Abstract
Invasive infections caused by Candida krusei are a significant concern because this organism is intrinsically resistant to fluconazole. Voriconazole is more active than fluconazole against C. krusei in vitro. One mechanism of fluconazole resistance in C. krusei is diminished sensitivity of the target enzyme, cytochrome P450 sterol 14α-demethylase (CYP51), to inhibition by this drug. We investigated the interactions of fluconazole and voriconazole with the CYP51s of C. krusei (ckCYP51) and fluconazole-susceptible Candida albicans (caCYP51). We found that voriconazole was a more potent inhibitor of both ckCYP51 and caCYP51 in cell extracts than was fluconazole. Also, the ckCYP51 was less sensitive to inhibition by both drugs than was caCYP51. These results were confirmed by expressing the CYP51 genes from C. krusei and C. albicans in Saccharomyces cerevisiae and determining the susceptibility of the transformants to voriconazole and fluconazole. We constructed homology models of the CYP51s of C. albicans and C. krusei based on the crystal structure of CYP51 from Mycobacterium tuberculosis. These models predicted that voriconazole is a more potent inhibitor of both caCYP51 and ckCYP51 than is fluconazole, because the extra methyl group of voriconazole results in a stronger hydrophobic interaction with the aromatic amino acids in the substrate binding site and more extensive filling of this site. Although there are multiple differences in the predicted amino acid sequence of caCYP51 and ckCYP51, the models of the two enzymes were quite similar and the mechanism for the relative resistance of ckCYP51 to the azoles was not apparent.
Candida krusei is an opportunistic pathogen that can cause serious infections in immunocompromised patients (1, 6, 7). This organism is intrinsically resistant to fluconazole. The new triazole voriconazole has greater in vitro activity than fluconazole against C. krusei (5, 15). Two mechanisms of azole resistance in C. krusei have been described. Isolates of C. krusei that are resistant to itraconazole exhibit reduced drug accumulation, suggesting that resistance to this drug is due to the activity of one or more drug efflux pumps (29). Recently, two ATP binding cassette transporters have been identified in C. krusei. Increased expression of these transporters is associated with reduced susceptibility to miconazole (9).
A second mechanism of azole resistance in C. krusei is diminished sensitivity of the target enzyme, cytochrome P450 sterol 14α-demethylase (CYP51), to inhibition by an azole antifungal agent. We have determined previously that fluconazole resistance in some strains of C. krusei is mediated predominantly by this mechanism (17).
In the present study, we cloned the full-length C. krusei CYP51 and examined its contribution to the differential sensitivity of C. krusei to fluconazole and voriconazole. We also used computer-assisted molecular modeling to examine the interactions of fluconazole and voriconazole with the predicted Candida albicans and C. krusei CYP51s.
MATERIALS AND METHODS
Organisms.
C. krusei strains 91-1158, 91-1159, and 91-1161 are clinical isolates that were generously provided by Michael Rinaldi (San Antonio, Tex.). C. albicans SC5314 is also a clinical isolate and was kindly supplied by William Fonzi (Georgetown School of Medicine, Washington, D.C.). Saccharomyces cerevisiae AH22 (MATα leu2-3,2-112 his3-11,3-15 Canr) was a generous gift of Steven Kelly (University of Wales, Aberystwyth, United Kingdom) (10). S. cerevisiae TY310 (MATα pdr1 pdr3::URA3 pdr5::TRP1 ade2-101 lys2-801 his3-Δ200 leu2::PET56) was a kind gift from Martine Raymond (Institut de Recherches Cliniques de Montreal, Montreal, Quebec, Canada) (27). Escherichia coli XL10-Gold (Stratagene, La Jolla, Calif.) was used for plasmid construction and amplification.
Ergosterol biosynthesis by cell extracts.
The concentration of voriconazole required to inhibit by 50% (IC50) the synthesis of ergosterol from [14C]mevalonic acid in cell extracts was determined exactly as described previously (17). Briefly, the organisms were grown overnight in Sabouraud dextrose broth at 37°C in a shaking incubator to an optical density at 600 nm of approximately 0.8. They were collected by centrifugation and then broken by vortexing with glass beads in 0.1 M potassium phosphate, pH 7.5, at a ratio of 4 g of beads to 1 g of organisms (wet weight). The resulting cell extract was separated from debris and unbroken cells by differential centrifugation. To measure sterol biosynthesis, 925 μl of cell extract was added to 75 μl of cofactor buffer (with or without voriconazole) to achieve the following final concentrations: 7 μM glucose-6-phosphate, 5 μM ATP, 3 μM reduced glutathione, 2 μM MnCl2, 1 μM NADP, 1 μM NADPH, 1 μM NAD, and 0.25 μCi of [14C]-mevalonic acid (10, 12). The samples were incubated at 37°C for 2 h and then saponified in ethanolic KOH at 80°C for 45 min. The sterols were extracted with petroleum ether (boiling point, 40 to 60°C), dried under nitrogen, and redissolved in chloroform. Next, they were separated by thin-layer chromatography on silica gel LK6D (Whatman Inc., Clifton, N.J.) using petroleum ether/diethyl ether (3:1, vol/vol) (23). The bands were visualized by iodine staining and the sterols were identified by comparison with commercially available standards, which were run in parallel. Finally, the sterol-containing bands were scraped from the plates, and their 14C content was determined by liquid scintillation counting. All experiments were repeated at least three times using different batches of cell extracts.
Inverse PCR.
Inverse PCR was used to amplify the 5′ and 3′ regions of the ckCYP51 open reading frame (16, 28). We first amplified a fragment of ckCYP51 from genomic DNA of C. krusei 91-1161 by PCR using primers Y572-01 and Y572-02 (Table 1). These primers were designed using the published incomplete ckCYP51 sequence (GenBank accession number S75391) (4). This fragment was used to probe Southern blots of C. krusei genomic DNA to identify a restriction enzyme that was likely to cut outside of the open reading frame of ckCYP51. We determined that the ckCYP51 probe hybridized with a 2.8-kb fragment of C. krusei DNA that had been digested with XbaI. Therefore, we digested C. krusei genomic DNA with XbaI and then isolated fragments of 2.5 to 3.5 kb in size by agarose gel electrophoresis. These fragments were ligated under dilute conditions to promote self-ligation. The self-ligated fragments were used as a template for inverse PCR using primers IPCR-1 and IPCR-2 (Table 1). The 1.8-kb product that was amplified was cloned into pGEM-T Easy (Promega Corp., Madison, Wis.) and sequenced.
TABLE 1.
PCR primers used in this study
| Primer name | Sequence |
|---|---|
| Y572-01 | 5′ ATGCCGTGGGTCGGCTCCGCCG 3′ |
| Y572-02 | 5′ CACCACCAAAAGGTAAGTAAGGGG 3′ |
| IPCR-1 | 5′ GAGTGGTGAGATGGGTGTAAGCAT 3′ |
| IPCR-2 | 5′ GGTGTTGCATCCCCTTACTTACCT 3′ |
| CK14DM1 | 5′ CGGGATCCTCTCTAGCAACAACAATGTCCG 3′ |
| CK14DM2 | 5′ CGGGATCCTTGATTGTCGATTTTGGTTT 3′ |
| CA14DM1 | 5′ CGGGATCCAATATGGCTATTGTTGAAACT 3′ |
| CA14DM2 | 5′ CGGGATCCAAACATACAAGTTTCTCTTTTTTC 3′ |
Cloning of ckCYP51 and caCYP51 and heterologous expression in S. cerevisiae.
The full-length ckCYP51 and caCYP51 were PCR amplified from genomic DNA of C. krusei and C. albicans, respectively. The high-fidelity Pwo DNA polymerase (Boehringer Mannheim) was used and the primers were CK14DM1 and CK14DM2 for ckCYP51, and CA14DM1 and CA14DM2 for caCYP51 (Table 1). The CYP51s from the three strains of C. krusei and C. albicans SC5314 were first ligated into pGEM-T Easy for sequencing. Multiple independent clones were sequenced for each strain. Next, the CYP51s from C. krusei 91-1161 and C. albicans SC5314 were excised from their respective plasmids with BamHI and ligated into pESC-LEU (Stratagene) to enable expression of the candidal CYP51 in S. cerevisiae under control of the GAL1 promoter. In addition, PCR mutagenesis was used to change the CTG codons of caCYP51 to TCT. This step was not necessary for ckCYP51 because CTG encodes leucine in C. krusei (18). All mutations were confirmed by sequencing. The plasmids containing ckCYP51 and caCYP51 were transformed into S. cerevisiae AH22 and TY310 using the lithium acetate method (3). The transformants were grown on minimal medium (yeast nitrogen base without amino acids [Difco, Detroit, Mich.] with 2% glucose [wt/vol] and 2% agar [wt/vol]) supplemented with appropriate amino acids.
Susceptibility testing.
The susceptibility of the C. krusei and C. albicans isolates to fluconazole and voriconazole was determined by the NCCLS M-27A broth microdilution method (14). Both fluconazole and voriconazole were dissolved in water. The fluconazole and voriconazole susceptibilities of the S. cerevisiae transformants were determined by the broth microdilution method of Sanglard et al. (22). The inoculum was 103 organisms, and the medium was yeast nitrogen base broth without amino acids containing 2% raffinose (wt/vol), 2% galactose (wt/vol), and histidine, adenine, and lysine (each at a concentration of 50 μg/ml). The incubation temperature was 30°C. Because of the slow growth of the transformants, the endpoints were read at 3 days for strain AH22 and 6 days for strain TY310.
Construction of homology models of the CYP51s of C. albicans and C. krusei.
Consensus homology models of caCYP51 and ckCYP51 were constructed using the program Modeler (version 3; Rockefeller University, New York, N.Y.) (21), as implemented in Quanta98 (version 98.1111; Molecular Simulations Inc., San Diego, Calif.). Initially four available bacterial crystal structures (CYP101, CYP102, CYP107A, and CYP108) were used to generate models of caCYP51 (similar to the ones described by Ji et al. [8]) and ckCYP51. The bacterial P450s are considerably shorter in sequence than caCYP51 and ckCYP51, making it very difficult to model the active site reliably. The publication of a crystal structure of CYP51 from Mycobacterium tuberculosis (mtCYP51 and 1ea1.pdb) (19) provided a better template for the construction of homology models. However, the structures of ckCYP51 and caCYP51 have an insertion just before the cysteine-binding pocket, and this insertion is not present in mtCYP51. The Protein Data Bank (2, 24) was searched for structures with a similar amino acid composition to the insertion, but none were obtained. Therefore, the area of this insertion could not be modeled reliably and limited conclusions about the structure of this area can be drawn.
A set of 20 models was produced. The best model was selected based on a PROCHECK (11) analysis of stereochemical quality and a visual inspection in Quanta98 to assess the location of the iron relative to the heme moiety, and the orientation of the amino acids involved in hydrogen bonds with the heme propionate moieties (e.g., R381 stabilizing the heme propionate moiety in ckCYP51).
Docking of inhibitors in the homology models for caCYP51 and ckCYP51.
Fluconazole was docked in the models of caCYP51 and ckCYP51 using the orientation derived from the crystal structure of mtCYP51 (1ea1.pdb) (19). The program Sybyl (Version 6.7; Tripos Inc., St. Louis, Mo.) was used to construct and energy minimize voriconazole and then superimpose it onto fluconazole in these models. The solvent accessible surfaces were generated using Quanta98.
Nucleotide sequence accession number of ckCYP51.
The GenBank accession number of the ckCYP51 sequence is AF531428.
RESULTS
Voriconazole is a more potent inhibitor of the C. krusei CYP51 than is fluconazole.
We compared the susceptibilities of intact C. krusei and C. albicans cells to growth inhibition by fluconazole and voriconazole. As reported previously, all three strains of C. krusei exhibited decreased susceptibility to fluconazole, while C. albicans SC5314 was highly susceptible to this drug (Table 2) (17). Voriconazole was considerably more active than fluconazole against both species of Candida (Table 2). However, the MIC of voriconazole for the three isolates of C. krusei was 16-fold higher than its MIC for C. albicans (Table 2).
TABLE 2.
Concentrations of fluconazole and voriconazole required to inhibit the growth and CYP51s of C. krusei and C. albicans
| Organism | MIC (μg/ml)
|
Mean IC50 (μg/ml) ± SD
|
||
|---|---|---|---|---|
| Fluconazolea | Voriconazole | Fluconazolea | Voriconazole | |
| C. krusei 91-1161 | 32 | 0.5 | 0.243 ± 0.130 | 0.014 ± 0.004 |
| C. krusei 91-1159 | 32 | 0.5 | 0.152 ± 0.064 | 0.020 ± 0.005 |
| C. krusei 91-1158 | 64 | 0.5 | 0.230 ± 0.118 | 0.020 ± 0.005 |
| C. albicans SC5314 | 1 | 0.031 | 0.010 ± 0.002 | 0.0020 ± 0.0006 |
Results are from our previous work (17).
Next, we determined the concentrations of voriconazole and fluconazole required to cause a 50% inhibition in ergosterol synthesis by cell extracts of C. krusei and C. albicans. In these cell extracts, any effects of drug efflux pumps on azole susceptibility were obviated because the cell membranes were disrupted. Also, we have shown previously that the strains of C. krusei and C. albicans used in these experiments have similar amounts of CYP51 (17). Therefore, the experiments with the cell extracts determined the sensitivity of the CYP51s of C. krusei and C. albicans to inhibition by the two drugs. We found that the IC50 of voriconazole was 8- to 17-fold lower than the IC50 of fluconazole for the three isolates of C. krusei (Table 2). The IC50 of voriconazole was also fivefold lower than the IC50 of fluconazole for C. albicans. These results indicate that voriconazole is more effective at inhibiting the CYP51s of both species of Candida, and that it is particularly active against the CYP51 of C. krusei.
We also found that the IC50s of both fluconazole and voriconazole were significantly higher for C. krusei than for C. albicans. Therefore, the CYP51 of C. krusei is less susceptible than CYP51 of C. albicans to inhibition by both drugs.
The predicted amino acid sequence of the C. krusei CYP51 is significantly different from that of other species of Candida.
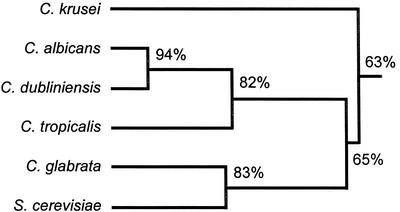
To investigate further the differential activity of voriconazole and fluconazole against the C. krusei CYP51, we cloned the full-length C. krusei CYP51. A fragment of ckCYP51 had previously been cloned by Burgener-Kairuz et al. (4). Using this sequence information, we amplified the flanking regions of the ckCYP51 fragment by inverse PCR and then sequenced this amplicon. We used this new sequence information to PCR amplify the full-length open reading frame of ckCYP51. The predicted protein encoded by this gene is comprised of 528 amino acids and it contains many of the conserved regions seen in the CYP51s of C. albicans and M. tuberculosis (Fig. 1A). However, the predicted C. krusei CYP51 is also markedly divergent from the CYP51s of other Candida species and S. cerevisiae, as it shares only 63% homology with these other enzymes (Fig. 1B).
FIG. 1.
Sequence comparison of the predicted CYP51 of C. krusei with other microbial CYP51s. (Top) Sequence alignment of ckCYP51, caCYP51, and mtCYP51. Amino acids conserved in all three sequences are indicated by an asterisk. Amino acids that are predicted to be within 12 Å of voriconazole are indicated in boldface type. (Bottom) Homology tree showing the relatedness of the predicted CYP51s from different species of Candida and S. cerevisiae. Numbers indicate the percent homology at the amino acid level.
We sequenced several copies of CYP51 from all three isolates of C. krusei. The predicted amino acid sequence of the CYP51 of strain 91-1161 was identical to that of strain 91-1158. However, the predicted CYP51 of strain 91-1159 had two amino acid substitutions, D434A and K456R (using the single-letter amino acid designation, where the first letter denotes the amino acid present in strains 91-1161 and 91-1158 and the second letter denotes the amino acid present in strain 91-1159).
Overexpression of ckCYP51 caCYP51 in S. cerevisiae.
Next, we examined the effects of overexpressing CYP51s from C. krusei and C. albicans on the susceptibility of two different strains of S. cerevisiae to fluconazole and voriconazole. Strain AH22 was used because it has very low expression of its native CYP51 (10). However, this strain had a relatively high fluconazole MIC, even when its was transformed with either caCYP51 or the empty plasmid, pESC-LEU (Table 3). Therefore, we confirmed the results obtained with this strain by expressing caCYP51 and ckCYP51 in strain TY310, which does not express several drug efflux pumps and is highly susceptible to fluconazole (27). As predicted by our biochemical studies, the MIC of fluconazole for S. cerevisiae expressing ckCYP51 was 8- to 16-fold higher than that of yeast expressing caCYP51 (Table 3). Yeast strains expressing either caCYP51 or ckCYP51 were highly susceptible to voriconazole (Table 3). However, strains expressing ckCYP51 were fourfold less susceptible to voriconazole than were strains expression caCYP51.
TABLE 3.
Antifungal susceptibilities of S. cerevisiae clones expressing ckCYP51, caCYP51, or the backbone vector
| Gene or vector expressed | MIC (μg/ml)
|
|||
|---|---|---|---|---|
|
S. cerevisiae AH22
|
S. cerevisiae TY310
|
|||
| Fluconazole | Voriconazole | Fluconazole | Voriconazole | |
| ckCYP51 | 256 | 0.25 | 32 | 0.03 |
| caCYP51 | 32 | 0.06 | 2 | 0.008 |
| pESC-LEU | 16 | 0.03 | 0.5 | <0.004 |
Molecular modeling of the interactions of caCYP51 and ckCYP51 with fluconazole and voriconazole.
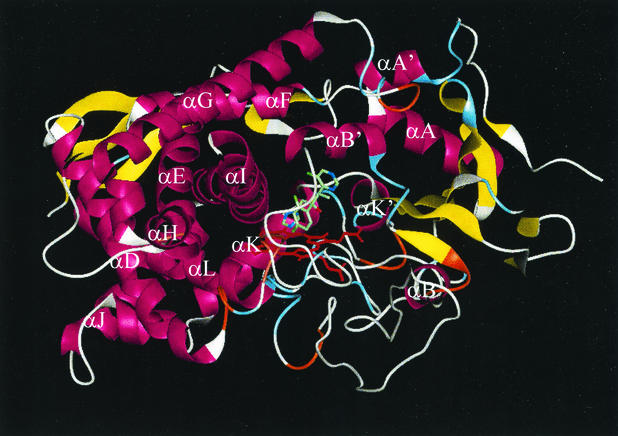
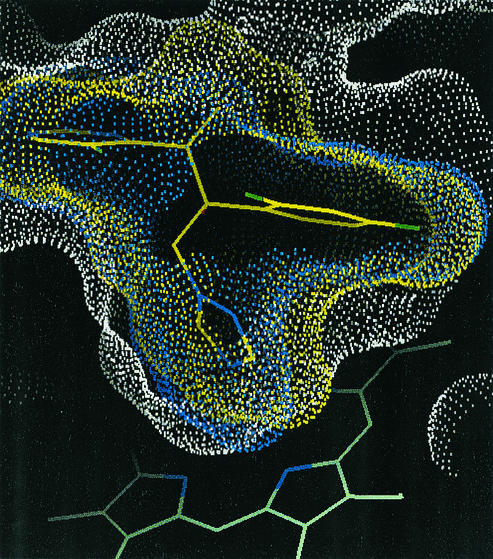
To analyze the interactions of caCYP51 and ckCYP51 with fluconazole and voriconazole, homology models of these enzymes were constructed based on the crystal structure of mtCYP51, using the alignment shown in Fig. 1. The docking of fluconazole and voriconazole with ckCYP51 and caCYP51 was modeled as described in Materials and Methods. The predicted secondary structure of ckCYP51 when it is bound by voriconazole is shown in Fig. 2. We compared the amino acids within an arbitrary 12-Å radius of the azole-binding site of ckCYP51 and caCYP51 to identify residues that are likely to mediate the differential binding of fluconazole and voriconazole to these enzymes. Our models suggest that the amino acids within 12 Å of the bound voriconazole or fluconazole are those from A122 to F134 and W145 to M148 in ckCYP51 and A114 to F126 and S137 to K147 in caCYP51 (Fig. 1 and 3). In models of both caCYP51 and ckCYP51, the N1 of voriconazole and fluconazole interacts with the heme moiety, while two aromatic residues (Y126 and F134 in ckCYP51, and Y118 and F126 in caCYP51) mediate hydrophobic interactions with each drug. This hydrophobic interaction is predicted to be stronger with voriconazole than it is for fluconazole, due to the extra methyl group on voriconazole. As a result, voriconazole fills the available space in the active site more extensively than does fluconazole (Fig. 4), explaining why voriconazole is a more potent inhibitor of both caCYP51 and ckCYP51 than is fluconazole.
FIG. 2.
Secondary structure of ckCYP51 docked with voriconazole. At this level of detail, caCYP51 and ckCYP51 are identical, as the side-chains are not shown. α-Helices are shown in purple, β-sheets are shown in yellow, three-turns are shown in aqua, four-turns are shown in orange, the heme moiety is shown in red, and regions with no secondary structure are shown in white. Voriconazole is shown in green.
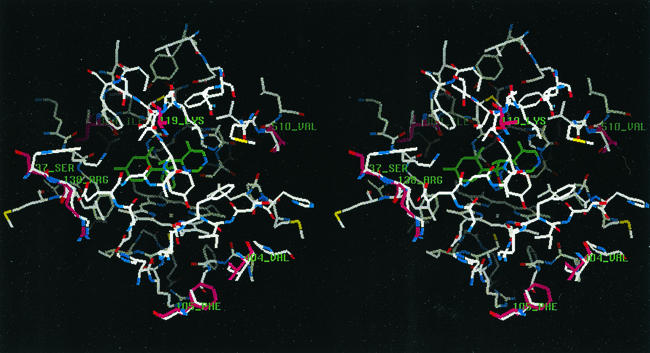
FIG. 3.
Stereo display of active site region of ckCYP51 within 12 Å of voriconazole. The carbon atoms of voriconazole are shown in green. Amino acid side chains that are different in caCYP51 are shown with purple carbon atoms and are labeled (see also Table 4 and Fig. 1).
FIG. 4.
Solvent-accessible surface of the ckCYP51 active site (white) with the surfaces of fluconazole (blue) and voriconazole (yellow). Voriconazole (shown with yellow carbon atoms) fills more of the available space in the active site than does fluconazole. The “pocket” highlighted is formed mainly by the side chains of Y126 and F134 (corresponding to Y118 and F126 in caCYP51) and the backbone of T130 (T122 in caCYP51). The heme moiety is shown as the green stick figure at the bottom of the figure to indicate the orientation.
The amino acids that are within 12 Å of the bound drug and that differ between ckCYP51 and caCYP51 are listed in Table 4 and shown in Fig. 3. None of these amino acids are predicted to interact with either voriconazole or fluconazole through their side chains. The amino acids that are the most different in the two CYP51s, K119 versus T127 and S137 versus W145 (caCYP51 versus ckCYP51), are not orientated towards the active site. Therefore, the present model does not provide a clear explanation for the reduced sensitivity of ckCYP51 to both drugs.
TABLE 4.
Differences between caCYP51 and ckCYP51 within 12-Å radius from active sitea
| caCYP51 | ckCYP51 | Difference (caCYP51 → ckCYP51) |
|---|---|---|
| Phe105 | Leu113 | Smaller, remains hydrophobic |
| Lys119 | Thr127 | Loss of positive charge |
| Ser137 | Trp145 | Polar residue changed to bulky hydrophobic |
| Arg138 | Lys146 | Conservative change |
| Ile304 | Val306 | Conservative change |
| Ala313 | Ser315 | Becomes more polar |
| Val404 | Ile404 | Conservative change |
| Val510 | Thr512 | Becomes more polar |
None of these changed residues are part of the active site (Fig. 1A).
DISCUSSION
Previous biochemical studies had indicated that fluconazole resistance in some strains of C. krusei occurs because the ckCYP51 is resistant to inhibition by this drug (17). This conclusion is supported by our present findings that S. cerevisiae strains that express ckCYP51 are significantly less susceptible to fluconazole than are strains that express caCYP51. We also found that voriconazole was considerably more active than fluconazole in inhibiting the growth of C. krusei in vitro. These results are consistent with those of other investigations (5, 15). Both the biochemical and gene overexpression studies demonstrated that the increased antifungal activity of voriconazole is due in part to the enhanced ability of this drug to inhibit ckCYP51.
We found that voriconazole was 64- to 128-fold more active than fluconazole at inhibiting the growth of C. krusei, whereas in the experiments with cell extracts, voriconazole was only 8- to 17-fold more potent than fluconazole in inhibiting ckCYP51. Therefore, an additional factor other than the enhanced inhibition of ckCYP51 must contribute to the increased activity of voriconazole against C. krusei. This additional factor may be the ability of voriconazole to achieve a high intracellular concentration within the fungal cell. At present, it is not possible to directly compare the intracellular accumulation of fluconazole and voriconazole because radiolabeled voriconazole is not available.
The activity of drug efflux pumps significantly influences intracellular accumulation of azoles and contributes to antifungal resistance in some isolates of C. krusei (9, 29). Thus, it is possible that voriconazole is poorer substrate for such pumps than is fluconazole. However, using radiolabeled fluconazole, we have previously determined that the fluconazole uptake of the three strains of C. krusei used in the present study was similar to that of C. albicans SC5314, which is susceptible to fluconazole (17). Thus, these strains of C. krusei do not appear to have high-level expression of azole efflux pumps.
We used computer-assisted homology modeling to compare the predicted structures of ckCYP51 and caCYP51, and to determine the structural basis for the differences in activity of fluconazole and voriconazole against ckCYP51 and caCYP51. Several models have been derived for caCYP51 previously, and these different models yield different predictions about how fluconazole interacts with caCYP51 (8, 25, 26). Our models of caCYP51 and ckCYP51 agree with the one developed by Talele and Kulkarni (26). In this model, the N-1 atom of fluconazole (or voriconazole) interacts with the heme moiety as well as Y118 and F126 of caCYP51. In ckCYP51, the two aromatic amino acids that are predicted to interact with fluconazole are Y126 and F134. Our analysis predicts that voriconazole is a more potent inhibitor of both caCYP51 and ckCYP51 than is fluconazole because the extra methyl group of voriconazole results in a stronger hydrophobic interaction with these aromatic amino acids and more extensive filling of the substrate binding site.
Although some of the amino acids that were within 12 Å of the active site differed between caCYP51 and ckCYP51, none of the these amino acids were predicted to interact with voriconazole or fluconazole through their side chains. Therefore, our models of caCYP51 and ckCYP51 do not provide a clear explanation for the relative resistance of the ckCYP51 to inhibition by fluconazole and voriconazole. It is possible that subtle differences in the active sites of the two enzymes do contribute significantly to fluconazole resistance in C. krusei. For example, F105 is predicted to be within 12 Å of the active site of caCYP51 and the F105L substitution is associated with fluconazole resistance in C. albicans (12). Interestingly, the F105L substitution is also present in the ckCYP51 (13), suggesting that this substitution may contribute to fluconazole resistance in C. krusei.
Analysis of other mutations in caCYP51 that are associated with azole resistance in C. albicans indicates that many of the amino acid substitutions are located in regions of the protein that are predicted to be outside of the substrate binding site (13, 20). These substitutions may change the conformation of the binding site and/or limit access to it. The use of site-directed mutagenesis to change the key amino acids that differ between caCYP51 and ckCYP51 will enable us to refine the three-dimensional models of both enzymes, so that the relative resistance of ckCYP51 to azoles can be explained. These investigations are currently in progress.
Acknowledgments
We thank Quynh T. Phan and Angela Sanchez for technical assistance.
This work was supported in part by an unrestricted grant from Pfizer, Inc. S. G. Filler was supported by the Burroughs-Wellcome Fund New Investigator Award in Molecular Pathogenic Mycology.
REFERENCES
- 1.Abbas, J., G. P. Bodey, H. A. Hanna, M. Mardani, E. Girgawy, D. Abi-Said, E. Whimbey, R. Hachem, and I. Raad. 2000. Candida krusei fungemia. An escalating serious infection in immunocompromised patients. Arch. Intern. Med. 160:2659-2664. [DOI] [PubMed] [Google Scholar]
- 2.Abola, E. E., J. L. Sussman, J. Prilusky, and N. O. Manning. 1997. Protein Data Bank archives of three-dimensional macromolecular structures. Methods Enzymol. 277:556-571. [DOI] [PubMed] [Google Scholar]
- 3.Agatep, R., R. Kirkpatrick, D. Parchaliuk, R. Woods, and R. Gietz. 1998. Transformation of Saccharomyces cerevisiae by the lithium acetate/single-stranded carrier DNA/polyethylen glycol (LiAc/ss-DNA/PEG) protocol. Tech. Tips Online [Online.] http://tto.trends.com.
- 4.Burgener-Kairuz, P., J. P. Zuber, P. Jaunin, T. G. Buchman, J. Bille, and M. Rossier. 1994. Rapid detection and identification of Candida albicans and Torulopsis (Candida) glabrata in clinical specimens by species-specific nested PCR amplification of a cytochrome P-450 lanosterol-α-demethylase (L1A1) gene fragment. J. Clin. Microbiol. 32:1902-1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Espinel-Ingroff, A., K. Boyle, and D. J. Sheehan. 2001. In vitro antifungal activities of voriconazole and reference agents as determined by NCCLS methods: review of the literature. Mycopathologia 150:101-115. [DOI] [PubMed] [Google Scholar]
- 6.Goldman, M., J. C. Pottage, Jr., and D. C. Weaver. 1993. Candida krusei fungemia. Report of 4 cases and review of the literature. Medicine (Baltimore) 72:143-150. [PubMed] [Google Scholar]
- 7.Iwen, P. C., D. M. Kelly, E. C. Reed, and S. H. Hinrichs. 1995. Invasive infection due to Candida krusei in immunocompromised patients not treated with fluconazole. Clin. Infect. Dis. 20:342-347. [DOI] [PubMed] [Google Scholar]
- 8.Ji, H., W. Zhang, Y. Zhou, M. Zhang, J. Zhu, Y. Song, and J. Lu. 2000. A three-dimensional model of lanosterol 14alpha-demethylase of Candida albicans and its interaction with azole antifungals. J. Med. Chem. 43:2493-2505. [DOI] [PubMed] [Google Scholar]
- 9.Katiyar, S. K., and T. D. Edlind. 2001. Identification and expression of multidrug resistance-related ABC transporter genes in Candida krusei. Med. Mycol. 39:109-116. [DOI] [PubMed] [Google Scholar]
- 10.Lamb, D. C., D. E. Kelly, T. C. White, and S. L. Kelly. 2000. The R467K amino acid substitution in Candida albicans sterol 14α-demethylase causes drug resistance through reduced affinity. Antimicrob. Agents Chemother. 44:63-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Laskowski, R. A., M. W. McArthur, D. S. Moss, and J. M. Thornton. 1993. PROCHECK: a program to check the stereochemical quality of protein structures. J. Appl. Crystallogr. 26:283-291. [Google Scholar]
- 12.Loffler, J., S. L. Kelly, H. Hebart, U. Schumacher, C. Lass-Florl, and H. Einsele. 1997. Molecular analysis of cyp51 from fluconazole-resistant Candida albicans strains. FEMS Microbiol. Lett. 151:263-268. [DOI] [PubMed] [Google Scholar]
- 13.Marichal, P., L. Koymans, S. Willemsens, D. Bellens, P. Verhasselt, W. Luyten, M. Borgers, F. C. Ramaekers, F. C. Odds, and H. V. Bossche. 1999. Contribution of mutations in the cytochrome P450 14alpha-demethylase (Erg11p, Cyp51p) to azole resistance in Candida albicans. Microbiology 145:2701-2713. [DOI] [PubMed] [Google Scholar]
- 14.National Committee for Clinical Laboratory Standards. 1997. Reference method for broth dilution antifungal susceptibility testing of yeasts: approved standard. NCCLS document M27-A. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 15.Nguyen, M. H., and C. Y. Yu. 1998. Voriconazole against fluconazole-susceptible and resistant candida isolates: in-vitro efficacy compared with that of itraconazole and ketoconazole. J. Antimicrob. Chemother. 42:253-256. [DOI] [PubMed] [Google Scholar]
- 16.Ochman, H., A. S. Gerber, and D. L. Hartl. 1988. Genetic applications of an inverse polymerase chain reaction. Genetics 120:621-623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Orozco, A. S., L. M. Higginbotham, C. A. Hitchcock, T. Parkinson, D. Falconer, A. S. Ibrahim, M. A. Ghannoum, and S. G. Filler. 1998. Mechanism of fluconazole resistance in Candida krusei. Antimicrob. Agents Chemother. 42:2645-2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pesole, G., M. Lotti, L. Alberghina, and C. Saccone. 1995. Evolutionary origin of nonuniversal CUGSer codon in some Candida species as inferred from a molecular phylogeny. Genetics 141:903-907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Podust, L. M., T. L. Poulos, and M. R. Waterman. 2001. Crystal structure of cytochrome P450 14alpha-sterol demethylase (CYP51) from Mycobacterium tuberculosis in complex with azole inhibitors. Proc. Natl. Acad. Sci. USA 98:3068-3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Podust, L. M., J. Stojan, T. L. Poulos, and M. R. Waterman. 2001. Substrate recognition sites in 14alpha-sterol demethylase from comparative analysis of amino acid sequences and X-ray structure of Mycobacterium tuberculosis CYP51. J. Inorg. Biochem. 87:227-235. [DOI] [PubMed] [Google Scholar]
- 21.Sali, A., and T. L. Blundell. 1993. Comparative protein modelling by satisfaction of spatial restraints. J. Mol. Biol. 234:779-815. [DOI] [PubMed] [Google Scholar]
- 22.Sanglard, D., F. Ischer, L. Koymans, and J. Bille. 1998. Amino acid substitutions in the cytochrome P-450 lanosterol 14α-demethylase (CYP51A1) from azole-resistant Candida albicans clinical isolates contribute to resistance to azole antifungal agents. Antimicrob. Agents Chemother. 42:241-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sorkhoh, N. A., M. A. Ghannoum, A. S. Ibrahim, R. J. Stretton, and S. S. Radwan. 1991. Growth of Candida albicans in the presence of hydrocarbons: a correlation between sterol concentration and hydrocarbon uptake. Appl. Microbiol. Biotechnol. 34:509-512. [Google Scholar]
- 24.Sussman, J. L., D. Lin, J. Jiang, N. O. Manning, J. Prilusky, O. Ritter, and E. E. Abola. 1998. Protein Data Bank (PDB): database of three-dimensional structural information of biological macromolecules. Acta Crystallogr. D Biol. Crystallogr. 54:1078-1084. [DOI] [PubMed] [Google Scholar]
- 25.Talele, T. T., S. S. Kulkarni, and V. M. Kulkarni. 1999. Development of pharmacophore alignment models as input for comparative molecular field analysis of a diverse set of azole antifungal agents. J. Chem. Infect. Comput. Sci. 39:958-966. [Google Scholar]
- 26.Talele, T. T., and V. M. Kulkarni. 1999. Three-dimensional quantitative structure-activity relationship (QSAR) and receptor mapping of cytochrome P-450(14 alpha DM) inhibiting azole antifungal agents. J. Chem. Infect. Comput. Sci. 39:204-210. [DOI] [PubMed] [Google Scholar]
- 27.Talibi, D., and M. Raymond. 1999. Isolation of a putative Candida albicans transcriptional regulator involved in pleiotropic drug resistance by functional complementation of a pdr1 pdr3 mutation in Saccharomyces cerevisiae. J. Bacteriol. 181:231-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Triglia, T., M. G. Peterson, and D. J. Kemp. 1988. A procedure for in vitro amplification of DNA segments that lie outside the boundaries of known sequences. Nucleic Acids Res. 16:8186.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Venkateswarlu, K., D. W. Denning, N. J. Manning, and S. L. Kelly. 1996. Reduced accumulation of drug in Candida krusei accounts for itraconazole resistance. Antimicrob. Agents Chemother. 40:2443-2446. [DOI] [PMC free article] [PubMed] [Google Scholar]