genetic crosses between strains of opposite mating types were performed as described previously (13). Briefly, genetic crosses were made by mixing equal volumes of cells (derived either from a YPD liquid preculture or from a colony resuspension) of each sexually compatible parent. Aliquots of 5 μl were spotted onto solid YCB, as were controls, which were prepared by spotting a pure cellular suspension of each parent. After 48 to 96 h of incubation at room temperature, the presence of ascospores in the spot derived from the cross and the lack of ascospores in the spots derived from each pure parental suspension were verified by observing cells resuspended in a drop of water with a light microscope (magnification, ×400). The procedure for ascospore isolation was based on the fact that they are more resistant than blastospores to spheroplast formation and subsequent osmotic lysis. For that, all cells contained in each spot (crosses and parental controls) were harvested from YCB and separately resuspended in 1 ml of 50 mM sodium phosphate buffer (pH 6.5)-1 M sorbitol, to which was added simultaneously 10 μl of 2-mercaptoethanol and 3 μl of lyticase (10 U/μl; Sigma). After 1 h of incubation at 37°C, the cells were pelleted (2,000 × g, 5 min) and resuspended in 1 ml of 0.2% (wt/vol) sodium dodecyl sulfate. Cell lysis was performed at 37°C for 30 min. The suspensions were then centrifuged, washed twice with 1 ml of sterile distilled water, and finally resuspended in 500 μl of water. Aliquots of 100 μl were spread onto YPD agar plates by using glass beads, and the plates were incubated at 30°C. Ascospore isolation was considered successful if colonies derived from ascospore germination developed from the cross within 48 to 72 h of incubation and if no colony could be observed from the parental controls within the same period of time. To ascertain that a true progeny made of meiotic products was used during the genetic analysis, segregation of the mating-type genes was always coanalyzed with segregation of the phenotypes of interest. Alternatively, genetic crosses were performed with parents carrying different recessive auxotrophic mutations on solid adequately supplemented YCB. Cell suspensions containing ascospores were spread onto solid YNB medium, on which only recombinant prototrophic meiotic products that had inherited both wild-type alleles from the parents were able to grow. Segregation ratios were all analyzed by the χ2 test.
Cytosine deaminase assay.
Yeast cells from a 6-h culture in YNB liquid medium (35°C, 250 rpm) were harvested by centrifugation and washed with 10 mM Tris-HCl (pH 7.5). The cellular pellet was frozen at −20°C and ground in a mortar under liquid nitrogen. The homogenate was resuspended in 2 ml of 10 mM Tris-HCl (pH 7.5) and centrifuged (10,000 × g, 4°C, 30 min). The supernatant constituted the crude extract, the protein concentration of which was measured by the method of Bradford (3). The cytosine deaminase assay was performed as described previously (17). The standard assay mixture contained, in a total volume of 150 μl, 100 μl of 20 mM cytosine (Sigma), 20 μl of 1 M sodium phosphate buffer (pH 7), and 30 μl of protein extract. The cytosine deaminase assay was stopped after 5, 10, 15, 20, and 30 min of incubation at 30°C by adding 10 μl of the reaction mixture to 1.5 ml of ice-cold 0.5 N perchloric acid. After a short centrifugation step (10,000 × g, 2 min), the optical density of 1 ml of supernatant was measured at 282 nm. The specific activity of cytosine deaminase was expressed as nanomoles of deaminated cytosine per minute per milligram of protein by using a millimolar extinction coefficient of 6.1 at 282 nm. Three independent assays were performed for each strain.
Uptake measurements.
Uptake measurements were made as described previously (4) by using [2-14C]5FC (1.92 GBq mmol−1) and [2-14C]cytosine (1.96 GBq mmol−1), from Moravek Biochemicals (Brea, Calif.), and [8-14C]adenine (1.96 GBq mmol−1), from Isotopchim (Ganagogie-Peyrus, France). Strains were grown in YPD medium at 35°C under agitation. The cells were harvested by centrifugation during the exponential phase (5 × 107 to 7 × 107 cells per ml) and resuspended at a density of 4 × 106 to 5 × 106 cells per ml in 50 mM sodium citrate (pH 5.0) containing 2% glucose at 30°C. The reaction was started by addition of radioactive ligand at concentrations ranging from 1 to 50 μM. As a function of time, aliquots (0.3 ml) were filtered through 0.8-μm-pore-size cellulose acetate filters from either the Pall Gelman Laboratory (Portsmouth, United Kingdom) or Sartorius (Goettingen, Germany), washed twice with 3 ml of cold water, dried, and assayed for radioactivity. As already reported (7), we verified that the initial rate of uptake was constant over the first 10 s of base accumulation. After this period of time, the rate of uptake decreased slowly due to the efflux of the solute that had accumulated. Accordingly, initial rates of uptake were measured after 4, 8, and 12 s of incubation. The maximal rate of uptake (Vmax) and the apparent Michaelis constant of transport [Kt(app)] were calculated by nonlinear regression analysis of the initial rates of uptake versus the solute concentrations by the method of Cleland (8).
RESULTS
Two-step screening for 5FC and FLC cross-resistance among clinical isolates.
The 60 clinical isolates were first screened for 5FC resistance on RPMI agar plates supplemented with 32 μg of 5FC per ml. Eight isolates were able to grow and were suspected to be resistant. The MICs of 5FC and four other antifungal drugs (AMB, FLC, ITC, KTC) were determined for each isolate by the microdilution method and compared to the MICs for two antifungal agent-susceptible reference strains, 6936 and 5094 (Table 2). This analysis confirmed that the clinical isolates selected were truly 5FC resistant, with the 5FC MICs for the isolates being greater than or equal to 128 μg/ml. Except for strain CL44, for which an elevated AMB MIC has already been described (25, 36), the isolates were susceptible to all other drugs tested.
TABLE 2.
MICs of antifungal agents for the C. lusitaniae 5FC-resistant isolates and reference strains
| Strain or isolate | MIC (μg/ml)
|
||||
|---|---|---|---|---|---|
| 5FC | AMB | FLC | ITC | KTC | |
| 6936 | ≤0.5 | 0.03 | 2 | 0.125 | ≤0.015 |
| 5094 | ≤0.5 | 0.06 | 1 | 0.125 | 0.03 |
| CI26 | 256 | 0.03 | 0.25 | 0.125 | ≤0.015 |
| CI29 | 128 | 0.03 | 2 | 0.06 | ≤0.015 |
| CI31 | 128 | 0.03 | 2 | 0.125 | ≤0.015 |
| CI32 | 256 | 0.06 | 0.25 | 0.125 | ≤0.015 |
| CI38 | 128 | 0.03 | 4 | 0.06 | ≤0.015 |
| CI42 | 128 | 0.25 | 2 | 0.125 | 0.03 |
| CI44 | ≥256 | 1 | 0.25 | ≤0.015 | ≤0.015 |
| CI60 | ≥256 | 0.06 | 8 | 0.125 | ≤0.015 |
In a second step, screening for cross-resistance among the eight 5FC-resistant isolates was performed by testing their ability to grow on RPMI agar plates containing 32 μg of 5FC per ml in combination with one of the four other antifungal agents, each of which was added at a concentration of four times the MIC for each isolate. Control experiments were performed to verify that four times the MIC of each of AMB, FLC, ITC, and KTC was sufficient to inhibit growth when each agent was used alone in RPMI agar plates. Growth was estimated after 48 h of incubation at 30°C by comparison with the growth observed on drug-free RPMI agar plates. The combination of antifungal agents led to complete growth inhibition in all cases except for one particular antifungal combination against four isolates. The growth of CL29, CL31, CL38, and CL42 was inhibited by four times the MIC of FLC but was restored when FLC was combined with 32 μg of 5FC per ml (data not shown). After 48 h of incubation, no difference in colony number or colony size could be observed between drug-free RPMI agar medium, 5FC-supplemented RPMI agar medium, and RPMI agar medium supplemented with 5FC and FLC for these four isolates.
Characterization of phenotype of 5FC and FLC cross-resistance.
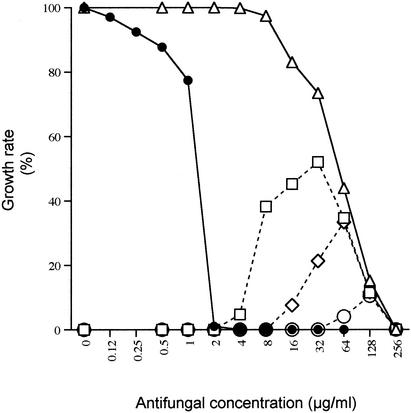
The growth of each isolate was measured by the twofold microdilution method in the presence of FLC alone (at a concentration gradient from 64 to 0.12 μg/ml), 5FC alone (at a concentration gradient from 256 to 0.5 μg/ml), and 5FC (at a concentration gradient from 256 to 0.5 μg/ml) in combination with three different concentrations of FLC (16, 32, and 64 μg/ml). A typical example of the results obtained after 48 h of incubation is shown for isolate CL42 (Fig. 1). Interestingly, cross-resistance to FLC did not occur over the entire range of 5FC concentrations. For example, 16 μg of FLC per ml was sufficient to inhibit the growth of CL42 in the presence of the lowest 5FC concentrations (from 0.5 to 2 μg/ml). Cross-resistance then developed progressively in the presence of 5FC at concentrations greater than 4 μg/ml, allowing growth of CL42 to reach a maximal rate (50% with regard to the growth rate in drug-free RPMI agar) in the presence of 32 μg of 5FC per ml. In the presence of greater concentrations, growth decreased in the same way as the decrease observed with 5FC alone, until complete inhibition occurred in the presence of 256 μg of 5FC per ml. Increases in the FLC concentration to 32 and 64 μg/ml resulted in concomitant increases in the threshold concentration of 5FC needed to trigger cross-resistance.
FIG. 1.
Growth rate of CL42 in the presence of different FLC and 5FC concentrations after 48 h of incubation. •, FLC alone; ▵, 5FC alone; □, 5FC gradient with a constant concentration of 16 μg of FLC per ml; ⋄, 5FC gradient with a constant concentration of 32 μg of FLC per ml; ○, 5FC gradient with a constant concentration of 64 μg of FLC per ml.
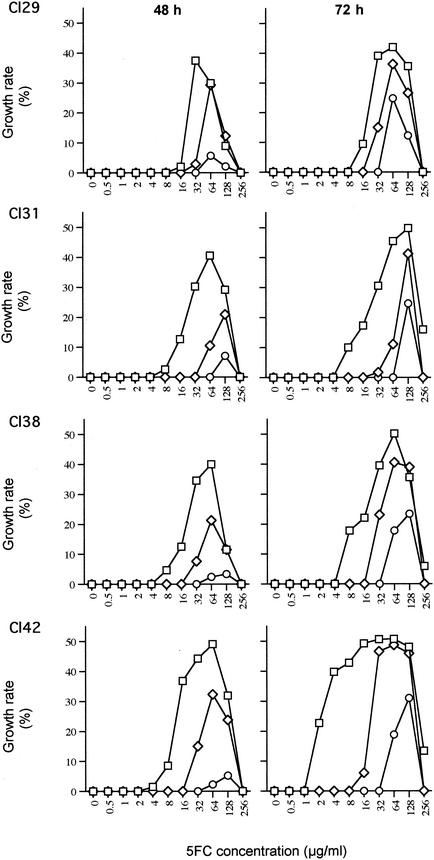
The cross-resistance patterns of the four clinical isolates varied slightly in terms of both the growth rates and the threshold concentration of 5FC from which they switched from an FLC-sensitive phenotype to an FLC-resistant phenotype (Fig. 2). Some of these variations could be related to the genotypes of the strains. For example, the cross-resistance of CL42 was consistently expressed over the higher 5FC concentration range. However, most of the variations routinely observed (±10% variation in the growth rate with a ±1 dilution in the threshold 5FC concentration) depended on environmental and physiological factors. Supplementation of RPMI with glucose and the use of larger inoculum sizes and fresh yeast cells (logarithmic growth phase) resulted in higher growth rates while the isolate exhibited cross-resistance and in concomitant lower threshold 5FC concentrations. The extent of these variations is not completely understood and contrasts with the weak variations generally observed during the routine determination of MICs for C. lusitaniae.
FIG. 2.
5FC and FLC cross-resistance patterns for each clinical isolate after 48 and 72 h of incubation. □, 5FC gradient with a concentration of 16 μg of FLC per ml; ⋄, 5FC gradient with a concentration of 32 μg of FLC per ml; ○, 5FC gradient with a concentration of 64 μg of FLC per ml.
Further experiments, summarized hereafter, were undertaken to better characterize the mechanism of cross-resistance (data not shown). First, we tried to trigger resistance to FLC (16, 32, and 64 μg/ml) by replacing 5FC with either cytosine or uracil, which were used at concentrations ranging from 512 to 1 μg/ml. Neither molecule was able to trigger resistance to FLC. Second, a 5FC gradient was used in an attempt to trigger cross-resistance to other azoles by replacing FLC by ITC or KTC at concentrations of 4, 8, and 16 times the MIC for each isolate. As expected from the results obtained during screening, cross-resistance was specific to the 5FC-FLC combination. Third, cross-resistant cells that had developed in the wells of a microplate with different concentrations of 5FC and FLC in combination were removed and inoculated in both liquid and solid RPMI supplemented with these combinations of 5FC and FLC and with FLC alone. Cells were able to grow only in the presence of both antifungals and failed to grow in the presence of FLC alone, indicating that FLC resistance could be expressed only in the presence of 5FC.
Genetic analyses of cross-resistance.
Genetic analyses were performed mainly to explore the possibility of whether the 5FC- and FLC-resistant (5FC + FLC)r phenotype could be disjoined through meiosis and yield progeny with an FLCr phenotype only. A first series of genetic crosses was carried out between antifungal agent-susceptible reference strain 6936 or 5094 and sexually compatible clinical isolates CL42 and CL38 (for the latter, the results of two different genetic crosses are reported in Table 3). Because of the technique used to isolate ascospores, the possibility of parental contamination of the progeny could not be excluded. For that reason, the segregation of the mating-type alleles was always analyzed in parallel with the segregation of antifungal resistance. The mating-type alleles segregated as an independent pair of alleles (segregation, 1/2:1/2) in each of the phenotypic groups observed in the progenies (Table 3), which confirmed that a pure progeny was isolated and analyzed in each cross. Additionally, crosses were performed with strains carrying auxotrophic markers. A first cross was carried out between CL38 MATα and strain 36/4 MATa Leu− in order to combine in a single progeny the antifungal resistance phenotype of CL38 and the auxotrophic phenotype of strain 36/4. Genetic analysis of the Leu− progeny of this cross allowed us to select strain 5/31 MATα Leu− 5FCr FLCs (5FC + FLC)r, which was further used in a cross with antifungal agent-susceptible strain 69/3 MATa Lys−. Segregation of the antifungal resistance phenotype was analyzed in the prototroph progeny of this cross.
TABLE 3.
Analysis of the segregation of the 5FC and (5FC + FLC) phenotypes and of the mating-type genes in the progeny from genetic crosses
| Cross
|
Phenotypes in progenya | No. of progeny | Segregation (no. of progeny)
|
|||
|---|---|---|---|---|---|---|
| No. | Strains | 5FCr:5FCs | (5FC + FLC)r: (5FC + FLC)s | MATa:MATα | ||
| 1 | CL42 MATa 5FCr FLCs (5FC + FLC)r × CL5094 MATα 5FCs FLCs (5FC + FLC)s | 5FCr FLCs (5FC + FLC)r 5FCs FLCs (5FC + FLC)s 5FCr FLCs (5FC + FLC)2 | 9 33 22 | 31:33 | 9:55b | 6:3 14:19 12:10 32:32c |
| 2 | CL38 MATα 5FCr FLCs (5FC + FLC)r × CL6936 MATa 5FCs FLCs (5FC + FLC)s | 5FCr FLCs (5FC + FLC)r 5FCs (5FC + FLC)s 5FCr FLCs (5FC + FLC)s | 24 259 | 33:25 | 24:34 | 11:13 11:14 5:4 27:31c |
| 3 | CL38 MATα 5FCr FLCs (5FC + FLC)r × CL6936 MATa 5FCs FLCs (5FC + FLC)s | 5FCr FLCs (5FC + FLC)r 5FCs FLCs (5FC + FLC)s 5FCr FLCs (5FC + FLC)s | 38 31 12 | 50:31b | 38:43 | 19:19 17:14 5:7 41:40c |
| 4 | CL38 MATα 5FC FLCs (5FC + FLC)r × 36/4 MATa Leu− 5FCs FLCs (5FC + FLC)s | 5FCr FLCs (5FC + FLC)r 5FCs FLCs (5FC + FLC)s | 15 14 | 15:14 | 15:14 | 7:8 4:10 11:18c |
| 5 | 5/31 MATα Leu− 5FCr FLCs (5FC + FLC)r × 69/3 MATa Lys− 5FCs FLCs (5FC + FLC)s | 5FCr FLCs (5FC + FLC)r 5FCs FLCs (5FC + FLC)5 | 30 43 | 30:43 | 30:43 | 17:13 22:21 39:34c |
False recombinant phenotype 5FCr FLCr (5FC + FLC)r was expressed on solid RPMI by one, one, and two progenies of crosses 1, 2, and 3, respectively. False recombinant phenotype 5FCs FLCr (5FC + FLC)s was expressed by two, three, and two progenies of crosses 1, 2, and 3, respectively. These phenotypes were not confirmed by the microdilution method. The phenotype 5FCr FLCr (5FC + FLC)r was reassigned to the phenotype 5FCr FLCs [5FC + FLC]r, and the phenotype 5FCs FLCr (5FC + FLC)s was reassigned to the phenotype 5FCs FLCs (5FC + FLC)s. No recombinant phenotype was observed in the progenies of the crosses involving at least one auxotrophic strain (crosses 4 and 5).
The segregation ratio statistically different from 1/2:1/2 at P = 0.05.
The values are totals.
To study the segregation of antifungal resistance phenotypes, the growth of the parents and the progenies was tested on RPMI agar plates supplemented with 5FC, FLC, and 5FC plus FLC (5FC and FLC concentrations, 64 and 32 μg/ml, respectively) and compared to the growth on drug-free RPMI agar plates. Initially, the results of the genetic analyses of the three first crosses reported in Table 3 were more complex than those presented. Besides both parental phenotypes and the nonparental phenotype 5FCr FLCs (5FC + FLC)s, two additional nonparental phenotypes were observed at a low frequency (see footnote a of Table 3): 5FCr FLCr (5FC + FLC)r and 5FCs FLCr (5FC + FLC)s. The profusion of nonparental phenotypes led us to check them by the microdilution method in liquid RPMI. Only the presence of isolates with the 5FCr FLCs (5FC + FLC)s phenotype was confirmed, with isolates of the two other phenotypes being falsely FLCr, behaving like the resistant parent and the susceptible parent, respectively. Accordingly, the phenotypic groups were revised.
In four of the five progenies analyzed, the 5FCr:5FCs phenotypes segregated 1/2:1/2. The 5FCr:5FCs segregation ratio differed significantly from 1/2:1/2 for only one progeny, obtained from clinical isolate CL38, but this was not confirmed by two other crosses involving CL38 (Table 3). It is therefore very likely that the 5FCr phenotype was derived from a single gene mutation. No FLC resistance gene could be characterized, since we never isolated a progeny resistant only to FLC among a total of 305 descendants analyzed from the five crosses. One may also reject the hypothesis that an FLC resistance gene could be tightly linked to the 5FC resistance gene, because some progenies had a 5FCr FLCs (5FC + FLC)s phenotype. Accordingly, the (5FC + FLC)r phenotype would be encoded only by the 5FC resistance gene. The fact that not all the 5FCr progenies expressed the (5FC + FLC)r phenotype would be due to a quantitative effect, which is further discussed below.
Recessiveness analysis.
When strain 5/31 MATα Leu− 5FCr FLCs (5FC + FLC)r and strain 69/3 MATa Lys− were plated on YCB, they entered conjugation within 12 h, whereas ascospore release had not yet begun. The conjugate-containing cellular mixture was removed from YCB, resuspended in water, and spread onto YNB medium, in which only heterocaryotic or diploid cells harboring two nuclei able to reciprocally complement the auxotrophic mutations could develop to form colonies. The heterocaryotic or diploid status of the prototroph colonies was confirmed by their ability to mate with each of the MATa and MATα test strains. When the prototroph colonies were replica plated onto YNB medium supplemented with 64 μg of 5FC per ml and leucine, they could no longer grow, while the growth of strain 5/31 on the same medium was normal, suggesting that the mutation conferring 5FC resistance was recessive.
Biochemical characterization of 5FC resistance.
Because resistance to 5FC was derived from the mutation of a single gene, it was of interest to identify the biochemical step whose dysfunction induced resistance. 5FC is a prodrug which is not toxic by itself. First, it enters the fungal cell through a cytosine permease and is then converted to 5FU by cytosine deaminase. Downstream, uracil phosphoribosyltransferase (UPRTase) converts 5FU to 5-fluorouridine monophosphate or 5-fluorodeoxyuridine monophosphate, two compounds that further interfere with protein and DNA synthesis, respectively (Fig. 3). To explore this metabolic pathway, we took advantage of the fact that 5FC can be replaced by 5FU to inhibit growth of the fungal cells as well as the fact that 5FU enters the cell through a permeation system distinct from that for cytosine permease. Measurement of the growth of the 5FC-resistant isolates in the presence of 5FU thus allowed assessment of the activities of two groups of enzymes (Fig. 3): those working upstream of the action of 5FU (cytosine permease and cytosine deaminase) and those working downstream of the action of 5FU (including UPRTase). The MICs of 5FU were determined for reference strains 6936 and 5094; clinical isolate CL60, which was 5FC resistant and 5FC and FLC susceptible; the four clinical isolates cross resistant to 5FC and FLC (isolates CL29, CL31, CL38, and CL42); and an EMS-induced cross-resistant mutant of C. lusitaniae (isolate CLE11-801). The results obtained with both 6936 and 5094 (Table 4) confirmed that 5FU had activity against C. lusitaniae cells. Isolate CL60 was 5FU resistant, whereas the isolates cross resistant to 5FC and FLC and strain CLE11-801 were all 5FU susceptible. These results indicate that the deficient enzymatic step is located at the UPRTase level or downstream in the CL60 isolate and concerned either cytosine deaminase or cytosine permease in all isolates cross resistant to 5FC and FLC.
FIG. 3.
Main enzymatic steps involved in uptake, conversion, and mechanism of action of fluoropyrimidines. 5FUMP, 5-fluorouridine monophosphate; 5FdUMP, 5-fluorodeoxyuridine monophosphate.
TABLE 4.
MICs of 5FU, cytosine deaminase activities, and kinetic parameters of [14C]5FC uptake for C. lusitaniae 5FC-resistant isolates and reference strains
| Strain or isolate | MIC (μg/ml) of 5FU | Cytosine deaminase activitya | 5FC uptake parametersb
|
|
|---|---|---|---|---|
| Kt(app) (μM) | Vmax (nmol min−1 107 cells−1) | |||
| 6936 | ≤0.5 | 7.89 ± 0.81 | 6.15 ± 0.18 | 1.20 ± 0.50 |
| 5094 | ≤0.5 | 20.98 ± 3.52 | 7.06 ± 3.03 | 1.09 ± 0.58 |
| CL60 | ≥256 | 48.93 ± 2.79 | 10.78 ± 0.32 | 1.63 ± 0.64 |
| CL29 | ≤0.5 | 23.78 ± 8.93 | NM | NM |
| CL31 | ≤0.5 | 17.38 ± 2.41 | NM | NM |
| CL38 | ≤0.5 | 12.83 ± 4.34 | NM | NM |
| CL42 | ≤0.5 | 25.15 ± 0.64 | NM | NM |
| CLE11-801 | ≤0.5 | 7.40 ± 0.11 | NM | NM |
Expressed as nanomoles of deaminated cytosine per minute per milligram of protein; values are averages of three independent assays.
5FC uptake was measured over a concentration range from 1 to 50 μM. If the transport could not be detected by using a 5FC concentration of 50 μM, it was noted as nonmeasurable (NM). Kt(app) and Vmax were calculated by nonlinear regression of the saturation curves obtained with at least five different solute concentrations. All experiments were done in triplicate.
Accordingly, all the strains and isolates listed above were assayed for cytosine deaminase activity. Despite great variations, cytosine deaminase activity was detectable in all the 5FC-resistant isolates, as well as 5FC-susceptible reference strains 6936 and 5094 (Table 4).
Finally, cytosine permease activity was assayed by the use of [14C]5FC uptake transport measurements. Prior to the development of this assay with C. lusitaniae, the uptake transport of pyrimidine and purine bases was briefly studied in reference strain 6936, as described previously (4). We first verified that [14C]5FC, [14C]cytosine, and [14C]adenine were efficiently transported into yeast cells (data not shown). Thereafter, 5FC uptake was subjected to competition by measuring the transport of 10 μM [14C]5FC in the presence of either 100 μM cytosine or 100 μM adenine. No Vmax or Kt(app) was measurable, indicating that 5FC transport is subjected to competitive inhibition by both pyrimidine and purine bases. A single permease is thus involved in the uptake transport of 5FC, cytosine, and adenine.
The uptake transport by the wild-type reference strains, together with the 5FC-resistant clinical isolates and the EMS-induced resistant mutant, was assayed by use of a range of [14C]5FC concentrations, and kinetic parameters were determined (Table 4). Strains 6936 and 5094 and clinical isolate CL60 showed detectable transport activity, with the Vmax and Kt(app) values for 5FC for the three strains being similar. This result was expected for CL60, because the deficient enzymatic step conferring 5FC resistance was localized downstream of the action of 5FU. The other clinical isolates tested (isolates CL29, CL31, CL38, and CL42) and strain CLE11-801 did not show any significant transport of 5FC compared to that for the wild-type controls. Lastly, we tried to determine whether FLC could act as a competitive inhibitor of 5FC uptake in wild-type strains. No significant change in Vmax or Kt(app) was observed during the transport of 10 μM [14C]5FC in the presence of 157 μM FLC (corresponding to 48 μg/ml) in strains 6936 and 5094.
In vitro selection of mutants of C. lusitaniae and other yeast species cross resistant to 5FC and FLC.
It was then of interest to determine whether the mechanism of cross-resistance described above was specific to C. lusitaniae or if it could affect other medically important yeast species. Since the recovery and the screening of large collections of clinical isolates of different species would have proved long and fastidious, a two-step in vitro procedure for the selection of spontaneous and EMS-induced resistant mutants was performed with C. lusitaniae, C. albicans, Candida tropicalis, Candida glabrata, and Cryptococcus neoformans. First, RPMI agar plates supplemented with 64 μg of 5FC per ml alone or with 64 μg of 5FC per ml plus 32 μg of FLC per ml were used to select resistant mutants (Table 5). RPMI agar plates supplemented with FLC alone were not used at this stage, since we knew that cross-resistance developed from 5FC-resistant and FLC-susceptible strains. The frequency of occurrence of resistant colonies corresponded to the average number of colonies growing on the selective medium (from 2 to 3,200, depending on the strain, the treatment, and the medium) versus the number of viable cells that were plated (from 0.5 × 107 to 3 × 107, according to the strain). The technical parameters of mutagenesis (mortality rates, mutant enrichment factors) are also reported. As shown in Table 5, colonies with spontaneous and EMS-induced resistance were selected on both types of media for each species except C. neoformans, colonies of which simultaneously resistant to 5FC and FLC could not be selected.
TABLE 5.
Frequency of resistant mutants selected on plates with 5FC and 5FC plus FLC plates after spontaneous and EMS-induced mutation
| Strain | Mutation frequency
|
Mutagenesis parameters
|
||||
|---|---|---|---|---|---|---|
| Spontaneous
|
EMS induced
|
% Mortality | Enrichment factor | |||
| 5FC | 5FC + FLC | 5FC | 5FC + FLC | |||
| C. lusitaniae 6936 | 1.3 × 10−6 | 4.1 × 10−7 | 2.2 × 10−4 | 6.9 × 10−5 | 42.7 | 172 |
| C. albicans 2091 | 6.4 × 10−6 | 9.2 × 10−7 | 5.4 × 10−5 | 4.3 × 10−5 | 39.8 | 8 |
| C. tropicalis 94 | 5.5 × 10−7 | 9.8 × 10−8 | 1.6 × 10−4 | 8.1 × 10−5 | 91.7 | 285 |
| C. glabrata 90030 | 1.5 × 10−6 | 8.4 × 10−7 | 4.0 × 10−4 | 2.1 × 10−4 | 96.8 | 278 |
| C. neoformans 135 | 1.1 × 10−6 | 0 | 2.8 × 10−5 | 0 | 27.1 | 26 |
For each strain, the resistance phenotype of the colonies was then studied with regard to cross-resistance to 5FC and FLC. For that, a set of colonies with spontaneous and induced resistance was randomly selected from each selective medium plate and tested for the ability to grow on RPMI agar plates supplemented with 64 μg of 5FC per ml alone, 32 μg of FLC per ml alone, or 64 μg of 5FC per ml plus 32 μg of FLC per ml. The results (Table 6) show that mutants cross-resistant to 5FC and FLC in a 5FCr FLCs phenotypic background could easily be selected from all Candida species but not C. neoformans. Confirmation of the cross-resistant phenotype was obtained for some mutants of each Candida species by the microdilution method. The growth curves of the isolates observed during cross-resistance were fully comparable to those of the clinical isolates of C. lusitaniae presented earlier (Fig. 1).
TABLE 6.
Phenotypes of mutant resistant colonies selected after spontaneous or EMS-induced mutation
| Strain | Mutant
|
No. of mutants in the following phenotypic categoryb:
|
||||
|---|---|---|---|---|---|---|
| Selected from: | Origina | No. analyzed | 5FCr FLCs (5FC + FLC)s | 5FCr FLCr (5FC + FLC)r | 5FCr FLCs (5FC + FLC)r | |
| C. lusitaniae | 5FC | S | 5 | 3 | 0 | 2 |
| EMS | 10 | 8 | 0 | 2 | ||
| 5FC + FLC | S | 2 | 0 | 0 | 2 | |
| EMS | 10 | 2 | 4 | 4 | ||
| C. albicans | 5FC | S | 16 | 15 | 1 | 0 |
| EMS | 10 | 9 | 0 | 1 | ||
| 5FC + FLC | S | 4 | 2 | 0 | 2 | |
| EMS | 10 | 1 | 1 | 8 | ||
| C. tropicalis | 5FC | S | 17 | 10 | 1 | 6 |
| EMS | 10 | 10 | 0 | 0 | ||
| 5FC + FLC | S | 3 | 0 | 0 | 3 | |
| EMS | 10 | 0 | 5 | 5 | ||
| C. glabrata | 5FC | S | 10 | 1 | 0 | 9 |
| EMS | 10 | 3 | 4 | 3 | ||
| 5FC + FLC | S | 10 | 0 | 0 | 10 | |
| EMS | 10 | 0 | 4 | 6 | ||
| C. neoformans | 5FC | S | 20 | 20 | 0 | 0 |
| EMS | 20 | 20 | 0 | 0 | ||
S, spontaneous mutant; EMS, EMS-induced mutant.
Resistance phenotypes were determined after replica plating of the mutant colonies on RPMI agar plates supplemented with 64 μg of 5FC alone per ml, 32 μg of FLC alone per ml, or 64 μg of 5FC per ml plus 32 μg of FLC per ml.
DISCUSSION
We identified four clinical 5FC-resistant isolates of C. lusitaniae that were further shown to be specifically cross-resistant to FLC when both 5FC and FLC were used simultaneously but FLC susceptible when FLC was used alone. The fact that FLC resistance could be expressed only in the presence of 5FC suggests that a single genetic event is responsible for the simultaneous resistance to both antifungals. This was confirmed by the genetic analyses: resistance to 5FC was shown to derive from a recessive mutation in a single gene, but no gene conferring resistance to FLC could be identified. It was therefore essential to characterize the mechanism of resistance to 5FC at the molecular level. We showed that key enzymes of the salvage pyrimidine pathway, such as cytosine deaminase and UPRTase, were unaffected. In fact, kinetic studies of [14C]5FC transport demonstrated that 5FC resistance was correlated with undetectable intracellular levels of the drug. Two hypotheses can explain this result. The first one is based on a very efficient efflux transport of 5FC, but this is highly improbable for two reasons. First, once 5FC has entered the cell, it would become the substrate of two proteins: the efflux transporter and the cytosine deaminase. Even though the efflux transporter had a greater affinity than cytosine deaminase for 5FC, a fraction of [14C]5FC should have been converted to [14C]5FU, which could have been measured. Second, it is assumed that this efflux mechanism would also transport FLC. Knowing that FLC resistance occurs only in the presence of 5FC, this would mean that the efflux mechanism is induced by 5FC or that 5FC and FLC are cotransported. This model does not fit the known efflux mechanisms described so far (6, 27, 33) and does not explain why we recovered progenies from genetic crosses that were only 5FC resistant and had lost the phenotype of 5FC and FLC cross-resistance.
The second hypothesis is based on the loss of activity of the cytosine permease. Accordingly, 5FC would not be transported inside the cells and its level would be maintained at the extracellular level. Cross-resistance to FLC would result from the competitive inhibition of FLC influx transport by extracellular 5FC, which supposes the occurrence of an uptake transporter for FLC. This model explains why with higher FLC concentrations, the 5FC concentration necessary to trigger cross-resistance is also higher, which is typical of competitive inhibition. Furthermore, we showed that withdrawal of 5FC again makes cells susceptible to FLC, which also supports the hypothesis. It is likely that FLC enters the cell not through the cytosine permease but through a distinct transporter, since we failed to inhibit or to slow 5FC uptake transport with FLC in wild-type strains. Additionally, if cytosine permease were also implicated in FLC transport, its deficiency would have resulted in resistance to FLC alone, which is not the case. On the other hand, 5FC competitively binds to the FLC transporter but is not itself transported and behaves as a strict inhibitor; otherwise, cells would have been killed by the conversion of intracellular 5FC to 5FU. Moreover, the FLC uptake transport is specifically challenged by 5FC and not by analogs such as cytosine or uracil. Interestingly, both 5FC and FLC molecules share a slight structural homology in their fluorinated six-atom cycle. With respect to this homology, it would be valuable to test the behavior of voriconazole, which is also fluorinated.
It is conceivable that the number of FLC uptake transporters varies by strain. This could explain the variations in the cross-resistance levels and in the threshold 5FC concentrations required for induction of cross-resistance in clinical isolates. Likewise, the nonparental phenotypes, which were observed in the progenies of some genetic crosses, are understandable only by consideration of the fact that 5FC and FLC cross-resistance varies quantitatively, depending on the ratio of the extracellular 5FC concentration to the number of FLC transporters. In particular, the nonparental phenotype 5FCr FLCs (5FC + FLC)s could be exhibited by progenies with a larger number of transporters, thus making the 5FC concentration insufficient to inhibit FLC transport.
Taken together, all these arguments strongly support the idea that a mere dysfunctioning of cytosine permease is responsible for 5FC and FLC cross-resistance. Although assays for the transport of guanine and hypoxanthine were not performed, it is likely that the cytosine permease of C. lusitaniae, which also transports adenine, behaves like the purine-cytosine permease of C. albicans (12) and Saccharomyces cerevisiae, which is encoded by the FCY2 gene (16). Accordingly, the fcy2 genotype was assigned to all of the cross-resistant C. lusitaniae strains.
The 5FC-resistant isolates in a collection of 60 clinical isolates that were supposedly genetically unrelated and that were collected over a period of 10 years from individual patients were screened. Nearly 13% of the isolates were resistant to 5FC, which is consistent with the prevalence of primary 5FC resistance reported in clinically important yeast species (1, 9, 35). The four 5FC and FLC cross-resistant isolates represented 50% of the 5FCr isolates and 6.5% of the whole collection. Among them, only isolate CL42 was implicated in a fungemia and documented as having been exposed to a combination of AMB and 5FC. The three others were derived from routine surveillance. 5FC and FLC cross-resistance is therefore rather frequent, even in strains that were not subjected to previous antifungal treatment. Furthermore, it is not restricted to a genetic trait of C. lusitaniae. From the in vitro selection of 5FCr mutants, we demonstrated its existence in other species of Candida, even in diploid species, at a frequency comparable to that observed in C. lusitaniae, thus opening the way to a systematic study of its occurrence in clinical specimens. If the failure to select cross-resistance in 5FCr mutants of C. neoformans is confirmed by further experiments, it will be difficult to interpret in any way because of the lack of information concerning purine and pyrimidine transport in C. neoformans and more generally in basidiomycetes.
A drug import defect may be a primary cause of resistance. However, except for the critical role of cytosine permease for the uptake of 5FC, very little is known about the way in which the other antifungal agents, especially azoles, enter the cell. So far, only one study, carried out with C. albicans, measured the uptake of [3H]KTC and concluded that the uptake system is energy dependent, at least for the transport of low KTC concentrations (2). Our study, which argues for the existence of the uptake transport of FLC, suggests that azole uptake transporters would be widespread in yeast species.
It is not known whether this mechanism of cross-resistance is of significance at the clinical level. Either way, for the first time, our results strongly suggest that, in Candida species, FLC probably enters the cell not only by passive diffusion, as has been believed thus far, but also through an uptake transporter and that FLC influx is subjected to competitive inhibition by 5FC. This finding has several implications. First, it means that a low intracellular FLC concentration does not necessarily derive from the activation of an efflux mechanism, unless it is obviously demonstrated by a molecular approach. Second, it points out the existence of a new possible mechanism of resistance to FLC, that is, through a defect in drug uptake. When [14C]FLC, the only marker suitable for accurate kinetic transport studies, becomes available, we should be able to provide direct evidence for the occurrence of this transporter.
Acknowledgments
We thank Annie Michel-Nguyen (Laboratoire de Microbiologie, Hôpital St Joseph, Marseille, France), who isolated and identified most of the strains used in this study, and A. Brulfert (Université Paris 5) for critical comments on the manuscript.
REFERENCES
- 1.Barchiesi, F., D. Arzeni, F. Caselli, and G. Scalise. 2000. Primary resistance to flucytosine among clinical isolates of Candida spp. J. Antimicrob. Chemother. 45:408-409. [DOI] [PubMed] [Google Scholar]
- 2.Boiron, P., E. Drouhet, B. Dupont, and L. Improvisi. 1987. Entry of ketoconazole into Candida albicans. Antimicrob. Agents Chemother. 31:244-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 4.Brèthes, D., M. C. Chirio, C. Napias, M. R. Chevallier, J. L. Lavie, and J. Chevallier. 1992. In vivo and in vitro studies of the purine-cytosine permease of Saccharomyces cerevisiae. Functional analysis of a mutant with an altered apparent transport constant of uptake. Eur. J. Biochem. 204:699-704. [DOI] [PubMed] [Google Scholar]
- 5.Burke, D., D. Dawson, and T. Stearns. 2000. Methods in yeast genetics: a Cold Spring Harbor Laboratory course manual, 2000 ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 6.Calabrese, D., J. Bille, and D. Sanglard. 2000. A novel multidrug efflux transporter gene of the major facilitator superfamily from Candida albicans (FLU1) conferring resistance to fluconazole. Microbiology 146:2743-2754. [DOI] [PubMed] [Google Scholar]
- 7.Chirio, M. C., D. Brèthes, C. Napias, X. Grandier-Vazeille, F. Rakotomanana, and J. Chevallier. 1990. Photoaffinity labelling of the purine-cytosine permease of Saccharomyces cerevisiae. Eur. J. Biochem. 194:293-299. [DOI] [PubMed] [Google Scholar]
- 8.Cleland, W. W. 1967. The statistical analysis of enzyme kinetic data. Adv. Enzymol. 29:1-32. [DOI] [PubMed] [Google Scholar]
- 9.Cuenca-Estrella, M., T. M. Diaz-Guerra, E. Mellado, and J. L. Rodriguez-Tudela. 2001. Flucytosine primary resistance in Candida species and Cryptococcus neoformans. Eur. J. Clin. Microbiol. Infect. Dis. 20:276-279. [DOI] [PubMed] [Google Scholar]
- 10.Ellis, D. 2002. Amphotericin B: spectrum and resistance. J. Antimicrob. Chemother. 49:7-10. [DOI] [PubMed] [Google Scholar]
- 11.Ernst, E. J., K. Yodoi, E. E. Roling, and M. E. Klepser. 2002. Rates and extents of antifungal activities of amphotericin B, flucytosine, fluconazole, and voriconazole against Candida lusitaniae determined by microdilution, Etest, and time-kill methods. Antimicrob. Agents Chemother. 46:578-581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fasoli, M. O., and D. Kerridge. 1990. Uptake of pyrimidines and their derivatives into Candida glabrata and Candida albicans. J. Gen. Microbiol. 136:1475-1481. [DOI] [PubMed] [Google Scholar]
- 13.Francois, F., T. Noël, R. Pépin, A. Brulfert, C. Chastin, A. Favel, and J. Villard. 2001. Alternative identification test relying upon sexual reproductive abilities of Candida lusitaniae strains isolated from hospitalized patients. J. Clin. Microbiol. 39:3906-3914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ghannoum, M. A., and L. B. Rice. 1999. Antifungal agents: mode of action, mechanisms of resistance, and correlation of these mechanisms with bacterial resistance. Clin. Microbiol. Rev. 12:501-517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guinet, R., J. Chanas, A. Goullier, G. Bonnefoy, and P. Ambroise-Thomas. 1983. Fatal septicemia due to amphotericin B-resistant Candida lusitaniae. J. Clin. Microbiol. 18:443-444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hopkins, P., M. R. Chevallier, R. Jund, and A. A. Eddy. 1988. Use of plasmid vectors to show that the uracil and cytosine permeases of yeast Saccharomyces cerevisiae are electrogenic protons symports. FEMS Microbiol. Lett. 49:173-177. [Google Scholar]
- 17.Jund, R., and F. Lacroute. 1970. Genetic and physiological aspects of resistance to 5-fluoropyrimidines in Saccharomyces cerevisiae. J. Bacteriol. 102:607-615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kelly, S. L., D. C. Lamb, D. E. Kelly, J. Loeffler, and H. Einsele. 1996. Resistance to fluconazole and amphotericin in Candida albicans from AIDS patients. Lancet 348:1523-1524. [DOI] [PubMed] [Google Scholar]
- 19.Lewis, R. E., and D. P. Kontoyiannis. 2001. Rationale for combination antifungal therapy. Pharmacotherapy 21:149S-164S. [DOI] [PubMed]
- 20.Merz, W. G. 1984. Candida lusitaniae: frequency of recovery, colonization, infection, and amphotericin B resistance. J. Clin. Microbiol. 20:1194-1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.National Committee for Clinical Laboratory Standards. 1997. Reference method for broth dilution antifungal susceptibility testing of yeasts. M-27A. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 22.Neely, M. N., and M. A. Ghannoum. 2000. The exciting future of antifungal therapy. Eur. J. Clin. Microbiol. Infect. Dis. 19:897-914. [DOI] [PubMed] [Google Scholar]
- 23.Nolte, F. S., T. Parkinson, D. J. Falconer, S. Dix, J. Williams, C. Gilmore, R. Geller, and J. R. Wingard. 1997. Isolation and characterization of fluconazole- and amphotericin B-resistant Candida albicans from blood of two patients with leukemia. Antimicrob. Agents Chemother. 41:196-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pappagianis, D., M. S. Collins, R. Hector, and J. Remington. 1979. Development of resistance to amphotericin B in Candida lusitaniae infecting a human. Antimicrob. Agents Chemother. 16:123-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rex, J. H., C. R. Cooper, Jr., W. G. Merz, J. N. Galgiani, and E. J. Anaissie. 1995. Detection of amphotericin B-resistant Candida isolates in a broth-based system. Antimicrob. Agents Chemother. 39:906-909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sanglard, D., F. Ischer, L. Koymans, and J. Bille. 1998. Amino acid substitutions in the cytochrome P-450 lanosterol 14α-demethylase (CYP51A1) from azole-resistant Candida albicans clinical isolates contribute to resistance to azole antifungal agents. Antimicrob. Agents Chemother. 42:241-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sanglard, D., F. Ischer, M. Monod, and J. Bille. 1997. Cloning of Candida albicans genes conferring resistance to azole antifungal agents: characterization of CDR2, a new multidrug ABC transporter gene. Microbiology 143:405-416. [DOI] [PubMed] [Google Scholar]
- 28.Sanglard, D., K. Kuchler, F. Ischer, J. L. Pagani, M. Monod, and J. Bille. 1995. Mechanisms of resistance to azole antifungal agents in Candida albicans isolates from AIDS patients involve specific multidrug transporters. Antimicrob. Agents Chemother. 39:2378-2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sanglard, D., and F. C. Odds. 2002. Resistance of Candida species to antifungal agents: molecular mechanisms and clinical consequences. Lancet Infect. Dis. 2:73-85. [DOI] [PubMed] [Google Scholar]
- 30.Sheehan, D. J., C. A. Hitchcock, and C. M. Sibley. 1999. Current and emerging azole antifungal agents. Clin. Microbiol. Rev. 12:40-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Siau, H., and D. Kerridge. 1999. 5-Fluorocytosine antagonizes the action of sterol biosynthesis inhibitors in Candida glabrata. J. Antimicrob. Chemother. 43:767-775. [DOI] [PubMed] [Google Scholar]
- 32.Siau, H., and D. Kerridge. 1998. The effect of antifungal drugs in combination on the growth of Candida glabrata in solid and liquid media. J. Antimicrob. Chemother. 41:357-366. [DOI] [PubMed] [Google Scholar]
- 33.Smriti, S. Krishnamurthy, B. L. Dixit, C. M. Gupta, S. Milewski, and R. Prasad. 2002. ABC transporters Cdr1p, Cdr2p and Cdr3p of a human pathogen Candida albicans are general phospholipid translocators. Yeast 19:303-318. [DOI] [PubMed] [Google Scholar]
- 34.Sugar, A. M. 1995. Use of amphotericin B with azole antifungal drugs: what are we doing? Antimicrob. Agents Chemother. 39:1907-1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vermes, A., H. J. Guchelaar, and J. Dankert. 2000. Flucytosine: a review of its pharmacology, clinical indications, pharmacokinetics, toxicity and drug interactions. J. Antimicrob. Chemother. 46:171-179. [DOI] [PubMed] [Google Scholar]
- 36.Wanger, A., K. Mills, P. W. Nelson, and J. H. Rex. 1995. Comparison of Etest and National Committee for Clinical Laboratory Standards broth macrodilution method for antifungal susceptibility testing: enhanced ability to detect amphotericin B-resistant Candida isolates. Antimicrob. Agents Chemother. 39:2520-2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.White, T. C., K. A. Marr, and R. A. Bowden. 1998. Clinical, cellular, and molecular factors that contribute to antifungal drug resistance. Clin. Microbiol. Rev. 11:382-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yoon, S. A., J. A. Vazquez, P. E. Steffan, J. D. Sobel, and R. A. Akins. 1999. High-frequency, in vitro reversible switching of Candida lusitaniae clinical isolates from amphotericin B susceptibility to resistance. Antimicrob. Agents Chemother. 43:836-845. [DOI] [PMC free article] [PubMed] [Google Scholar]