Abstract
Bacillus clausii SIN is one of the four strains of B. clausii composing a probiotic administered to humans for the prevention of gastrointestinal side effects due to oral antibiotic therapy. The strain is resistant to kanamycin, tobramycin, and amikacin. A gene conferring aminoglycoside resistance was cloned into Escherichia coli and sequenced. The gene, called aadD2, encoding a putative 246-amino acid protein, shared 47% identity with ant(4′)-Ia from Staphylococcus aureus, which encodes an aminoglycoside 4′-O-nucleotidyltransferase. Phosphocellulose paper-binding assays indicated that the gene product was responsible for nucleotidylation of kanamycin, tobramycin, and amikacin. The aadD2 gene was detected by DNA-DNA hybridization in the three other strains of the probiotic mixture and in the reference strain B. clausii DSM8716, although it did not confer resistance in these strains. Mutations in the sequence of the putative promoter for aadD2 from B. clausii SIN resulted in higher identity with consensus promoter sequences and may account for aminoglycoside resistance in that strain. The aadD2 gene was chromosomally located in all strains and was not transferable by conjugation. These data indicate that chromosomal aadD2 is specific to B. clausii.
Probiotics are administered to humans for the prevention of gastrointestinal side effects due to oral antibiotic therapy. Ingestion of high quantities of spores is thought to restore an intestinal flora following the destruction of the normal flora by antibiotics (17). Enterogermina is a preparation of Bacillus available in Italy (3, 18). It is composed of four antibiotic-resistant strains, OC, NR, T, and SIN (3), initially identified as Bacillus subtilis but recently assigned to the Bacillus clausii species (26). For the prevention of intestinal disorders, the strains of Bacillus used in the Enterogermina preparation were rendered multiantibiotic resistant to survive in the presence of the antibiotics which are coadministered (3, 18). One of the strains, B. clausii SIN, is resistant to kanamycin, tobramycin, and amikacin (3). Resistance to aminoglycosides is generally due to enzymatic modification of the drugs and is widespread in a variety of bacterial pathogens (27). However, aminoglycoside inactivation has rarely been reported for Bacillus spp. An aph(3′)-IVa gene from a butirosin-producing strain of Bacillus circulans was reported and subsequently sequenced (4, 10). This gene encodes an aminoglycoside 3′-O-phosphotransferase type IV which confers resistance to butirosin, gentamicin B, kanamycin, lividomycin, neomycin, paromomycin, and ribostamycin by modifying these aminoglycosides at the 3′-hydroxyl group (10). B. subtilis 168 possesses a chromosomal aadK gene which encodes an aminoglycoside 6-O-adenylyltransferase that confers resistance to streptomycin (23). A kanamycin 4′-O-nucleotidyltransferase encoded by plasmid pTB913 from a thermophilic Bacillus sp. was found to be similar to that encoded by the gene ant(4′)-Ia or aadD borne by plasmid pUB110 from Staphylococcus aureus (16). The aim of this study was to characterize the mechanism of resistance of B. clausii SIN to aminoglycosides and to elucidate the genetic basis of this mechanism.
MATERIALS AND METHODS
Bacterial strains.
The four B. clausii strains OC, NR, SIN, and T used for the production of Enterogermina were obtained from Sanofi-Synthelabo OTC SpA (Milan, Italy) as separate spore suspensions. B. clausii DSM8716 was used as a reference strain.
Susceptibility to antibiotics.
Antibiotic susceptibility was tested by the disk diffusion method, and MICs were determined by agar dilution according to the recommendations of the National Committee for Clinical Laboratory Standards (21). Susceptible, intermediate, and resistant categories were defined according to the recommendations of the National Committee for Clinical Laboratory Standards (22).
Mating experiments.
Enterococcus faecalis JH2-2 (11) and Enterococcus faecium HM1070 (1), both resistant to rifampin and fusidic acid, and B. subtilis UCN19, resistant to ciprofloxacin, were used as recipients in mating experiments (5, 11). The latter strain was obtained by stepwise selection of a mutant from B. subtilis 168 (12) on agar plates containing increasing concentrations of ciprofloxacin. In every transfer experiment, E. faecalis BM4110 containing the conjugative plasmid pAMβ1 (14) was used as a control. Agar plates for the selection of transconjugants contained rifampin (50 μg/ml) plus fusidic acid (20 μg/ml) or ciprofloxacin (8 μg/ml) combined with 128 μg of kanamycin per ml for enterococci and 20 μg/ml for B. subtilis. All mating experiments were repeated a minimum of three times.
PCR.
The deoxynucleotide primers ANT4-1 (5′ TAAATATGGGGATGATGTTAAGGC 3′) and ANT4-2 (5′ TGGTATGCGTTTTGACACATCCAC 3′), specific for ant(4′)-Ia, were used. S. aureus BM3002 was used as a control (6).
DNA manipulations.
Total and plasmid DNA from Bacillus strains was extracted as described previously for enterococci (2). Cloning was carried out by standard techniques (25). Total DNA from B. clausii strains was digested with various restriction enzymes, cloned into plasmid pUC18, and introduced by electrotransformation into Escherichia coli DH10B (2). Recombinant plasmids were selected on agar plates containing ampicillin (200 μg/ml) and kanamycin (20 μg/ml). A DNA fragment conferring kanamycin resistance was then subcloned into the shuttle plasmid pJIM2246 conferring resistance to chloramphenicol (24) and introduced into E. faecalis JH2-2 by electrotransformation (1). Sequencing of DNA was performed on ABI PRISM 377 (Perkin-Elmer Corp., Norwalk, Conn.) with big dye terminator according to the protocol supplied by the manufacturer. Nucleotide and amino acid sequences were analyzed by using the software available online at Pedro's Biomolecular Research Tools website (http://www.up.univ-mrs.fr/∼wabim/pedro/research_tools.html) and at the National Center for Biotechnology Information website (http://www.ncbi.nlm.nih.gov/). Putative promoters were identified by using the Neural Network Promoter Prediction software (www.fruitfly.org/seq_tools/). Score predictions of >0.9 were considered to be significant.
Southern hybridization.
DNA from B. clausii strains was digested with I-CeuI or SmaI (New England Biolabs, Beverly, Mass.), separated by pulsed-field gel electrophoresis with a technique used for enterococci (2), transferred onto a nylon membrane, and hybridized to a probe specific for aadD2 of B. clausii and consisting of the entire gene amplified by PCR and labeled with digoxigenin (Boehringer Mannheim). DNA was then hybridized to a probe specific for the 16S rRNA. The 16S rRNA probe was obtained by labeling the DNA fragment amplified from B. clausii DSM8716 with deoxynucleotide primers 5′ AACTGGAGGAAGGTGGGGAT 3′ and 5′ AGGAGGTGATCCAACCGCA 3′ (7).
Aminoglycoside-modifying enzyme assays.
Bacteria were lysed by ultrasonic disintegration, and the resulting extracts were centrifuged at 100,000 × g for 45 min. Aminoglycoside-modifying activity in the supernatant (S100) was tested by the phosphocellulose paper-binding technique with [α-32P]ATP (specific activity, 30 Ci/mmol) as described previously (8). Aminoglycosides (67 μg/ml, i.e., 1.4 × 10−1 mM kanamycin or tobramycin) were incubated with S100 extracts at 30°C for 30 min in TMND buffer (0.06 M Tris-HCl, 0.04 M MgCl2, 0.4 M NH4Cl, 1.6 × 10−4 M dithiothreitol [pH 7.1]).
Nucleotide sequence accession number.
The 1,006-bp fragment containing aadD2 was submitted to GenBank and assigned accession no. AF539790.
RESULTS AND DISCUSSION
Antibiotic resistance in B. clausii strains.
By disk diffusion, all B. clausii strains were found to be resistant to penicillin G, cefalotin, cefotaxime, erythromycin, and lincomycin. Resistance to aminoglycosides was detected only in B. clausii SIN, and the MICs of certain aminoglycosides for the B. clausii strains are shown in Table 1. B. clausii SIN was resistant to kanamycin, tobramycin, and amikacin but susceptible to gentamicin and netilmicin.
TABLE 1.
MICs of aminoglycosides for B. clausii strains and transformants
| Strain | MIC (μg/ml)
|
||||
|---|---|---|---|---|---|
| Kanamycin | Tobramycin | Amikacin | Gentamicin | Netilmicin | |
| B. clausii DSM8716 | 0.5 | 0.5 | 1 | 0.5 | 0.5 |
| B. clausii OC | 4 | 2 | 1 | 0.5 | <0.5 |
| B. clausii NR | 2 | 1 | 0.5 | 0.5 | <0.5 |
| B. clausii SIN | >1,000 | 516 | 16 | 0.5 | <0.5 |
| B. clausii T | 4 | 4 | 0.5 | 0.5 | <0.5 |
| E. coli DH10B | 0.5 | 1 | 1 | 1 | 0.25 |
| E. coli DH10B/pUV12 | 32 | 64 | 4 | 1 | <0.5 |
| E. faecalis JH2-2 | 16 | 8 | 64 | 8 | 1 |
| E. faecalis JH2-2/pUV13 | >1,000 | >1,000 | 128 | 8 | 1 |
Characterization of the aadD2 gene from B. clausii SIN.
An 8-kb EcoRI DNA fragment originating from the DNA of B. clausii SIN was cloned into plasmid pUC18. The recombinant plasmid, pUV11, conferred resistance to ampicillin and kanamycin. A 2.2-kb Sau3A fragment that also conferred resistance to kanamycin was subsequently subcloned into pUC18, generating plasmid pUV12, and sequenced. Analysis of the sequence revealed the presence of an open reading frame of 765 bp or an alternative open reading frame starting 6 bp upstream and putatively encoding 254- or 256-amino-acid proteins. Both ATG start codons were preceded at 9 and 10 bp by ribosome-binding site-like sequences, 5′ GAGATGGAAG 3′ and 5′ CAAAAGGAGA 3′, complementary to five and seven bases (underlined), respectively, of the 3′-OH-terminal sequence (5′ UCUUUCCUCC 3′) of B. subtilis 16S rRNA (19).
A search for the presence of motifs in the deduced amino acid sequence detected a nucleotidyltransferase domain between amino acids 14 and 59 that is shared by a large family of nucleotidyltransferases (15). In this family, highest homology (identity, 47%; similarity, 65%) was found with the enzyme encoded by ant(4′)-Ia or aadD (GenBank accession no. V01282) of plasmid pUB110 from S. aureus (Fig. 1) (20, 27). As previously mentioned, a gene that is identical, except for a 1-bp substitution, was reported in a thermophilic Bacillus sp., where it was borne by plasmid pTB913 (16). This nucleotidyltransferase modifies kanamycin, tobramycin, and amikacin and confers a resistance phenotype similar to those of B. clausii SIN and the E. coli transformant (Table 1).
FIG. 1.
Amino acid sequence comparison of AadD2 from B. clausii SIN with Ant(4′)-Ia or AadD encoded by plasmid pUB110 from S. aureus (16). Sequence identity is indicated by asterisks.
The aadD2 gene was subcloned into shuttle vector pJIM2246, and the recombinant plasmid, pUV13, was electrotransformed into E. faecalis JH2-2. For the transformants, the MICs of kanamycin and tobramycin were elevated and that of amikacin was moderately higher but the MICs of gentamicin and netilmicin remained unchanged. The resistance phenotype was consistent with modification of the 4′-hydroxyl group of aminoglycosides.
Analysis, by the phosphocellulose paper-binding assay, of bacterial extracts from E. coli DH10B/pUV12 indicated that aminoglycoside resistance was due to a nucleotidylating activity. Kanamycin A nucleotidylation was defined as 100%, and tobramycin (91%), amikacin (16%), isepamicin (14%), and neomycin B (100%) were modified at the indicated percentages. Gentamicin C1 and netilmicin were not substrates for the enzyme. On the basis of the substrate profile, the enzyme encoded by pUV12 was an aminoglycoside 4′-O-nucleotidyltransferase. Similar results were obtained with B. clausii SIN extracts and, surprisingly, also for B. clausii DSM8716, although the enzyme activity was much weaker in the latter strain. According to the nomenclature proposed by Shaw et al. (27), the kanamycin nucleotidyltransferase of B. clausii was assigned to the Ant(4′)-I enzyme family and the corresponding new gene was tentatively called aadD2.
Distribution and localization of aadD2.
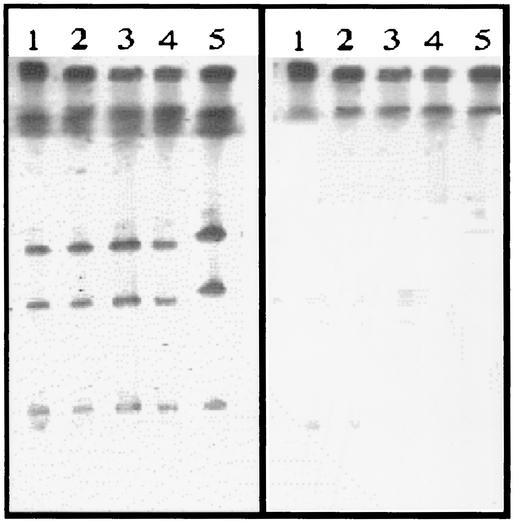
After digestion with the I-CeuI enzyme, which recognizes a sequence which is specific for rRNA operons (13), total DNA of the B. clausii strains yielded apparently six fragments, indicating that this species contains a minimum of six rRNA operons (Fig. 2). Southern experiments showed that all fragments hybridized with an rrs probe specific for 16S rRNA. The aadD2 probe hybridized to a single large chromosomal fragment of each strain. B. clausii OC, NR, SIN, and T had indistinguishable SmaI-generated patterns, and hybridization showed that the aadD2-like genes were borne by a fragment approximately 30 kb in size in the probiotic strains and by a larger fragment of ca. 50 kb in B. clausii DSM8716 (data not shown). Thus, aadD2-like genes were present in all the strains of B. clausii studied but were phenotypically expressed only in B. clausii SIN.
FIG. 2.
Localization of aadD2 in B. clausii. Total DNA from B. clausii strains NR (lanes 1), OC (lanes 2), T (lanes 3), SIN (lanes 4), and DSM8716 (lanes 5) was digested with restriction enzyme I-CeuI and subjected to pulsed-field gel electrophoresis. DNA was transferred to a nylon membrane and hybridized successively with rrs (16S rRNA) (left) and aadD2 (right) probes labeled with digoxigenin. The low-molecular-weight I-CeuI band is barely visible in lanes 2, 3, 4, and 5 (left).
Sequence analysis of the 8-kb EcoRI insert of plasmid pUV11 revealed the presence, 3′ downstream from the aadD2 gene, of genes homologous to blaZ, blaI, and blaR from Staphylococcus, responsible for the production and regulation of a penicillinase (our unpublished data), a ytrA-like gene encoding a putative 135-amino-acid protein homologous to tRNA synthetases, the structural gene for an ABC transporter, and a gntR-like gene putatively encoding a transcriptional regulator. The presence of aadD2 adjacent to genes known to be part of the chromosome confirms its chromosomal location. In addition, repeated attempts to transfer resistance to aminoglycosides and to beta-lactams from B. clausii strains to E. faecalis JH2-2, E. faecium HM1070, and B. subtilis UCN19 by conjugation were unsuccessful.
Analysis of the putative promoter of aadD2 from B. clausii SIN.
There was a discrepancy between the presence of aadD2 in all strains of B. clausii studied and the various levels of aminoglycoside resistance of the strains. We thus amplified and analyzed a DNA sequence of nearly 200 bp upstream from the aadD2 genes of B. clausii SIN (resistant to aminoglycosides), NR, OC, T, and DSM8716 (susceptible to aminoglycosides). Potential −35 and −10 consensus sequences were identified, one of which may be the recognition site for a DNA-dependent RNA polymerase (Fig. 3). In all strains, one promoter sequence was confidently predicted (prediction score, 0.93). Comparison of the sequences revealed three nucleotide substitutions in the B. clausii SIN sequence. Two of these substitutions were located in putative −35 and −10 regions. A C-to-A mutation rendered the −35 sequence (TTAGCA) identical to the consensus promoter sequences of E. coli and B. subtilis (9, 19), which might result in a stronger promoter. Another mutation was located in the −10 sequence (Fig. 3). This mutation led to the prediction (score, 0.95) of an additional putative promoter. The consequence, if any, of the third substitution remains unknown.
FIG. 3.
Sequence comparison of putative promoters for the aadD2 genes in B. clausii NR, OC, SIN, T, and DSM8716. The putative −35 and −10 sequences are indicated in bold. The −35 consensus sequence is TTGACA. A putative transcription start site is indicated by a letter in larger font. Two putative ribosome-binding sites are underlined (single and double lines). Putative start codons are shown in bold italics. Sequence identity is indicated by asterisks.
Although the origin of B. clausii SIN is not known, the identity of the SmaI restricted DNA profiles of strains composing Enterogermina strongly supports the notion that SIN is a derivative of one of the other B. clausii probiotic strains or of a common parental strain.
Acknowledgments
This study was supported in part by a grant from Sanofi-Synthelabo OTC SpA, Milan, Italy.
REFERENCES
- 1.Bozdogan, B., and R. Leclercq. 1999. Effects of genes encoding resistance to streptogramins A and B on the activity of quinupristin-dalfopristin against Enterococcus faecium. Antimicrob. Agents Chemother. 43:2720-2725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bozdogan, B., L. Berrezouga, M. S. Kuo, D. A. Yurek, K. A. Farley, B. J. Stockman, and R. Leclercq. 1999. A new resistance gene, linB, conferring resistance to lincosamides by nucleotidylation in Enterococcus faecium HM1025. Antimicrob. Agents Chemother. 43:925-929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ciffo, F. 1984. Determination of the spectrum of antibiotic resistance of the “Bacillus subtilis” strains of Enterogermina. Chemioterapia 3:45-52. [PubMed] [Google Scholar]
- 4.Courvalin, P., B. Weisblum, and J. Davies. 1977. Aminoglycoside-modifying enzyme of an antibiotic-producing bacterium acts as a determinant of antibiotic resistance in Escherichia coli. Proc. Natl. Acad. Sci. USA 74:999-1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ehrenfeld, E. E., and D. B. Clewell. 1987. Transfer functions of the Streptococcus faecalis plasmid pAD1: organization of plasmid DNA encoding response to sex pheromone. J. Bacteriol. 169:3473-3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.El Solh, N., J. M. Fouace, J. Pillet, and Y. A. Chabbert. 1981. Plasmid DNA content of multiresistant Staphylococcus aureus strains. Ann. Microbiol. (Paris) 132B:131-156. [PubMed]
- 7.Greisen, K., M. Loeffelholz, A. Purohit, and D. Leong. 1994. PCR primers and probes for the 16S rRNA gene of most species of pathogenic bacteria, including bacteria found in cerebrospinal fluid. J. Clin. Microbiol. 32:335-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haas, M. J., and J. E. Dowding. 1975. Aminoglycoside-modifying enzymes. Methods Enzymol. 43:611-628. [DOI] [PubMed] [Google Scholar]
- 9.Haldenwang, W. G. 1995. The sigma factors of Bacillus subtilis. Microbiol. Rev. 59:1-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Herbert, C. J., M. Sarwar, S. S. Ner, I. G. Giles, and M. Akhtar. 1986. Sequence and interspecies transfer of an aminoglycoside phosphotransferase gene (APH) of Bacillus circulans. Self-defence mechanism in antibiotic-producing organisms. Biochem. J. 233:383-393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jacob, A. E., and S. J. Hobbs. 1974. Conjugal transfer of plasmid-borne multiple antibiotic resistance in Streptococcus faecalis var. zymogenes. J. Bacteriol. 117:360-372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kunst, F., N. Ogasawara, I. Moszer, A. M. Albertini, G. Alloni, V. Azevedo, M. G. Bertero, P. Bessieres, A. Bolotin, S. Borchert, R. Borriss, L. Boursier, A. Brans, M. Braun, S. C. Brignell, S. Bron, S. Brouillet, C. V. Bruschi, B. Caldwell, V. Capuano, N. M. Carter, S. K. Choi, J. J. Codani, J. F. Connerton, A. Danchin, et al. 1997. The complete genome sequence of the gram-positive bacterium Bacillus subtilis. Nature 390:249-256. [DOI] [PubMed] [Google Scholar]
- 13.Liu, S. L., A. Hessel, and K. E. Sanderson. 1993. Genomic mapping with I-Ceu I, an intron-encoded endonuclease specific for genes for ribosomal RNA, in Salmonella spp., Escherichia coli, and other bacteria. Proc. Natl. Acad. Sci. USA 90:6874-6878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martin, B., G. Alloing, V. Mejean, and J. P. Claverys. 1987. Constitutive expression of erythromycin resistance mediated by the ermAM determinant of plasmid pAM β1 results from deletion of 5′ leader peptide sequences. Plasmid 18:250-253. [DOI] [PubMed] [Google Scholar]
- 15.Martin, G., and W. Keller. 1996. Mutational analysis of mammalian poly(A) polymerase identifies a region for primer binding and catalytic domain, homologous to the family X polymerases, and to other nucleotidyltransferases. EMBO J. 15:2593-2603. [PMC free article] [PubMed] [Google Scholar]
- 16.Matsumura, M., Y. Katakura, T. Imanaka, and S. Aiba. 1984. Enzymatic and nucleotide sequence studies of a kanamycin-inactivating enzyme encoded by a plasmid from thermophilic bacilli in comparison with that encoded by plasmid pUB110. J. Bacteriol. 160:413-420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mazza, P. 1994. The use of Bacillus subtilis as an antidiarrhoeal microorganism. Boll. Chim. Farm. 133:3-18. [PubMed] [Google Scholar]
- 18.Mazza, P., F. Zani, and P. Martelli. 1992. Studies on the antibiotic resistance of Bacillus subtilis strains used in oral bacteriotherapy. Boll. Chim. Farm. 131:401-408. [PubMed] [Google Scholar]
- 19.Moran, C. P., Jr., N. Lang, and R. Losick. 1981. Nucleotide sequence of a Bacillus subtilis promoter recognized by Bacillus subtilis RNA polymerase containing sigma 37. Nucleic Acids Res. 9:5979-5990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Muller, R. E., T. Ano, T. Imanaka, and S. Aiba. 1986. Complete nucleotide sequences of Bacillus plasmids pUB110dB, pRBH1 and its copy mutants. Mol. Gen. Genet. 202:169-171. [DOI] [PubMed] [Google Scholar]
- 21.National Committee for Clinical Laboratory Standards. 1997. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 4th ed. Approved standard, document M7-A4. National Committee for Clinical Laboratory Standards, Villanova, Pa.
- 22.National Committee for Clinical Laboratory Standards. 1997. Performance standards for antimicrobial susceptibility testing. Sixth information supplement M100-S7. National Committee for Clinical Laboratory Standards, Villanova, Pa.
- 23.Noguchi, N., M. Sasatsu, and M. Kono. 1993. Genetic mapping in Bacillus subtilis 168 of the aadK gene which encodes aminoglycoside 6-adenylyltransferase. FEMS Microbiol. Lett. 114:47-52. [DOI] [PubMed] [Google Scholar]
- 24.Renault, P., G. Corthier, N. Goupil, C. Delorme, and S. D. Ehrlich. 1996. Plasmid vectors for Gram-positive bacteria switching from high to low copy number. Gene 183:175-182. [DOI] [PubMed] [Google Scholar]
- 25.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 26.Senesi, S., F. Celandroni, A. Tavanti, and E. Ghelardi. 2001. Molecular characterization and identification of Bacillus clausii strains marketed for use in oral bacteriotherapy. Appl. Environ. Microbiol. 67:834-839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shaw, K. J., P. N. Rather, R. S. Hare, and G. H. Miller. 1993. Molecular genetics of aminoglycoside resistance genes and familial relationships of the aminoglycoside-modifying enzymes. Microbiol. Rev. 57:138-163. [DOI] [PMC free article] [PubMed] [Google Scholar]