Abstract
We tested the efficacy of micafungin (FK) alone or in combination with other antifungals against systemic murine aspergillosis. FK alone at 10 mg/kg of body weight/dose prolonged survival (P = 0.01) and reduced CFU in the brain and kidney. Combination therapy that used suboptimal FK with amphotericin B or itraconazole prolonged survival. Although no survivors were free of infection, no antagonism was seen. Nikkomycin Z with FK showed significantly greater potency (P < 0.01) than either alone.
With the continued rise in the number of fungal infections, particularly in immunocompromised patients, there remains a critical need for improved therapeutic agents (3-5, 8). Micafungin (FK463; Fujisawa) is of the echinocandin class of antifungals (7, 9, 14, 23). The mode of action is the inhibition of beta-glucan synthesis in the cell wall. This compound has been reported to have both in vitro and in vivo activity, primarily against Candida spp. and Aspergillus spp. (14, 16-19, 22-24), which is similar to the profiles of activity of other echinocandins (9, 25). As has been shown in studies that used anidulafungin (20), in vitro micafungin acts in synergy with nikkomycin Z, an inhibitor of chitin synthesis (1).
Micafungin (FK) is efficacious in murine models of candidosis and aspergillosis in immunosuppressed murine models (14, 16, 17) and is effective prophylactically against Pneumocystis carinii (15). In light of the in vitro data showing synergistic activity (1) and studies indicating synergy between cilofungin and conventional amphotericin B (AMB) against systemic aspergillosis (6, 11), the aim of the present studies was to examine the efficacy of micafungin alone or in combination with other agents in a model of systemic aspergillosis by using nonimmunosuppressed mice.
Systemic aspergillosis model.
Murine models of systemic aspergillosis were established in 7-week-old female CD-1 mice (Charles River Laboratories, Wilmington, Del.) by intravenous (i.v.) inoculation of Aspergillus fumigatus 10AF conidia (2, 6, 10, 11). In the initial study, mice (mean weight of 24.9 g) were infected with 8 × 106 conidia. For the combination therapy study, mice (mean weight of 26.4 g) were infected with 6.7 × 106 conidia.
Dose escalation study treatments.
The initial study determined the efficacy of FK (Fujisawa Healthcare, Inc., Deerfield, Ill.) as sole therapy. Groups of 10 mice received no treatment (untreated controls), saline (FK diluent), or FK in saline at 1, 3, or 10 mg/kg of body weight/dose given subcutaneously (s.c.) twice daily for 12 days and beginning 1 day after infection.
Combination therapy study.
The efficacy of FK in combination with other antifungal therapeutic agents also was assessed. Therapy began 1 day postinfection and continued for 12 days. Groups of 10 animals were given no treatment, 3 mg/kg/dose of FK in sterile saline given s.c. twice daily, 0.8 mg/kg of AMB (PharmaTek, Inc., Huntington, N.Y.) given i.v. once daily in sterile 5% dextrose, 100 mg of itraconazole (ICZ)/kg in 2-hydroxypropyl-β-cyclodextrin (12, 13) given orally once daily, 200 mg of nikkomycin Z (NIK)/kg (provided by Richard F. Hector) given s.c. once daily in dimethylsulfoxide (DMSO):polyethylene glycol 200 (10% [vol/vol] DMSO), or a combination of FK with one of the other three drugs.
Deaths were tallied through day 17 postinfection, all surviving mice were euthanatized by CO2 asphyxia, and the number of CFU in the brains and kidneys was determined (2, 6, 10, 11).
Statistical evaluation of survival was done by using a log rank test, and comparison of CFU was done with a Mann-Whitney U test. For the comparison of CFU, a value of log10 5 was assigned to data points missing due to the death of the animal. This value approximates the CFU in an organ just prior to death and allows for the nonparametric comparison of CFU in the organs between groups.
In vitro activity.
In vitro studies were done by using a previously described method (21) to determine activities against the strain of A. fumigatus 10AF used in these studies. FK had a MIC and minimal fungicidal concentration (MFC) of >16 μg/ml, AMB had a MIC and MFC of 1 μg/ml, and ITZ had a MIC and MFC of 1.56 and 3.13 μg/ml, respectively. The MIC and MFC of NIK has been reported previously as 800 μg/ml (20).
FK alone in vivo.
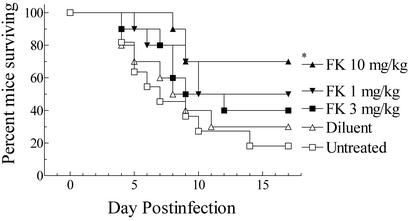
The cumulative mortality of FK alone in vivo is presented in Fig. 1. The first deaths occurred at day 4 postinfection, with 70 and 80% of the control mice succumbing to infection. Fewer mice treated with FK died of infection. By a log rank test, only FK at 10 mg/kg/dose significantly prolonged survival compared to that with no treatment (P = 0.011) or saline treatment (P = 0.046). No other comparisons were significant.
FIG. 1.
Cumulative mortality of mice infected systemically with A. fumigatus given one of the indicated treatments. All treatments began on day 1 postinfection and continued for 12 days. Treatments were twice daily at the dosage (milligrams per kilogram) indicated. An asterisk indicates a P value of 0.011 versus untreated controls and a P value of 0.046 versus diluent controls. No other comparisons were significant at the P = 0.05 level.
The CFU recovered from the organs of surviving mice showed that animals given FK had 30- to 50-fold lower fungal burdens in the kidneys (log10 geometric mean of 1.3 to 1.5 for treated mice versus a log10 geometric mean of 3.1 for surviving untreated controls), the target organ, than did surviving controls. However, no animals were free of detectable infection in the kidneys. Statistical comparisons showed that only FK at 10 mg/kg/dose was significantly efficacious (for brain, P = 0.027 versus untreated; for kidney, P = 0.013 versus untreated and P = 0.038 versus saline controls).
FK combination therapy.
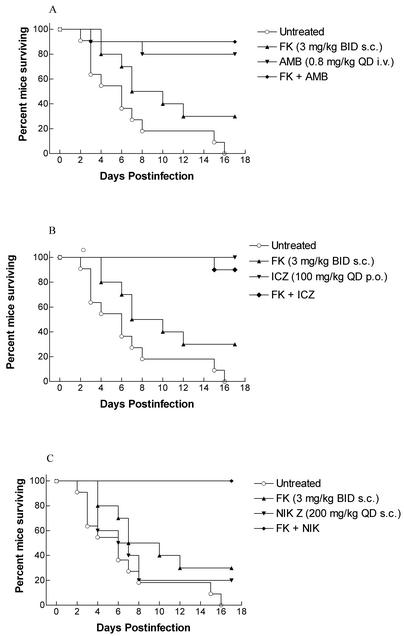
The design of the FK combination therapy was to determine whether FK given in combination with AMB, ICZ, or NIK showed improved therapeutic activity. FK was deliberately used at a suboptimal dosage of 3 mg/kg/dose, whereas AMB and ICZ regimens may have been closer to their optima. Mice in the control group began to succumb to infection on day 2, with most deaths occurring between days 4 and 8 (Fig. 2). Only those treated with FK or NIK alone had significant mortality. Eighty to 100% of mice given AMB, ICZ, or one of the combination regimens survived (P = 0.0001 versus controls). The 3 mg/kg/dose of FK alone showed a trend toward efficacy (P = 0.057), similar to the results of the initial study reported here. Statistically there were no differences among AMB, ICZ, or any of the combinations (P > 0.05). Because of the high survival percentage of mice receiving AMB or ICZ alone, no significant survival advantage could be demonstrated with the addition of FK. The combination of FK and NIK significantly improved efficacy in prolonging survival versus either drug given alone (P = 0.0012 and 0.0003, respectively).
FIG. 2.
Cumulative mortality of mice infected systemically with A. fumigatus given one of the indicated treatments. All treatments began on day 1 postinfection and continued for 12 days. (A) Treatments with FK and AMB. (B) Treatments with FK and ICZ. (C) Treatments with FK and NIK. BID, twice daily; QD, once a day.
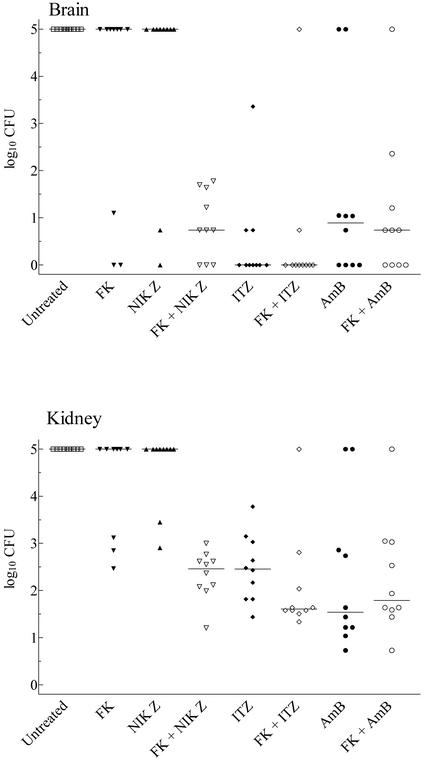
We obtained similar results in comparing the number of CFU recovered from the brains and kidneys of surviving mice. No animal in any treatment regimen cleared kidney infection, whereas some animals in all groups cleared the cerebral infection (Fig. 3). This clearance was greatest in ICZ-treated animals (7 of 10) and FK-plus-ICZ-treated animals (8 of 10). Four or fewer animals were free of infection in the brain in other regimens. Although not significantly different from results with ICZ alone, animals given the combination of FK and ICZ carried the lowest mean burdens in the brain. FK plus NIK showed significant efficacy over either drug alone (P = 0.023 and 0.004, respectively).
FIG. 3.
Scattergram of the CFU recovered from the brains and kidneys of surviving mice in the combination therapy study. Animals succumbing to infection were assigned a log10 value of 5 for each organ, and organs with no detectable infection were assigned a log10 value of 0. The bar represents the median value for the group. No surviving mouse was free of infection in both organs.
Although no treated animals were free of infection in the kidneys, efficacy was exhibited by the combination regimens as well as AMB or ICZ alone (Fig. 3). FK and NIK in combination showed significant efficacy (P = 0.0007 and 0.0002 versus FK or NIK alone), whereas neither ICZ nor AMB in combination with FK caused a significant improvement in clearing infection from the kidneys over sole treatment. It should be noted that the combination of FK and ICZ did cause a reduction of CFU that approached significance compared to reduction of CFU with ICZ alone (P = 0.0588). A higher dosage of FK might further improve the regimen.
These results demonstrate that FK has therapeutic efficacy against systemic aspergillosis in nonimmunosuppressed animals, corroborating results of previous studies on immunosuppressed animals (14). Our results do differ from those of previous studies somewhat in that we achieved significant efficacy by FK alone at about a 20-fold higher daily dosages than those previously reported effective (14). This may be due to our route of FK administration (s.c. rather than i.v.) (14) and the substantially larger inoculum used in our system (ca. 40- to 200-fold higher in our studies).
From the dose escalation data, we determined that 3 mg of FK per kg of body weight would be a suboptimal dose to be used in the combination therapy study, chosen to allow for the demonstration of improved efficacy. Overall, a significant improvement in efficacy between FK and NIK was found in our study, which correlates well with results from in vitro studies (1). The effectiveness of AMB or ICZ alone did not allow for the demonstration of significant enhancement in prolonging survival by the combinations. Previous studies found significantly improved efficacy in the model that used a combination of AMB and cilofungin (6). The difference in our present study showing no improved efficacy may be the change to daily i.v. dosing of AMB rather than intraperitoneal dosing every other day. In spite of the efficacy of AMB or ICZ alone, neither in combination with FK showed any significant improvement in the reduction of CFU, particularly in the kidneys, nor did these combinations cure any animals of infection from both organs. No antagonism was observed with the FK plus AMB or FK plus ICZ combinations. Cure by combination regimens might have been seen with a higher dosage of FK. Regardless, these results indicate than FK has efficacy against systemic aspergillosis when given in combination with ICZ, AMB, or NIK. Additional studies to determine optimal treatment regimens that might potentially result in cures are warranted.
Acknowledgments
These studies were funded in part by a grant from Fujisawa Inc.
REFERENCES
- 1.Chiou, C. C., N. Mavrogiorgos, E. Tillem, R. Hector, and T. J. Walsh. 2001. Synergy, pharmacodynamics, and time-sequenced ultrastructural changes of the interaction between nikkomycin Z and the echinocandin FK463 against Aspergillus fumigatus. Antimicrob. Agents Chemother. 45:3310-3321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clemons, K. V., G. Grunig, R. A. Sobel, L. F. Mirels, D. M. Rennick, and D. A. Stevens. 2000. Role of IL-10 in invasive aspergillosis: increased resistance of IL-10 gene knockout mice to lethal systemic aspergillosis. Clin. Exp. Immunol. 122:186-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Denning, D. W. 1998. Invasive aspergillosis. Clin. Infect. Dis. 26:781-803. [DOI] [PubMed] [Google Scholar]
- 4.Denning, D. W. 1994. Treatment of invasive aspergillosis. J. Infect. 28(Suppl. 1):25-33. [DOI] [PubMed] [Google Scholar]
- 5.Denning, D. W., and D. A. Stevens. 1990. Antifungal and surgical treatment of invasive aspergillosis: review of 2,121 published cases. Rev. Infect. Dis. 12:1147-1201. [DOI] [PubMed] [Google Scholar]
- 6.Denning, D. W., and D. A. Stevens. 1991. Efficacy of cilofungin alone and in combination with amphotericin B in a murine model of disseminated aspergillosis. Antimicrob. Agents Chemother. 35:1329-1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Georgopapadakou, N. H. 2001. Update on antifungals targeted to the cell wall: focus on beta-1,3-glucan synthase inhibitors. Expert Opin. Investig. Drugs 10:269-280. [DOI] [PubMed] [Google Scholar]
- 8.Granier, F. 2000. Invasive fungal infections. Epidemiology and new therapies. Presse Med. 29:2051-2056. [PubMed] [Google Scholar]
- 9.Graybill, J. R. 2001. Hitting a new target with echinocandins. Why chase something else? Curr. Opin. Investig. Drugs 2:468-471. [PubMed] [Google Scholar]
- 10.Hanson, L. H., K. V. Clemons, D. W. Denning, and D. A. Stevens. 1995. Efficacy of oral saperconazole in systemic murine aspergillosis. J. Med. Vet. Mycol. 33:311-317. [DOI] [PubMed] [Google Scholar]
- 11.Hanson, L. H., A. M. Perlman, K. V. Clemons, and D. A. Stevens. 1991. Synergy between cilofungin and amphotericin B in a murine model of candidiasis. Antimicrob. Agents Chemother. 35:1334-1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hostetler, J. S., L. H. Hanson, and D. A. Stevens. 1992. Effect of cyclodextrin on the pharmacology of antifungal oral azoles. Antimicrob. Agents Chemother. 36:477-480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hostetler, J. S., L. H. Hanson, and D. A. Stevens. 1993. Effect of hydroxypropyl-beta-cyclodextrin on efficacy of oral itraconazole in disseminated murine cryptococcosis. J. Antimicrob. Chemother. 32:459-463. [DOI] [PubMed] [Google Scholar]
- 14.Ikeda, F., Y. Wakai, S. Matsumoto, K. Maki, E. Watabe, S. Tawara, T. Goto, Y. Watanabe, F. Matsumoto, and S. Kuwahara. 2000. Efficacy of FK463, a new lipopeptide antifungal agent, in mouse models of disseminated candidiasis and aspergillosis. Antimicrob. Agents Chemother. 44:614-618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ito, M., R. Nozu, T. Kuramochi, N. Eguchi, S. Suzuki, K. Hioki, T. Itoh, and F. Ikeda. 2000. Prophylactic effect of FK463, a novel antifungal lipopeptide, against Pneumocystis carinii infection in mice. Antimicrob. Agents Chemother. 44:2259-2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maesaki, S., M. A. Hossain, Y. Miyazaki, K. Tomono, T. Tashiro, and S. Kohno. 2000. Efficacy of FK463, a (1,3)-beta-D-glucan synthase inhibitor, in disseminated azole-resistant Candida albicans infection in mice. Antimicrob. Agents Chemother. 44:1728-1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matsumoto, S., Y. Wakai, T. Nakai, K. Hatano, T. Ushitani, F. Ikeda, S. Tawara, T. Goto, F. Matsumoto, and S. Kuwahara. 2000. Efficacy of FK463, a new lipopeptide antifungal agent, in mouse models of pulmonary aspergillosis. Antimicrob. Agents Chemother. 44:619-621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mikamo, H., Y. Sato, and T. Tamaya. 2000. In vitro antifungal activity of FK463, a new water-soluble echinocandin-like lipopeptide. J. Antimicrob. Chemother. 46:485-487. [DOI] [PubMed] [Google Scholar]
- 19.Muller, F. M., O. Kurzai, J. Hacker, M. Frosch, and F. Muhlschlegel. 2001. Effect of the growth medium on the in vitro antifungal activity of micafungin (FK-463) against clinical isolates of Candida dubliniensis. J. Antimicrob. Chemother. 48:713-715. [DOI] [PubMed] [Google Scholar]
- 20.Stevens, D. A. 2000. Drug interaction studies of a glucan synthase inhibitor (LY 303366) and a chitin synthase inhibitor (nikkomycin Z) for inhibition and killing of fungal pathogens. Antimicrob. Agents Chemother. 44:2547-2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stevens, D. A., and B. H. Aristizabal. 1997. In vitro antifungal activity of novel azole derivatives with a morpholine ring, UR-9746 and UR-9751, and comparison with fluconazole. Diagn. Microbiol. Infect. Dis. 29:103-106. [DOI] [PubMed] [Google Scholar]
- 22.Tawara, S., F. Ikeda, K. Maki, Y. Morishita, K. Otomo, N. Teratani, T. Goto, M. Tomishima, H. Ohki, A. Yamada, K. Kawabata, H. Takasugi, K. Sakane, H. Tanaka, F. Matsumoto, and S. Kuwahara. 2000. In vitro activities of a new lipopeptide antifungal agent, FK463, against a variety of clinically important fungi. Antimicrob. Agents Chemother. 44:57-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tomishima, M., H. Ohki, A. Yamada, H. Takasugi, K. Maki, S. Tawara, and H. Tanaka. 1999. FK463, a novel water-soluble echinocandin lipopeptide: synthesis and antifungal activity. J. Antibiot. (Tokyo) 52:674-676. [DOI] [PubMed] [Google Scholar]
- 24.Uchida, K., Y. Nishiyama, N. Yokota, and H. Yamaguchi. 2000. In vitro antifungal activity of a novel lipopeptide antifungal agent, FK463, against various fungal pathogens. J. Antibiot. (Tokyo) 53:1175-1181. [DOI] [PubMed] [Google Scholar]
- 25.Walsh, T. J., M. A. Viviani, E. Arathoon, C. Chiou, M. Ghannoum, A. H. Groll, and F. C. Odds. 2000. New targets and delivery systems for antifungal therapy. Med. Mycol. 38(Suppl. 1):335-347. [PubMed] [Google Scholar]