Abstract
Biofilms formed by Klebsiella pneumoniae resisted killing during prolonged exposure to ampicillin or ciprofloxacin even though these agents have been shown to penetrate bacterial aggregates. Bacteria dispersed from biofilms into medium quickly regained most of their susceptibility. Experiments with free-floating bacteria showed that stationary-phase bacteria were protected from killing by either antibiotic, especially when the test was performed in medium lacking carbon and nitrogen sources. These results suggested that the antibiotic tolerance of biofilm bacteria could be explained by nutrient limitation in the biofilm leading to stationary-phase existence of at least some of the cells in the biofilm. This mechanism was supported by experimental characterization of nutrient availability and growth status in biofilms. The average specific growth rate of bacteria in biofilms was only 0.032 h−1 compared to the specific growth rate of planktonic bacteria of 0.59 h−1 measured in the same medium. Glucose did not penetrate all the way through the biofilm, and oxygen was shown to penetrate only into the upper 100 μm. The specific catalase activity was elevated in biofilm bacteria to a level similar to that of stationary-phase planktonic cells. Transmission electron microscopy revealed that bacteria were affected by ampicillin near the periphery of the biofilm but were not affected in the interior. Taken together, these results indicate that K. pneumoniae in this system experience nutrient limitation locally within the biofilm, leading to zones in which the bacteria enter stationary phase and are growing slowly or not at all. In these inactive regions, bacteria are less susceptible to killing by antibiotics.
Bacteria growing in biofilms resist killing by antibiotics. A flurry of recent review articles attest to the growing interest in this problem (5, 11, 12, 15), but the fundamental biology and physics behind this phenomenon remain obscure. Two hypotheses have historically dominated this debate. The first is that antibiotics fail to fully penetrate biofilms (14). The second hypothesis supposes that at least some of the bacteria in a biofilm enter a slow- or nongrowing state in which the bacteria are less susceptible to killing (4, 19). Reduced growth in parts of the biofilm could be due to incomplete penetration of metabolic substrates into the biofilm. The only satisfactory means of discriminating among the contributions of these two resistance mechanisms is to measure, in the same experimental system, antibiotic penetration, nutrient delivery, and bacterial growth rates.
In two previous articles, we reported that ciprofloxacin readily penetrated biofilms formed by Klebsiella pneumoniae but failed to kill bacteria in the biofilm (1, 20). Ampicillin was likewise shown to penetrate biofilms formed by a β-lactamase-negative mutant strain but killed these bacteria, which were highly sensitive to ampicillin when tested in free aqueous suspension, only very slowly (1, 20). These results demonstrate convincingly that poor penetration of antibiotics is an insufficient explanation for biofilm resistance in this system. Some other protective mechanism must be at work.
This article describes experimental measurements of bacterial killing, average specific growth rate, specific catalase activity, glucose penetration, and oxygen penetration in the same K. pneumoniae biofilm model system with which we previously measured antibiotic penetration. We also report measurements of planktonic cell susceptibility as a function of inoculum growth status and medium nutrient level. Our hypothesis was that some of the bacteria in these biofilms experience nutrient limitation and enter a stationary-phase state. It was further hypothesized that bacteria that had entered stationary phase would be protected from killing by antibiotics as long as they lacked key nutrients.
MATERIALS AND METHODS
Bacterial strains, media, and antibiotics.
A β-lactamase-positive wild-type K. pneumoniae strain (Kp1) and a β-lactamase-deficient mutant (Kp102M) were used in pure culture (1). The medium used throughout was a phosphate-buffered minimal medium with glucose as the sole carbon and energy source. The medium contained 6.25 g of anhydrous dextrose, 0.360 g of NH4Cl, 0.100 g of MgSO4 · 7H2O, 5.68 g of Na2HPO4 (anhydrous), 5.44 g of KH2PO4, 250 μl of trace elements (1), and 15.0 g of Bacto agar (Difco Laboratories, Detroit, Mich.) per liter of deionized water. Liquid cultures were grown in the same medium without agar. Phosphate-buffered water (PBW; 0.31 mM phosphate, 2.0 mM magnesium) was prepared as described elsewhere (1) and used for culture dilutions. Ampicillin sodium salt was purchased from Sigma Chemical Company (St. Louis, Mo.). The Bayer Corporation (Leverkusen, Germany) donated ciprofloxacin hydrochloride powder. Powdered antibiotics were dissolved in sterile water and added to molten culture medium (≈50°C) to create antibiotic-amended agar for biofilm experiments or added to culture broth for planktonic experiments.
Planktonic susceptibility.
Overnight planktonic cultures of K. pneumoniae were diluted to an optical density at 600 nm, 1-cm path length, of 0.200 with PBW. One milliliter of diluted culture was used to inoculate 100 ml of medium for a final population of ca. 106 CFU per ml. In some experiments complete medium was used, while in other experiments glucose and ammonium chloride were omitted from the medium. After removing the time-zero sample, 0.500 g of ampicillin or 180 μg of ciprofloxacin, dissolved in sterile nanopure water, was added to the subculture to attain antibiotic concentrations of 5,000 μg/ml and 1.8 μg/ml, respectively. These concentrations of antibiotics were approximately 10 times the MICs (1). The culture was placed on a 37°C orbital shaker and sampled every 30 min for 4 h. The sampling procedure was as follows. A 1.5-ml sample of the culture was pipetted into a 2.0-ml conical microcentrifuge tube (Fisher Scientific, San Francisco, Calif.). The bacteria were pelleted at 10,000 rpm for 7.5 min at room temperature with a Micro14 microcentrifuge (Fisher Scientific, San Francisco, Calif.). The supernatant was removed with a pipette. The bacteria were washed with 1.5 ml of PBW and repelleted as described above, and the supernatant was removed and discarded. The bacteria were suspended in 1.5 ml of PBW and then serially diluted in PBW. Viable bacteria were enumerated as described below.
Biofilm preparation.
Overnight planktonic cultures of K. pneumoniae were diluted to an optical density at 600 nm, 1-cm path length, of 0.200 in PBW and used to inoculate individual sterile, black, polycarbonate membrane filters (25-mm diameter, 0.2-μm pore; Poretics Corp., Livermore, Calif.) resting on agar culture medium. A 5-μl inoculum was used for biofilm susceptibility experiments, whereas a 50-μl inoculum was used for penetration studies. Membranes were sterilized by UV exposure (15 min per side) prior to inoculation. Plates were inverted and incubated at 37°C for 48 h, and the membrane-supported biofilms were transferred to fresh culture medium every 8 to 10 h.
Biofilm susceptibility.
Colony biofilms were transferred to antibiotic-containing agar and incubated at 37°C. Biofilms were sampled every 30 min for 4 h or at approximately daily intervals for longer-term experiments. When sampled, each membrane-supported biofilm was placed in 9.0 ml of PBW and vortexed on high speed for 2.0 min with a Maxi Mix II Vortex mixer (Barnstead/Thermolyne, Dubuque, Iowa) and then serially diluted in PBW. Viable bacteria were enumerated as described below. Because the volume of fluid carried with the biofilm was only a few microliters, residual antibiotic was diluted by a factor of approximately 1,000 in the first step of the sampling process.
Viable-cell enumeration.
Serially diluted samples were plated on R2A agar (Difco Laboratories, Detroit, Mich.) with the drop plating method (8) and incubated at 37°C for 18 to 20 h. The change in CFU before and after treatment was calculated as a log reduction of CFU. The log reduction of CFU, or simply log reduction, at a particular sampling time was defined as the negative log10 of the quotient of the CFU at that time and the CFU prior to treatment. A positive log reduction represented a decrease in CFU. Where three or more replicate experiments were performed, log reduction values were averaged, and the standard error of the mean was calculated. Mean log reductions were compared with a two-tailed, two-sample t test assuming unequal variances.
Specific growth rates.
Viable-cell numbers in colony biofilms were measured over the 48-h development period following inoculation of the membrane by the methods described above. Specific growth rates were computed by linear regression of natural log cell number versus time data; the slope of this line was the estimated specific growth rate.
Catalase activity.
The catalase activity of mid-log-phase planktonic, stationary-phase planktonic, and 72-h colony biofilm cultures was quantified with a spectrophotometric hydrogen peroxide assay. Samples of planktonic cultures or colony biofilms suspended in phosphate-buffered saline were centrifuged at 6,000 rpm for 10 min. The bacteria were washed twice with cold 50 mM potassium phosphate buffer (pH 7.0). After washing, the cells were resuspended in the buffer and stored at −70°C until sonication. Cells were lysed by administering five 30-s sonication pulses (4 W) to 1-ml aliquots of the cell suspension. Sonicated samples were centrifuged at 13,000 × g for 10 min. An aliquot of the cell lysate was mixed with 18 mM hydrogen peroxide in 50 mM potassium phosphate buffer in a 1:5 (vol/vol) ratio. Immediately after mixing, the catalase activity was quantified by tracking the change in absorbance at 240 nm for 5 min, measuring every 20 s. Potassium phosphate buffer served as the blank, and 18 mM hydrogen peroxide mixed 1:5 with sterile potassium phosphate buffer served as the negative control. The catalase reaction rate was estimated by linear regression of the absorbance measurements over time. The reaction rate was normalized by dividing the rate by the total protein concentration of the cell lysate, which was quantified with a Lowry protein assay kit (Sigma, St. Louis, Mo.). Units of specific catalase activity were defined as the change in absorbance at 240 nm per minute per microgram of protein per milliliter.
Transmission electron microscopy.
A β-lactamase-negative K. pneumoniae colony biofilm was treated with ampicillin for 12 h. This sample was fixed, stained, dehydrated, poststained, embedded in epoxy resin, sectioned, and examined by transmission electron microscopy as detailed elsewhere (20).
Glucose penetration.
The permeation of glucose through colony biofilms was measured with essentially the same technique that we used previously to measure antibiotic penetration through these colony biofilms (1); 13-mm-diameter, 0.2-μm-pore black polycarbonate membrane filters (Poretics Corp., Livermore, Calif.) were placed on top of 48-h-old K. pneumoniae biofilms. A concentration disk (catalog no. 1599-33-6; Difco Laboratories, Detroit, Mich.) was moistened with 24 μl of buffered water prior to placement on top of the 13-mm-diameter membranes. The biofilm sandwiched between the membranes and the moistened disk were transferred to glucose-containing agar medium. Sampled disks were placed in 1 ml of buffered water. Glucose in the resulting solution was assayed with a commercial glucose oxidase kit (Sigma, St. Louis, Mo.).
Oxygen penetration.
Oxygen concentration profiles in colony biofilms were measured with a dissolved oxygen microelectrode. The oxygen microelectrode is based on the principle of the common amperometric Clark oxygen electrode and is described in more detail by Jorgensen and Revsbech (9). It consists of an outer casing sealed at the sensor tip with an oxygen-permeable silicone membrane. The casing is fabricated from a Pasteur pipette that is tapered down to an active sensor tip of 15 μm. A carbonate buffer electrolyte fills the internal cavity. Three electrodes occupy the internal cavity as well: a gold-tipped, glass-encased platinum cathode, where oxygen diffusing in through the silicone membrane is reduced; a silver/silver chloride counterelectrode, which serves as the current return; and a guard electrode, which reduces unwanted oxygen entering from the back of the electrode. A potential of −0.8 V direct current is applied between the cathode and the common electrode. The current from the cathode, which is proportional to the concentration of oxygen in the bulk external solution, is measured with a picoammeter in the range of 0 to 3 nA. The same potential is applied between the guard electrode and the common electrode to reduce the background signal while measuring low concentrations of oxygen.
The oxygen microelectrode was lowered into the biofilm by a computer-controlled stepping motor with equipment that has been detailed elsewhere (12). The electrode was calibrated in air, and a zero level was obtained by placing the electrode tip in a 0.5% agar containing a suspension (0.2%) of freshly prepared ferrous sulfide (3).
RESULTS
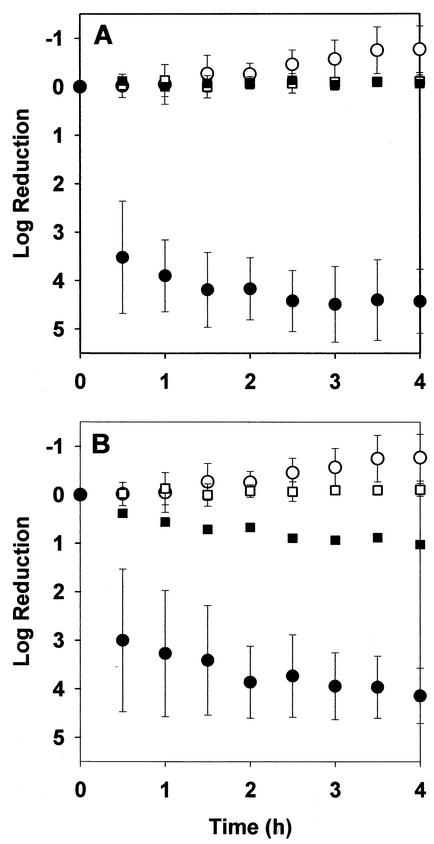
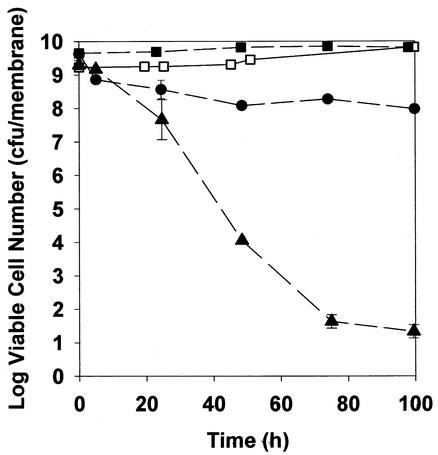
K. pneumoniae in 48-h-old colony biofilms resisted killing by ciprofloxacin or ampicillin even when biofilms were subjected to biocidal concentrations of the antibiotics for prolonged periods. Intact biofilms exposed to 1.8 μg of ciprofloxacin per ml experienced approximately a 1-log reduction in viable-cell numbers within 4 h, with no additional killing upon continued treatment for up to 100 h (Fig. 1B and Fig. 2). In experiments with growing cells in free aqueous suspension, the same concentration of ciprofloxacin reduced the number of viable bacteria by more than four orders of magnitude within 4 h (Fig. 1B). Ampicillin at 5,000 μg/ml also effectively killed growing free-floating bacteria in 4 h (Fig. 1A). Colony biofilms formed by the β-lactamase-positive parent strain were untouched by treatment with 5,000 μg of ampicillin per ml for 100 h (Fig. 2). This antibiotic treatment prevented the growth that was evident in the untreated control, but no decrease in viable-cell numbers could be measured in treated biofilms. Even a β-lactamase-negative derivative strain was relatively protected from killing by ampicillin when grown as colony biofilms. The β-lactamase-negative mutant was challenged with the same concentration of ampicillin (5,000 μg/ml) used in experiments with the wild-type parent. This concentration of antibiotic, which was approximately 2,500 times the MIC of the β-lactamase-deficient mutant, resulted in only a 0.12-log reduction in viable-cell numbers after 4 h of treatment in biofilms.
FIG. 1.
Comparison of susceptibility of suspended and biofilm K. pneumoniae to 5,000 μg of ampicillin per ml (A) and 1.8 μg of ciprofloxacin per ml (B). Symbols: ○, untreated planktonic control; •, treated planktonic cells; □, untreated biofilm control; ▪, treated biofilm. Biofilms were 48 h old when tested. Error bars show standard deviations. Positive values of the log reduction represent killing, and negative values represent growth.
FIG. 2.
Susceptibility of K. pneumoniae biofilms to 5,000 μg of ampicillin per ml or 1.8 μg of ciprofloxacin per ml during prolonged exposure. Symbols: □, untreated β-lactamase-positive control; ▪, ampicillin-treated β-lactamase-positive strain; •, ciprofloxacin-treated β-lactamase-positive strain; ▴, ampicillin-treated β-lactamase-negative strain. Data from duplicate experiments are shown; error bars show standard deviations.
To investigate the role of growth status and nutrient provision on bacterial susceptibility, batch planktonic cultures were challenged in two different media with inocula from two different states. The medium was either the full minimal medium or a version from which glucose and ammonium chloride were omitted. The latter medium contained no carbon or nitrogen source to support bacterial growth. The inoculum was taken either from a batch culture in exponential phase or from a similar culture that had entered stationary phase for at least 36 h. In all experiments, the initial cell density was adjusted to similar levels of approximately 106 CFU/ml.
Free-floating bacteria were most susceptible when challenged in the complete medium with an inoculum taken from a growing culture. These were the conditions used to generate the planktonic data shown in Fig. 1. When bacteria were challenged in medium lacking a carbon or nitrogen source or when the experiment was performed in complete medium but with a stationary-phase inoculum, killing was diminished (Table 1). Planktonic bacteria were least susceptible when bacteria taken from a stationary-phase culture were challenged with antibiotic in a carbon- and nitrogen-deficient medium. These experimental conditions, which are the least favorable to growth, resulted in log reductions of 1.8 after 4 h of treatment with ciprofloxacin and −0.21 after 4 h of treatment with ampicillin. These efficacies were not much different from the log reductions measured in intact biofilms of 1.02 for ciprofloxacin and −0.06 for ampicillin. When exponential-phase bacteria were resuspended in spent medium, they were susceptible to both antibiotics (Table 1). The pH of spent medium ranged from 5.1 to 5.9. When the spent medium was amended with fresh nutrients, the susceptibility was the same as when bacteria were resuspended in fresh medium (Table 1).
TABLE 1.
Killing of K. pneumoniae by ampicillin (5,000 μg/ml) and ciprofloxacin (1.8 μg/ml) after 4 h of exposurea
| Strain | Inoculum | Medium | Log reduction in no. of viable cells
|
||
|---|---|---|---|---|---|
| Control | Ampicillin | Ciprofloxacin | |||
| Kp1 | Exponential-phase culture | Complete | −0.77 ± 0.48 | 4.43 ± 0.66 | 4.14 ± 0.57 |
| Stationary-phase culture | Glucose and nitrogen omitted | −0.25 ± 0.30 | −0.21 ± 0.40 | 1.80 ± 0.28 | |
| Exponential-phase culture | Glucose and nitrogen omitted | −0.42 | 4.54 | 2.17 | |
| Stationary-phase culture | Complete | −0.92 | 3.01 | 2.69 | |
| Exponential-phase culture | Spent medium | −0.81 | 4.69 | 2.92 | |
| Exponential-phase culture | Spent medium, glucose and nitrogen added | −0.89 | 5.05 | 4.66 | |
| Resuspended biofilm | Complete | −2.50 | 2.27 ± 0.67 | 3.94 | |
| Resuspended biofilm | Glucose and nitrogen omitted | 0.79 | 0.65 ± 0.62 | 1.26 ± 0.51 | |
| Resuspended antibiotic-treated biofilm | Complete | −0.60 | 4.05 | 3.30 | |
| Kp102 | Exponential-phase culture | Complete | −1.48 | 4.62 | NDb |
| Kp1 | Intact biofilm | Complete | −0.11 ± 0.13 | −0.06 ± 0.08 | 1.02 ± 0.06 |
| Kp102 | Intact biofilm | Complete | −0.19 ± 0.13 | 0.12 ± 0.14 | 0.90 ± 0.17 |
The data are log reductions in viable-cell numbers. When three or more replicates were performed, the standard error of the mean is reported. All of the experiments were performed in batch suspension culture tests except for the intact biofilm data reported in the last two rows.
ND, not determined.
When bacteria were dispersed from colony biofilms and treated with antibiotic in aqueous suspension in complete medium, they regained most of their susceptibility (Table 1). Only a fraction of the normal planktonic susceptibility was restored by resuspension in medium lacking carbon and nitrogen. Bacteria from colony biofilms that had been treated with either ciprofloxacin or ampicillin were rendered sensitive to these agents by resuspension in complete medium.
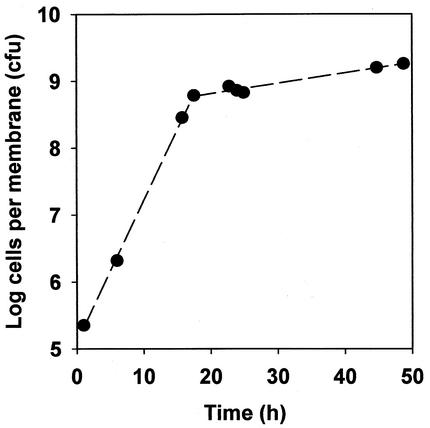
Accumulation curves of colony biofilms (Fig. 3) resembled batch culture growth curves. A period of relatively rapid exponential growth during the first 12 to 16 h was followed by a period of much slower growth. The average specific growth rate during the first 12 h was 0.49 h−1, whereas it was only 0.032 h−1 during the last 24 h of colony biofilm development. For comparison, the average specific growth rate of planktonic bacteria in the same medium was 0.59 h−1. The population-averaged specific growth rate of bacteria in 48-h-old colony biofilms was therefore only about 5% of the maximum specific growth rate of this microorganism.
FIG. 3.
Typical accumulation curve of a K. pneumoniae colony biofilm.
As a second indicator of the stationary-phase character of K. pneumoniae in colony biofilms, catalase activities were compared between exponentially growing planktonic cells, stationary-phase planktonic cells, and mature (48-h) colony biofilms. No catalase activity was detectable in growing planktonic cells (less than 0.2 U). Colony biofilms had a mean specific catalase activity of 1.78 U, and stationary-phase planktonic cells had a mean specific catalase activity of 1.73 U. The specific catalase activity measured in biofilms was statistically significantly greater than that measured in exponential-phase planktonic cells (P = 0.008) but was not statistically significantly different from the specific activity measured in free-floating stationary-phase bacteria (P = 0.89).
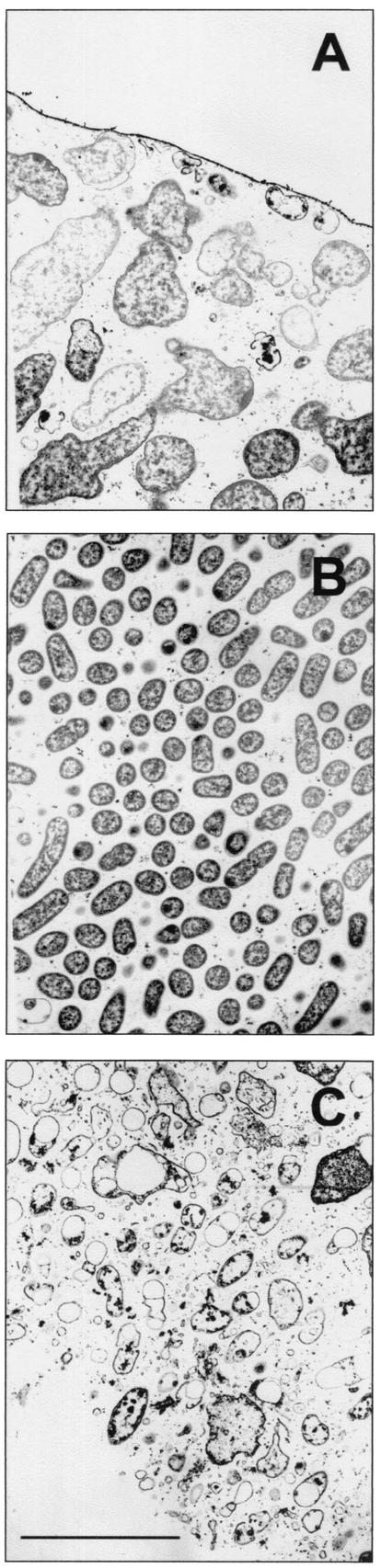
It is well known that penicillin antibiotics affect growing bacteria but are unable to kill nongrowing bacteria (17). Visual evidence of cell destruction by ampicillin could indicate that the cell was actively growing, whereas unaffected cells may have been in a slow-growing or nongrowing state. Transmission electron microscopy was used to visualize the effects of 12 h of ampicillin treatment of a β-lactamase-negative K. pneumoniae colony biofilm (Fig. 4). Bloated cells, lysed cells, and cell debris were evident near the air interface and near the membrane, while the middle of the colony appeared unaffected (Fig. 4). Bacteria in an untreated control appeared normal throughout the biofilm (not shown).
FIG. 4.
Transmission electron microscope images of ampicillin-treated, β-lactamase-negative K. pneumoniae colony biofilm. The biofilm was treated for 12 h. Enlargement and destruction of bacterial cells are evident near both boundaries of the colony biofilm (A, air interface; C, membrane interface) but not in the most central part of the colony interior (B). Bar, 5 μm.
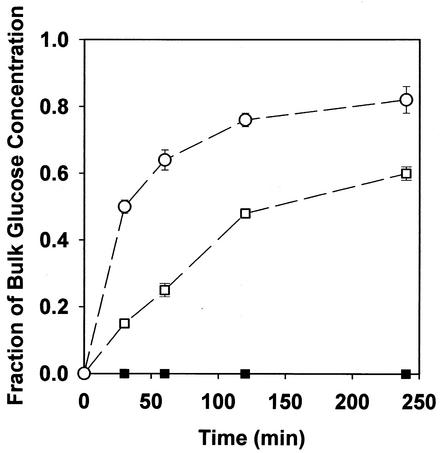
Glucose failed to penetrate colony biofilms of K. pneumoniae when formulated at the standard concentration of 6.25 g/liter in agar medium (Fig. 5). Glucose did penetrate in a sterile control experiment performed with two membranes but no bacterial colony. Glucose also penetrated colony biofilms that had been deactivated by overnight exposure to 5% formaldehyde.
FIG. 5.
Penetration of glucose through K. pneumoniae biofilms. Symbols: ▪, intact biofilm; ○, control with no biofilm; □, formaldehyde-killed biofilm.
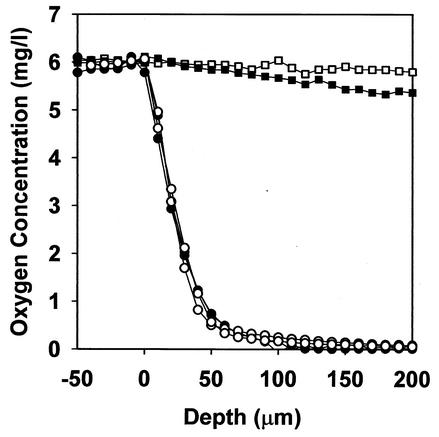
Oxygen penetrated about 50 to 100 μm into the biofilm from the air interface (Fig. 6). Below a depth of approximately 100 μm, the biofilm was anaerobic. Nearly identical oxygen profiles were measured in biofilms formed by β-lactamase-positive and β-lactamase-negative strains. There was no such oxygen gradient in a sterile agar control or in a glutaraldehyde-killed colony biofilm.
FIG. 6.
Penetration of oxygen into K. pneumoniae biofilms. Symbols: •, β-lactamase-positive biofilm; ○, β-lactamase-negative biofilm; ▪, glutaraldehyde-killed biofilm; □, 0.5% sterile agar control.
DISCUSSION
The K. pneumoniae colony biofilm model is one of the few in vitro biofilm models in which antibiotic penetration has been conclusively demonstrated (1, 20). Despite the fact that the antibiotics penetrate, bacteria in these biofilms survive prolonged chemotherapy (Fig. 2) by antibiotic treatments that rapidly dispatch growing free-floating cells (Fig. 1). These results oblige one to turn from physics to biology in search of an explanation for the reduced antibiotic susceptibility of the biofilm in this system.
One long-standing explanation for reduced susceptibility in the biofilm state is the possibility that some of the bacteria in a biofilm grow slowly or enter a stationary-phase state in which they are protected. If this is the sole explanation, then it should be possible to mimic the reduced susceptibility in a biofilm with planktonic cultures in which growth has been throttled by restricting nutrients and starting with a stationary-phase inoculum. Indeed, no killing by ampicillin could be discerned when treating K. pneumoniae inoculated from a stationary-phase culture into medium lacking carbon and nitrogen sources. On the other hand, reduced killing by ciprofloxacin was measured under these conditions, but it did not match the level of protection afforded by growth in the biofilm. These results are hardly surprising for ampicillin, given the tight coupling between bacterial growth and β-lactam killing (17). The growth dependence of killing by fluoroquinolones in enteric bacteria is less clear, with mixed conclusions in the literature (2, 6, 7, 13, 21). A combination of stationary-phase existence and nutrient limitation appears to be sufficient to explain the ampicillin resistance of these K. pneumoniae biofilms. The same conditions of restricted growth can only explain about half of the reduced susceptibility of biofilms to ciprofloxacin.
If nutrient limitation is the key to reduced susceptibility in biofilms, then bacteria resuspended from a biofilm into medium lacking nutrients should retain their protection. Preliminary data support this prediction (Table 1). Bacteria dispersed from biofilms into complete medium were more susceptible than bacteria resuspended in medium lacking carbon and nitrogen. These results are consistent with a role for nutrient limitation in protecting the bacteria in biofilms from killing by antibiotics. Bacterial survivors from antibiotic-treated biofilms remained sensitive to antibiotics, showing that the resistance in the biofilm state was not due to mutation or acquisition of a resistance gene.
Having demonstrated that the antibiotic susceptibility of planktonic bacteria can be substantially reduced when they enter stationary phase and are starved for nutrients, it becomes interesting to know whether bacteria in biofilms have a stationary-phase character and experience nutrient limitation.
Measurements of average specific growth rate and specific catalase activity, and transmission electron microscopy images of β-lactam action in the biofilm support the idea that many of the bacteria in the biofilm exist in a stationary-phase or nongrowing state. The biofilm accumulation curve looks much like that of a batch culture in which the culture enters stationary phase (Fig. 3). In the early hours of biofilm development, the specific growth rate of bacteria in the colony was 0.49 h−1, which is similar to the specific growth rate of planktonic bacteria of 0.59 h−1. As the colony matured, however, the specific growth rate slowed dramatically. When colonies were 48 h old, the age at which antibiotic treatments commenced, the average specific growth rate was an order of magnitude slower, 0.032 h−1.
Catalase activity has been widely used as an indicator of stationary phase in enteric microorganisms because catalase is induced upon entry into stationary phase (16). Specific catalase levels in the biofilm were significantly greater than the catalase levels measured in growing planktonic cells and were comparable to those of stationary-phase planktonic cells. Transmission electron microscopy images of an ampicillin-treated colony biofilm suggest a nonuniform pattern of growth (Fig. 4). Bacteria near the membrane and bacteria near the air interface were obviously affected by ampicillin, whereas bacteria in the middle layer of the colony were not visibly affected. This suggests that bacteria were growing near the membrane and near the air interface but were not growing in the interior of the aggregate. One explanation for this pattern is that bacteria near the membrane grow by fermenting glucose, while bacteria near the air interface grow aerobically on the waste products of the fermentation. Bacteria in the middle of the colony may experience simultaneous limitation for both glucose and oxygen, effectively preventing metabolism and growth. This interpretation is consistent with the incomplete penetration of glucose (Fig. 5) and oxygen (Fig. 6). It is also supported by a related study of growth patterns in K, pneumoniae colony biofilms (18).
In summary, the data reported here for K. pneumoniae colony biofilms support a model in which some bacteria in the biofilm experience limitation for oxygen and glucose, causing them to enter a stationary-phase state. The bacteria that have entered stationary phase and remain in a region where nutrients are lacking are protected from killing by ampicillin and ciprofloxacin. This model is sufficient to explain all of the resistance to ampicillin and much of the protection against ciprofloxacin. Proof of this mechanism, in this and other systems, depends on the application of techniques to characterize, in time and space, the heterogeneous physiological status of bacteria in biofilms (19).
Acknowledgments
This work was supported through cooperative agreement EEC-8907039 between the National Science Foundation and Montana State University and by the industrial partners of the Center for Biofilm Engineering.
Andy Blixt assisted with electron microscopy.
REFERENCES
- 1.Anderl, J. N., M. J. Franklin, and P. S. Stewart. 2000. Role of antibiotic penetration limitation in Klebsiella pneumoniae biofilm resistance to ampicillin and ciprofloxacin. Antimicrob. Agents Chemother. 44:1818-1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ashby, M. J., J. E. Neale, S. J. Knott, and I. A. Critchley. 1994. Effect of antibiotics on non-growing planktonic cells and biofilms of Escherichia coli. J. Antimicrob. Chemother. 33:443-452. [DOI] [PubMed] [Google Scholar]
- 3.Brock, T. D., and K. O'Dea. 1977. Amorphous ferrous sulfide as a reducing agent for culture of anaerobes. Appl. Environ. Microbiol. 33:254-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown, M. R. W., D. G. Allison, and P. Gilbert. 1988. Resistance of bacterial biofilms to antibiotics: a growth rate-related effect? J. Antimicrob. Chemother. 22:777-783. [DOI] [PubMed] [Google Scholar]
- 5.Costerton, J. W., P. S. Stewart, and E. P. Greenberg. 1999. Bacterial biofilms: a common cause of persistent infections. Science 284:1318-1322. [DOI] [PubMed] [Google Scholar]
- 6.Dalhoff, A., S. Matutat, and U. Ullmann. 1995. Effect of quinolones against slowly growing bacteria. Chemotherapy 41:92-99. [DOI] [PubMed] [Google Scholar]
- 7.Evans, D. J., D. G. Allison, M. R. W. Brown, and P. Gilbert. 1991. Susceptibility of Pseudomonas aeruginosa and Escherichia coli biofilms to ciprofloxacin: effect of specific growth rate. J. Antimicrob. Chemother. 27:177-184. [DOI] [PubMed] [Google Scholar]
- 8.Herigstad, B., M. Hamilton, and J. Heersink. 2001. How to optimize the drop plate methods for enumerating bacteria. J. Microbiol. Methods 44:121-129. [DOI] [PubMed] [Google Scholar]
- 9.Jorgensen, B. B., and N. P. Revsbech. 1988. Microsensors. Methods Enzymol. 167:639-659. [Google Scholar]
- 10.Lewis, K. 2001. Riddle of biofilm resistance. Antimicrob. Agents Chemother. 45:999-1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mah, T.-F. C., and G. A. O'Toole. 2001. Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol. 9:34-39. [DOI] [PubMed] [Google Scholar]
- 12.Rasmussen, K., and Z. Lewandowski. 1998. Microelectrode measurements of local mass transport rates in heterogeneous biofilms. Biotechnol. Bioeng. 59:302-309. [DOI] [PubMed] [Google Scholar]
- 13.Roosendaal, R., I. A. Bakker-Woudenberg, M. van den Berghe-van Raffe, J. C. Vink-van den Berg, and M. F. Michel. 1987. Comparative activities of ciprofloxacin and ceftazidime against Klebsiella pneumoniae in vitro and in experimental pneumoniae in leukopenic rats. Antimicrob. Agents Chemother. 31:1809-1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stewart, P. S. 1996. Theoretical aspects of antibiotic diffusion into microbial biofilms. Antimicrob. Agents Chemother. 40:2517-2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stewart, P. S., and J. W. Costerton. 2001. Antibiotic resistance of bacteria in biofilms. Lancet 358:135-138. [DOI] [PubMed] [Google Scholar]
- 16.Switala, J., B. L. Triggs-Raine, and P. C. Loewen. 1990. Homology among bacterial catalase genes. Can. J. Microbiol. 36:728-731. [DOI] [PubMed] [Google Scholar]
- 17.Tuomanen, E., R. Cozens, W. Tosch, O. Zak, and A. Tomasz. 1986. The rate of killing of Escherichia coli by β-lactam antibiotics is strictly proportional to the rate of bacterial growth. J. Gen. Microbiol. 132:1297-1304. [DOI] [PubMed] [Google Scholar]
- 18.Wentland, E. J., P. S. Stewart, C.-T. Huang, and G. A. McFeters. 1996. Spatial variations in growth rate within Klebsiella pneumoniae colonies and biofilm. Biotechnol. Prog. 12:316-321. [DOI] [PubMed] [Google Scholar]
- 19.Xu, K. D., G. A. McFeters, and P. S. Stewart. 2000. Biofilm resistance to antimicrobial agents. Microbiology 146:547-549. [DOI] [PubMed] [Google Scholar]
- 20.Zahller, J., and P. S. Stewart. 2002. A transmission electron microscopic study of antibiotic action on Klebsiella pneumoniae biofilm. Antimicrob. Agents Chemother. 46:2679-2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zeiler, H. J. 1985. Evaluation of the in vitro bactericidal action of ciprofloxacin on cells of Escherichia coli in the logarithmic and stationary-phases of growth. Antimicrob. Agents Chemother. 28:524-527. [DOI] [PMC free article] [PubMed] [Google Scholar]