Abstract
TRPP2 is a member of the transient receptor potential (TRP) superfamily of cation channels, which is mutated in autosomal dominant polycystic kidney disease (ADPKD). TRPP2 is thought to function with polycystin 1—a large integral protein—as part of a multiprotein complex involved in transducing Ca2+-dependent information. TRPP2 has been implicated in various biological functions including cell proliferation, sperm fertilization, mating behaviour, mechanosensation and asymmetric gene expression. Although its function as a Ca2+-permeable cation channel is well established, its precise role in the plasma membrane, the endoplasmic reticulum and the cilium is controversial. Recent studies suggest that TRPP2 function is highly dependent on the subcellular compartment of expression, and is regulated by many interactions with adaptor proteins. This review summarizes the most pertinent evidence about the properties of TRPP2 channels, focusing on the compartment-specific functions of mammalian TRPP2.
Keywords: polycystin, TRP cation channel, calcium ion, mechanosensation, endoplasmic reticulum, polycystic kidney disease
Introduction
Transient receptor potential (TRP) channels belong to a superfamily of multifunctional cation channels that are present in virtually all mammalian cell types and which are mutated in autosomal dominant polycystic kidney disease (ADPKD). The TRP superfamily (Fig 1) includes 56 related six-transmembrane domain channels, which are classified into seven families: TRPC (Canonical), TRPV (Vanilloid), TRPM (Melastatin), TRPN (Nompc, from no mechanoreceptor potential-c), TRPA (Ankyrin-like with transmembrane domain 1), TRPML (Mucolipin) and TRPP (Polycystin; for reviews see Clapham et al, 2005; Delmas, 2004a, 2005; Montell, 2005; Nilius & Voets, 2005).
Figure 1.
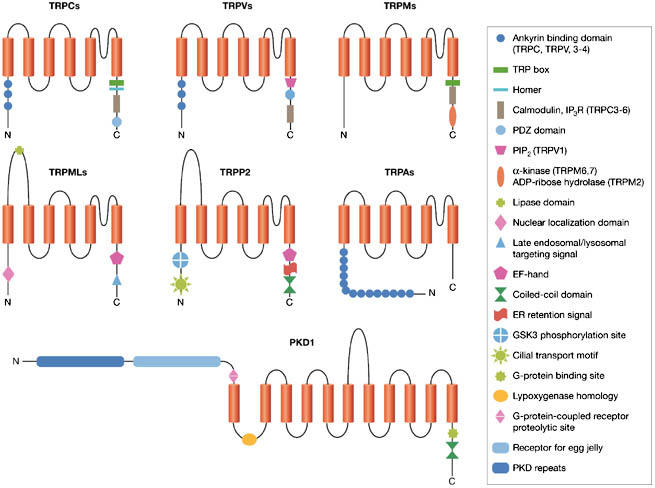
Structural elements of the mammalian transient receptor potential cation channels and polycystin 1. ER, endoplasmic reticulum; GSK3, glycogen synthase kinase 3; PKD, polycystin; TRP, transient receptor potential family (TRPA, TRPC, TRPM, TRPML, TRPP, TRPV).
The polycystin family is divided structurally and functionally into two subfamilies, the polycystin 1 (PKD1)-like and polycystin 2 (PKD2)-like proteins, both of which have a modest degree of sequence similarity between subfamilies (Delmas, 2004a, 2005; Igarashi & Somlo, 2002; Nauli & Zhou, 2004). In humans, the PKD2-like subgroup contains three homologous proteins—PKD2, PKD2L1 and PKD2L2—which are now referred to as TRPP2, TRPP3 and TRPP5. The PKD1-like subgroup contains five homologous proteins, all with an 11-transmembrane topology, and—by virtue of their structure—are not considered as members of the TRP superfamily (Fig 1).
TRPP2 has been shown to assemble with PKD1 to form a receptor–ion channel complex (Delmas et al, 2004a; Hanaoka et al, 2000). Both proteins localize to primary cilia of renal epithelial cells, where they are implicated in mechanosensitive transduction signals (Pazour et al, 2002; Yoder et al, 2002; Nauli et al, 2003). TRPP2 has been also documented at two other subcellular locations—the plasma membrane (PM) and the endoplasmic reticulum (ER)—but it is still disputed whether TRPP2 functions as an intracellular or a plasmalemmal channel. The finding that TRPP2 is retained in the ER of most cell systems has supported the view that TRPP2 might function as a reticular Ca2+-release channel (Koulen et al, 2002). Conversely, TRPP2 has been shown to reside and act at the PM, notably in Madine–Darby canine kidney (MDCK) cells derived from cortical collecting ducts (Luo et al, 2003; Scheffers et al, 2002). These apparently incongruent views might be reconciled by the recent demonstration that subcellular transport and localization of TRPP2 are controlled by many interactions with adaptor proteins and enzymes (Hidaka et al, 2004; Köttgen et al, 2005a; Köttgen & Walz, 2005; Streets et al, 2006; Geng et al, 2006). Such varied transport behaviour provides a mechanism for the dynamic regulation of TRPP2 channel density at the ER, PM and cilial localizations, and for different subcellular TRPP2 signalling functions. Thus, the knowledge of the compartment-specific regulations of TRPP2 is of crucial importance for the understanding of its roles in health and disease.
Subcellular localization and transport of TRPP2
A long-standing debate has surrounded the subcellular localization of TRPP2. In most cell-based systems, TRPP2 concentrates in intracellular compartments, most notably in the ER (Cai et al, 1999). TRPP2 encompasses an ER-retention signal within its carboxyl terminus (Fig 2), which seems to prevent transport to the cell surface when expressed alone (Cai et al, 1999). Deletion mutants for this ER-retention signal translocate to the cell surface and can be detected by immunological and electrophysiological methods (Chen et al, 2001). Opposing these findings are reports that TRPP2 is present at the PM of MDCK cells (Scheffers et al, 2002) or after treatment with chemical chaperones/proteasome inhibitors (Gonzalez-Perrett et al, 2001; Luo et al, 2003; Vassilev et al, 2001). TRPP2 has been also localized to basolateral PMs, lamellipodia, primary cilia and mitotic spindles (Cai et al, 1999; Foggensteiner et al, 2000; Nauli et al, 2003; Newby et al, 2002; Rundle et al, 2004; Yoder et al, 2002).
Figure 2.
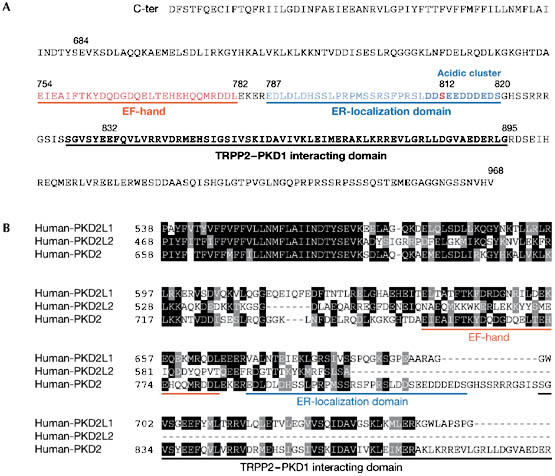
Regulatory carboxy-terminal domains of transient receptor potential polycystin proteins. (A) Sequence of human TRPP2 C-terminus. (B) Sequence alignment of the variable C-termini of human PKD2L1 (TRPP3), PKD2L2 (TRPP5) and PKD2 (TRPP2). Identical and similar residues are highlighted in black and grey, respectively. TRPP, transient receptor potential polycystin.
Recent data have begun to clarify the location of TRPP2. First, Köttgen et al (2005a) reported that TRPP2 transport between the ER, Golgi and PM compartments might be directed by the phosphofurin acidic cluster proteins PACS1 and PACS2—two sorting proteins that bind to an acidic cluster in the C-terminal domain of TRPP2 (Fig 2). Binding of these adaptor proteins to TRPP2 is promoted by casein kinase 2 (CK2)-dependent phosphorylation of Ser 812. Mutation of Ser 812 to alanine or destruction of the acidic cluster abrogates the interaction between TRPP2 and the PACS proteins, and increases whole-cell TRPP2 currents. Thus, mechanisms that regulate the interaction of PACS proteins with TRPP2 are likely to have a key role in TRPP2 transport. Importantly, TRPP3 and TRPP5 lack the PACS-binding acidic cluster (Fig 2B), suggesting that their transport is regulated in a different way to that of TRPP2. This might explain why TRPP3 is targeted at the cell surface, whereas TRPP2 is retained in the ER. Second, a recently discovered protein called PIGEA14 (polycystin-2 interactor, Golgi- and ER-associated protein) has been shown to interact with the C-terminus of TRPP2. Co-expression of both proteins in HeLa cells and in pig kidney LLC-PK1 cells causes a redistribution of TRPP2 as well as PIGEA14 from the ER to a putative trans-Golgi compartment (Hidaka et al, 2004), indicating that transport of TRPP2 is regulated at the ER and the trans-Golgi network. Finally, the TRPP2 amino-terminus contains signal domains that are required for PM and cilial localizations. PM, but not cilial, localization of TRPP2 is regulated by glycogen synthase kinase 3 phosphorylation of Ser 76 (Streets et al, 2006), whereas another N-terminal motif (R6V7xP8) is necessary for localization in cilia (Fig 1; Geng et al, 2006). The study by Geng et al (2006) also provides evidence that TRPP2 transport to cilia is independent of PKD1 because TRPP2 mutants lacking the PKD1 interaction region still transport to cilia.
TRPP2 as an intracellular Ca2+-permeable cation channel
TRPP2 might act as a Ca2+-release channel in ER membranes, which amplifies Ca2+ transients initiated by inositol-1,4,5-trisphosphate (Ins(1,4,5)P3)-generating PM receptors (Koulen et al, 2002). This led to the suggestion that TRPP2 is a novel type of intracellular receptor that might be involved in mediating Ca2+-induced Ca2+ release (Fig 3). TRPP2 seems to be directly activated by Ca2+ and displays a bell-shaped dependence on cytoplasmic Ca2+ (Koulen et al, 2002). Although it is not yet clear whether the Ca2+-binding EF-hand of TRPP2 is involved in Ca2+-dependent modulation of TRPP2, it is worth noting that pathogenic TRPP2 mutants with a premature termination of the peptide chain in their C-tail lost their ability to detect Ca2+. However, TRPP3 artificial truncation mutants lacking the EF-hand show Ca2+-activated channel activities, suggesting that, at least for TRPP3, the EF-hand and other parts of the C-tail are not key determinants of the Ca2+-dependent activation (Li et al, 2002).
Figure 3.
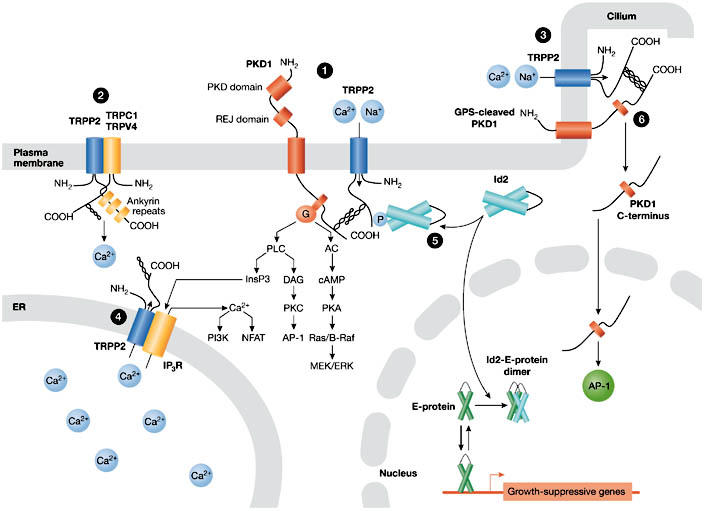
Compartment-specific functions of transient receptor potential polycystin 2. The various models for the localization-dependent functions of TRPP2 are shown. TRPP2 mediates Ca2+ influx at the plasma membrane (PM) and ciliary membrane (cilium), where it functions in a protein complex with polycystin 1 (PKD1) (1, 3) and possibly with other members of the TRP channel superfamily (TRPC1, TRPV4) (2). Ca2+ influx induced by shear flow in renal epithelial cells is triggered by bending of the luminal cilium and seemingly requires the presence of PKD1–TRPP2 complexes in the cilium (3). Mechanical stress results in activation of PKD1–TRPP2 complexes to allow Ca2+ influx either in the shaft or in the base of the cilium. TRPP2 acts as a Ca2+-release channel in the endoplasmic reticulum (ER), where it might interact with, and regulate, inositol-1,4,5-trisphosphate receptors (Ins(1,4,5)P3Rs) (4). Serine-phosphorylated TRPP2 sequesters Id2 in the cytoplasm and prevents it from entering the nucleus (5). PKD1 can undergo a proteolytic cleavage that releases its carboxy-terminal tail, which translocates to the nucleus and activates the transcription factor AP1 (6). Note that it is hypothesized that PKD1 targeted to the primary cilium is also cleaved at its GPCR proteolytic site (GPS). ERK, extracellular signal-regulated kinase; GPCR, G-protein coupled receptor; Id2, inhibitor of DNA binding 2; MEK, mitogen-activated protein kinase/ERK kinase; NFAT, nuclear factor of activated T-cells; PI3K, phosphatidyl-inositol 3-kinase; PKA, cAMP-dependent protein kinase; PKC, protein kinase C; REJ, receptor for egg jelly; TRPP2, transient receptor potential polycystin 2.
Phosphorylation of Ser 812 by a putative CK2 results in a significant increase in the sensitivity of the TRPP2 channel to Ca2+ stimulation (Cai et al, 2004). The S812A substitution shifts the Ca2+ dependence such that TRPP2-S812A has a maximum open probability at tenfold higher Ca2+ concentrations (∼3 μM [Ca2+]) than phosphorylated TRPP2. Reticular TRPP2 is therefore likely to have enhanced Ca2+ sensitivity, as CK2 is opportunely associated with the ER and most TRPP2 channels are phosphorylated in vivo (Cai et al, 2004).
In line with a role for ER-localized TRPP2 in regulating intracellular Ca2+, TRPP2+/− vascular smooth muscle cells have altered intracellular Ca2+ homeostasis (Qian et al, 2003). Moreover, TRPP2 has been recently shown to interact functionally and physically with Ins(1,4,5)P3R in an oocyte expression system (Fig 3; Li Y et al, 2005). The physiological relevance of these results remains to be clarified.
TRPP2 as a cell surface Ca2+-permeable cation channel
A key issue surrounding TRPP2 is the extent to which channel activity depends on the presence of PKD1. Hanaoka et al (2000) provided evidence that PKD1 and TRPP2 interact to form heteromeric complexes in vitro (Newby et al, 2002; Qian et al, 1997; Tsiokas et al, 1997, 1999). Co-expression of PKD1 and TRPP2 in Chinese hamster ovary cells, as well as in neurons, promotes the translocation of TRPP2 to the PM and generates a non-selective cation channel the selectivity of which is identical to that of homomeric TRPP2 (Delmas et al, 2004b; Hanaoka et al, 2000). Channel activity is not observed when the C-terminal interaction between PKD1 and TRPP2 is inhibited, indicating that co-assembly of PKD1 and TRPP2 is required for the proper targeting of TRPP2 to the PM. In this model, TRPP2 acts as the ion-translocating component because PKD1 alone cannot form an ion channel (Delmas et al, 2002; Hanaoka et al, 2000). In the complex, PKD1 seems to regulate TRPP2 channel activity (Delmas et al, 2004a; Xu et al, 2003). Conversely, TRPP2 binding to PKD1 reduces the ability of PKD1 to constitutively activate G-proteins possibly by competitive interaction (Delmas et al, 2002, 2004a). These data support the view that in addition to its ion-channel function, TRPP2 also regulates the downstream effects of PKD1 on its target effectors. Therefore the balance between TRPP2 and PKD1 expression, which is disrupted in ADPKD, might have a crucial role in normal PKD1–TRPP2 signalling. Functional regulation of PKD1 by TRPP2 has been further substantiated in the case of PKD1-mediated nuclear factor of activated T cells (NFAT) activation (Puri et al, 2004), the nuclear translocation of the PKD1 C-terminal domain (Chauvet et al, 2004) and Janus kinase (JAK)/signal transducers and activators of transcription (STAT) signalling (Fig 3; Bhunia et al, 2002).
The TRPP2–PKD1 complex regulates signalling by preventing the pro-proliferative helix–loop–helix protein Id2 from entering the nucleus (Fig 3; Li X et al, 2005). Id2 is known to associate with E-proteins and blocks their ability to turn on growth-suppressive genes. It is normally prevented from translocating to the nucleus through its association with the serine-phosphorylated C-terminal domain of TRPP2, which is promoted by PKD1. These data predict that loss-of-function mutations in either TRPP2 or PKD1, or disruption of their functional interaction, will cause Id2 to enter the nucleus and turn off growth-suppressive genes. Such a mechanism might be involved in the pathogenesis of ADPKD.
Activation of the PKD1–TRPP2 multiprotein complex
The idea that PKD1 and TRPP2 are interacting partners within a signalling pathway first arose from the observation that mutations in PKD1 or TRPP2 produce virtually identical clinical presentations. Other early evidence came from studies of Caenorhabditis elegans orthologues of ADPKD genes—lov-1 (location of vulva, a PKD1 orthologue) and pkd-2—which might be associated subunits of a sensory pathway necessary for normal mating behaviour (Barr et al, 2001; Barr & Sternberg, 1999). Reconstituted PKD1–TRPP2 complexes can be activated by applying antibodies directed against the extracellular receptor for egg jelly (REJ) domain of PKD1 (Delmas et al, 2004a). This leads to the concordant activation of TRPP2 and heterotrimeric G-proteins. Thus, PKD1 and TRPP2 can form functionally associated subunits of a receptor–ion channel complex in which PKD1 acts as a ‘receptor' that regulates TRPP2 activity and G-protein signalling. This mechanism might mimic a yet-to-be-determined extracellular signal that activates the polycystin complex.
This mechanism has fascinating parallels with the acrosome reaction in sea-urchin spermatozoa (Mengerink et al, 2000). The acrosome reaction requires activation of REJ1/REJ3—two PKD1 orthologues harbouring REJ modules—and which bind components of the egg jelly (Hirohashi & Vacquier, 2002). As for their mammalian counterparts, antibodies directed against the REJ domain of sea-urchin REJs induce the acrosome reaction by opening Ca2+-permeable channels (Moy et al, 1996). Recently, sea-urchin REJ3 has been shown to bind physically to the sea-urchin sperm orthologue of TRPP2 in the acrosome PM (Neill et al, 2004), raising the possibility that TRPP2 might be involved in the Ca2+-regulated acrosome reaction in sea urchins. Collectively, these findings add further weight to the primary importance of the REJ domain in activation of the PKD1–TRPP2 signalling complex.
TRPP2 might be involved in mechanosensitive functions
Recent studies have provided evidence that PKD1 and TRPP2 localize together in primary cilia of renal epithelial cells where they might function in transducing sensory information, such as shear fluid stress (McGrath et al, 2003; Nauli et al, 2003; Pazour et al, 2002). The primary cilium is proposed to act as a flow sensor because it was shown to be essential for the ability of MDCK cells to detect laminar fluid flow (Praetorius & Spring, 2001, 2003). Fluid shear-force bending of the cilium causes Ca2+ influx through mechanically sensitive channels. In this model, the PKD1–TRPP2 complex can be envisaged as a mechanosensor, which is used to transduce stimulus energy into a change in membrane permeability. However, the demonstration that the PKD1–TRPP2 complex is mechanosensitive still awaits direct experimental evidence.
A recent study provided the first evidence for the presence of single cation channel currents from isolated primary cilia of LLC-PK1 cells (Raychowdhury et al, 2005), although their molecular identity and mechanosensitivity remains to be established. In these cells, the epidermal growth factor receptor modulates TRPP2 and localizes with TRPP2 in the primary cilium (Ma et al, 2005), suggesting that TRPP2 might be involved in both mechanical and chemical transduction processes.
TRPP2 has been also shown to have a central role in the establishment of the left–right asymmetry of visceral organs (McGrath et al, 2003). TRPP2 is expressed in both motile and immotile monocilia of embryonic nodal cells. However, a perinodal Ca2+ signal is absent in Trpp2−/− mice embryos, suggesting that TRPP2 functions as a mechanotransducer in immotile monocilia, transducing leftward nodal flow into an increase in Ca2+. This function is consistent with the observation that targeted disruption in TRPP2 causes situs inversus, in addition to the hallmark cardiac and kidney defects (Pennekamp et al, 2002). It is interesting in this regard that the lack of laterality defects in PKD1-knockout embryos correlates with the absence of PKD1 in cilia (Karcher et al, 2005), favouring the view that TRPP2 might be involved in mechanosensation in the absence of PKD1. A similar mechanosensitive role for TRPP orthologues has been proposed in axonemal-based sperm flagella of Drosophila melanogaster (Gao et al, 2003; Watnick et al, 2003).
Interaction with other putative mechanosensitive channels
The recent identification of TRP channels as core components of mechanoreceptors in C. elegans, D. melanogaster and vertebrates might offer clues to the conservative mechanoreceptive structural elements of mechanotransducers (O'Neil & Heller, 2005; Pedersen et al, 2005). In the fruit fly, D. melanogaster, mechanoelectrical responses in bristle sensory neurons involve—among others—the Nompc channel, a member of the TRPN family. The nematode C. elegans can sense touch through the activation of the OSM-9 channel, a distant member of the TRPV family. Importantly, all these TRP channels have intracellular N-terminal tails harbouring several ankyrin repeats, which might anchor the channels to the cytoskeleton. Ankyrin repeats contain antiparallel α-helices that can stack to form a superhelix with spring-like behaviour (Lee et al, 2006). Atomic force microscopy measurements have revealed that tandem ankyrin repeats display linear elasticity and behave as a fully reversible nanospring, reinforcing the idea that ankyrin motifs might be key to mechanotransduction.
In vertebrates, TRPV2 and TRPV4 have been implicated in mechanosensation because they can sense membrane stretch (Muraki et al, 2003) and hypo-osmotic stress (Alessandri-Haber et al, 2003), respectively. Here again, it is worth noting that TRPV4 requires the N-terminal domain with the three ankyrin repeats to sense physical challenges (Liedtke et al, 2000).
Neither PKD1 nor TRPP2 have ankyrin repeats, raising the question as to whether these two proteins are putative mechanotransducers. On this issue, the extracellular domain of PKD1 has been recently shown to display a dynamic extensibility whereby its length might be regulated through unfolding/refolding of its immunoglobulin-like domains (Qian et al, 2005). This would provide the structural support for a tethered mechanism that might be required for mechanical gating. Although these mechanical properties of PKD1 are important in the context of mechanosensation, they seem more appropriate to providing structural support in cell–cell and/or cell–matrix interactions at basolateral membranes than in mechanotransduction in the solitary cilium.
It is therefore reasonable to consider an alternative model in which mechanical force is transmitted indirectly to the protein complex or through an auxiliary subunit. For example, TRPP2 might assemble with other TRP channels to form a mechanosensitive channel. A tantalizing link points to TRPV4 as it is expressed in renal epithelial cells, particularly in the distal nephron and collecting ducts, which are flow-sensitive segments (Tian et al, 2004). In this regard, preliminary evidence by Gerd Walz's group indicate that TRPV4 and TRPP2 interact and co-localize in the primary cilium (Köttgen et al, 2005b). Recently, TRPC1 was also found to be a component of the vertebrate mechanosensitive cation channel (Maroto et al, 2005). Interestingly, TRPC1 is known to interact with TRPP2 in expression systems (Tsiokas et al, 1999) and might form functional heteromers with TRPP2 (Delmas, 2004b), suggesting that TRPC1 might contribute to the mechanosensory TRPP2 apparatus.
Concluding remarks
In the past few years, considerable progress has been achieved in evaluating the distribution and functional characteristics of TRPP proteins. Most notably, TRPP2 channels have been shown to transport to different subcellular compartments and, as such, display specific subcellular functions. In renal primary cilia TRPP2 interacts with PKD1, a process that might be essential for the regulation of mechanosensation and the cell cycle. However, the available information does not support the candidacy of the PKD1–TRPP2 complex as the core component of the mechanosensitive apparatus of the primary cilium. In the ER, TRPP2 acts as an intracellular Ca2+ release channel. It will be important to identify ligands for PKD1 that affect the function of the PKD1–TRPP2 complex and to identify the factors interacting with TRPP2 that determine its compartment-specific functions. These studies will contribute not only to a better understanding of TRPP physiological functions but also to the development of new strategies for targeted therapeutic intervention in ADPKD.

Aurélie Giamarchi

Françoise Padilla

Bertrand Coste

Matthieu Raoux

Marcel Crest

Eric Honoré

Patrick Delmas
Acknowledgments
This work was supported by the Centre National de la Recherche Scientifique (CNRS) and by grants from the Agence Nationale de la Recherche (ANR; 05-PCOD-029), the Schlumberger Fundation and the French Research Ministry (ACI 5294).
References
- Alessandri-Haber N, Yeh JJ, Boyd AE, Parada CA, Chen X, Reichling DB, Levine JD (2003) Hypotonicity induces TRPV4-mediated nociception in rat. Neuron 39: 497–511 [DOI] [PubMed] [Google Scholar]
- Barr MM, Sternberg PW (1999) A polycystic kidney-disease gene homologue required for male mating behaviour in C. elegans. Nature 401: 386–389 [DOI] [PubMed] [Google Scholar]
- Barr MM, DeModena J, Braun D, Nguyen CQ, Hall DH, Sternberg PW (2001) The Caenorhabditis elegans autosomal dominant polycystic kidney disease gene homologs lov-1 and pkd-2 act in the same pathway. Curr Biol 11: 1341–1346 [DOI] [PubMed] [Google Scholar]
- Bhunia AK, Piontek K, Boletta A, Liu L, Qian F, Xu PN, Germino FJ, Germino GG (2002) PKD1 induces p21(waf1) and regulation of the cell cycle via direct activation of the JAK-STAT signaling pathway in a process requiring PKD2. Cell 109: 157–168 [DOI] [PubMed] [Google Scholar]
- Cai Y, Maeda Y, Cedzich A, Torres VE, Wu G, Hayashi T, Mochizuki T, Park JH, Witzgall R, Somlo S (1999) Identification and characterization of polycystin-2, the PKD2 gene product. J Biol Chem 274: 28557–28565 [DOI] [PubMed] [Google Scholar]
- Cai Y et al. (2004) Calcium dependence of polycystin-2 channel activity is modulated by phosphorylation at Ser812. J Biol Chem 279: 19987–19995 [DOI] [PubMed] [Google Scholar]
- Chauvet V et al. (2004) Mechanical stimuli induce cleavage and nuclear translocation of the polycystin-1 C terminus. J Clin Invest 114: 1433–1443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen XZ, Segal Y, Basora N, Guo L, Peng JB, Babakhanlou H, Vassilev PM, Brown EM, Hediger MA, Zhou J (2001) Transport function of the naturally occurring pathogenic polycystin-2 mutant, R742X. Biochem Biophys Res Commun 282: 1251–1256 [DOI] [PubMed] [Google Scholar]
- Clapham DE, Julius D, Montell C, Schultz G (2005) International Union of Pharmacology. XLIX. Nomenclature and structure–function relationships of transient receptor potential channels. Pharmacol Rev 57: 427–450 [DOI] [PubMed] [Google Scholar]
- Delmas P (2004a) Polycystins: from mechanosensation to gene regulation. Cell 118: 145–148 [DOI] [PubMed] [Google Scholar]
- Delmas P (2004b) Assembly and gating of TRPC channels in signalling microdomains. Novartis Found Symp 258: 75–89; discussion 89,–102, 263–266 [PubMed] [Google Scholar]
- Delmas P (2005) Polycystins: polymodal receptor/ion-channel cellular sensors. Pflugers Arch 451: 264–276 [DOI] [PubMed] [Google Scholar]
- Delmas P et al. (2002) Constitutive activation of G-proteins by polycystin-1 is antagonized by polycystin-2. J Biol Chem 277: 11276–11283 [DOI] [PubMed] [Google Scholar]
- Delmas P, Nauli SM, Li X, Coste B, Osorio N, Crest M, Brown DA, Zhou J (2004a) Gating of the polycystin ion channel signaling complex in neurons and kidney cells. FASEB J 18: 740–742 [DOI] [PubMed] [Google Scholar]
- Delmas P, Padilla F, Osorio N, Coste B, Raoux M, Crest M (2004b) Polycystins, calcium signaling, and human diseases. Biochem Biophys Res Commun 322: 1374–1383 [DOI] [PubMed] [Google Scholar]
- Foggensteiner L, Bevan AP, Thomas R, Coleman N, Boulter C, Bradley J, Ibraghimov-Beskrovnaya O, Klinger K, Sandford R (2000) Cellular and subcellular distribution of polycystin-2, the protein product of the PKD2 gene. J Am Soc Nephrol 11: 814–827 [DOI] [PubMed] [Google Scholar]
- Gao Z, Ruden DM, Lu X (2003) PKD2 cation channel is required for directional sperm movement and male fertility. Curr Biol 13: 2175–2178 [DOI] [PubMed] [Google Scholar]
- Geng L, Okuhara D, Yu Z, Tian X, Cai Y, Shibazaki S, Somlo S (2006) Polycystin-2 traffics to cilia independently of polycystin-1 by using an N-terminal RVxP motif. J Cell Sci 119: 1383–1395 [DOI] [PubMed] [Google Scholar]
- Gonzalez-Perrett S, Kim K, Ibarra C, Damiano AE, Zotta E, Batelli M, Harris PC, Reisin IL, Arnaout MA, Cantiello HF (2001) Polycystin-2, the protein mutated in autosomal dominant polycystic kidney disease (ADPKD), is a Ca2+-permeable nonselective cation channel. Proc Natl Acad Sci USA 98: 1182–1187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanaoka K, Qian F, Boletta A, Bhunia AK, Piontek K, Tsiokas L, Sukhatme VP, Guggino WB, Germino GG (2000) Co-assembly of polycystin-1 and -2 produces unique cation-permeable currents. Nature 408: 990–994 [DOI] [PubMed] [Google Scholar]
- Hidaka S, Konecke V, Osten L, Witzgall R (2004) PIGEA-14, a novel coiled-coil protein affecting the intracellular distribution of polycystin-2. J Biol Chem 279: 35009–35016 [DOI] [PubMed] [Google Scholar]
- Hirohashi N, Vacquier VD (2002) High molecular mass egg fucose sulfate polymer is required for opening both Ca2+ channels involved in triggering the sea urchin sperm acrosome reaction. J Biol Chem 277: 1182–1189 [DOI] [PubMed] [Google Scholar]
- Igarashi P, Somlo S (2002) Genetics and pathogenesis of polycystic kidney disease. J Am Soc Nephrol 13: 2384–2398 [DOI] [PubMed] [Google Scholar]
- Karcher C, Fischer A, Schweickert A, Bitzer E, Horie S, Witzgall R, Blum M (2005) Lack of a laterality phenotype in Pkd1 knock-out embryos correlates with absence of polycystin-1 in nodal cilia. Differentiation 73: 425–432 [DOI] [PubMed] [Google Scholar]
- Köttgen M, Walz G (2005) Subcellular localization and trafficking of polycystins. Pflugers Arch 451: 286–293 [DOI] [PubMed] [Google Scholar]
- Köttgen M et al. (2005a) Trafficking of TRPP2 by PACS proteins represents a novel mechanism of ion channel regulation. EMBO J 24: 705–716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köttgen M et al. (2005b) Polycystin-2 and TRPV4 form a functional heteromultimeric complex that might act as a cilial mechanosensor. J Am Soc Nephrol 16: TH–FC116 [Google Scholar]
- Koulen P, Cai Y, Geng L, Maeda Y, Nishimura S, Witzgall R, Ehrlich BE, Somlo S (2002) Polycystin-2 is an intracellular calcium release channel. Nat Cell Biol 4: 191–197 [DOI] [PubMed] [Google Scholar]
- Lee G, Abdi K, Jiang Y, Michaely P, Bennett V, Marszalek PE (2006) Nanospring behaviour of ankyrin repeats. Nature 440: 246–249 [DOI] [PubMed] [Google Scholar]
- Li Q, Liu Y, Zhao W, Chen XZ (2002) The calcium-binding EF-hand in polycystin-L is not a domain for channel activation and ensuing inactivation. FEBS Lett 516: 270–278 [DOI] [PubMed] [Google Scholar]
- Li X, Luo Y, Starremans PG, McNamara CA, Pei Y, Zhou J (2005) Polycystin-1 and polycystin-2 regulate the cell cycle through the helix–loop–helix inhibitor Id2. Nat Cell Biol 7: 1102–1112 [DOI] [PubMed] [Google Scholar]
- Li Y, Wright JM, Qian F, Germino GG, Guggino WB (2005) Polycystin 2 interacts with type I inositol 1,4,5-trisphosphate receptor to modulate intracellular Ca2+ signaling. J Biol Chem 280: 41298–41306 [DOI] [PubMed] [Google Scholar]
- Liedtke W, Choe Y, Marti-Renom MA, Bell AM, Denis CS, Sali A, Hudspeth AJ, Friedman JM, Heller S (2000) Vanilloid receptor-related osmotically activated channel (VR-OAC), a candidate vertebrate osmoreceptor. Cell 103: 525–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y, Vassilev PM, Li X, Kawanabe Y, Zhou J (2003) Native polycystin 2 functions as a plasma membrane Ca2+-permeable cation channel in renal epithelia. Mol Cell Biol 23: 2600–2607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma R, Li WP, Rundle D, Kong J, Akbarali HI, Tsiokas L (2005) PKD2 functions as an epidermal growth factor-activated plasma membrane channel. Mol Cell Biol 25: 8285–8298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroto R, Raso A, Wood TG, Kurosky A, Martinac B, Hamill OP (2005) TRPC1 forms the stretch-activated cation channel in vertebrate cells. Nat Cell Biol 7: 179–185 [DOI] [PubMed] [Google Scholar]
- McGrath J, Somlo S, Makova S, Tian X, Brueckner M (2003) Two populations of node monocilia initiate left–right asymmetry in the mouse. Cell 114: 61–73 [DOI] [PubMed] [Google Scholar]
- Mengerink KJ, Moy GW, Vacquier VD (2000) suREJ proteins: new signalling molecules in sea urchin spermatozoa. Zygote 8 (Suppl 1): S28–S30 [PubMed] [Google Scholar]
- Montell C (2005) The TRP superfamily of cation channels. Sci STKE 2005: re3. [DOI] [PubMed] [Google Scholar]
- Moy GW, Mendoza LM, Schulz JR, Swanson WJ, Glabe CG, Vacquier VD (1996) The sea urchin sperm receptor for egg jelly is a modular protein with extensive homology to the human polycystic kidney disease protein, PKD1. J Cell Biol 133: 809–817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muraki K, Iwata Y, Katanosaka Y, Ito T, Ohya S, Shigekawa M, Imaizumi Y (2003) TRPV2 is a component of osmotically sensitive cation channels in murine aortic myocytes. Circ Res 93: 829–838 [DOI] [PubMed] [Google Scholar]
- Nauli SM, Zhou J (2004) Polycystins and mechanosensation in renal and nodal cilia. Bioessays 26: 844–856 [DOI] [PubMed] [Google Scholar]
- Nauli SM et al. (2003) Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nat Genet 33: 129–137 [DOI] [PubMed] [Google Scholar]
- Neill AT, Moy GW, Vacquier VD (2004) Polycystin-2 associates with the polycystin-1 homolog, suREJ3, and localizes to the acrosomal region of sea urchin spermatozoa. Mol Reprod Dev 67: 472–477 [DOI] [PubMed] [Google Scholar]
- Newby LJ, Streets AJ, Zhao Y, Harris PC, Ward CJ, Ong AC (2002) Identification, characterization, and localization of a novel kidney polycystin–1–polycystin-2 complex. J Biol Chem 277: 20763–20773 [DOI] [PubMed] [Google Scholar]
- Nilius B, Voets T (2005) TRP channels: a TR(I)P through a world of multifunctional cation channels. Pflugers Arch 451: 1–10 [DOI] [PubMed] [Google Scholar]
- O'Neil RG, Heller S (2005) The mechanosensitive nature of TRPV channels. Pflugers Arch 451: 193–203 [DOI] [PubMed] [Google Scholar]
- Pazour GJ, San Agustin JT, Follit JA, Rosenbaum JL, Witman GB (2002) Polycystin-2 localizes to kidney cilia and the ciliary level is elevated in orpk mice with polycystic kidney disease. Curr Biol 12: R378–R380 [DOI] [PubMed] [Google Scholar]
- Pedersen SF, Owsianik G, Nilius B (2005) TRP channels: an overview. Cell Calcium 38: 233–252 [DOI] [PubMed] [Google Scholar]
- Pennekamp P, Karcher C, Fischer A, Schweickert A, Skryabin B, Horst J, Blum M, Dworniczak B (2002) The ion channel polycystin-2 is required for left–right axis determination in mice. Curr Biol 12: 938–943 [DOI] [PubMed] [Google Scholar]
- Praetorius HA, Spring KR (2001) Bending the MDCK cell primary cilium increases intracellular calcium. J Membr Biol 184: 71–79 [DOI] [PubMed] [Google Scholar]
- Praetorius HA, Spring KR (2003) Removal of the MDCK cell primary cilium abolishes flow sensing. J Membr Biol 191: 69–76 [DOI] [PubMed] [Google Scholar]
- Puri S, Magenheimer BS, Maser RL, Ryan EM, Zien CA, Walker DD, Wallace DP, Hempson SJ, Calvet JP (2004) Polycystin-1 activates the calcineurin/NFAT (nuclear factor of activated T-cells) signaling pathway. J Biol Chem 279: 55455–55464 [DOI] [PubMed] [Google Scholar]
- Qian F, Germino FJ, Cai Y, Zhang X, Somlo S, Germino GG (1997) PKD1 interacts with PKD2 through a probable coiled-coil domain. Nat Genet 16: 179–183 [DOI] [PubMed] [Google Scholar]
- Qian F, Wei W, Germino G, Oberhauser A (2005) The nanomechanics of polycystin-1 extracellular region. J Biol Chem 280: 40723–40730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian Q, Hunter LW, Li M, Marin-Padilla M, Prakash YS, Somlo S, Harris PC, Torres VE, Sieck GC (2003) Pkd2 haploinsufficiency alters intracellular calcium regulation in vascular smooth muscle cells. Hum Mol Genet 12: 1875–1880 [DOI] [PubMed] [Google Scholar]
- Raychowdhury MK, McLaughlin M, Ramos AJ, Montalbetti N, Bouley R, Ausiello DA, Cantiello HF (2005) Characterization of single channel currents from primary cilia of renal epithelial cells. J Biol Chem 280: 34718–34722 [DOI] [PubMed] [Google Scholar]
- Rundle DR, Gorbsky G, Tsiokas L (2004) PKD2 interacts and co-localizes with mDia1 to mitotic spindles of dividing cells: role of mDia1 in PKD2 localization to mitotic spindles. J Biol Chem 279: 29728–29739 [DOI] [PubMed] [Google Scholar]
- Scheffers MS, Le H, van der Bent P, Leonhard W, Prins F, Spruit L, Breuning MH, de Heer E, Peters DJ (2002) Distinct subcellular expression of endogenous polycystin-2 in the plasma membrane and Golgi apparatus of MDCK cells. Hum Mol Genet 11: 59–67 [DOI] [PubMed] [Google Scholar]
- Streets AJ, Moon DJ, Kane ME, Obara T, Ong AC (2006). Identification of an N-terminal glycogen synthase kinase 3 phosphorylation site which regulates the functional localization of polycystin-2 in vivo and in vitro. Hum Mol Genet 15: 1465–1473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian W, Salanova M, Xu H, Lindsley JN, Oyama TT, Anderson S, Bachmann S, Cohen DM (2004) Renal expression of osmotically responsive cation channel TRPV4 is restricted to water-impermeant nephron segments. Am J Physiol Renal Physiol 287: F17–F24 [DOI] [PubMed] [Google Scholar]
- Tsiokas L, Kim E, Arnould T, Sukhatme VP, Walz G (1997) Homo- and heterodimeric interactions between the gene products of PKD1 and PKD2. Proc Natl Acad Sci USA 94: 6965–6970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsiokas L, Arnould T, Zhu C, Kim E, Walz G, Sukhatme VP (1999) Specific association of the gene product of PKD2 with the TRPC1 channel. Proc Natl Acad Sci USA 96: 3934–3939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassilev PM et al. (2001) Polycystin-2 is a novel cation channel implicated in defective intracellular Ca2+ homeostasis in polycystic kidney disease. Biochem Biophys Res Commun 282: 341–350 [DOI] [PubMed] [Google Scholar]
- Watnick TJ, Jin Y, Matunis E, Kernan MJ, Montell C (2003) A flagellar polycystin-2 homolog required for male fertility in Drosophila. Curr Biol 13: 2179–2184 [DOI] [PubMed] [Google Scholar]
- Xu GM, Gonzalez-Perrett S, Essafi M, Timpanaro GA, Montalbetti N, Arnaout MA, Cantiello HF (2003) Polycystin-1 activates and stabilizes the polycystin-2 channel. J Biol Chem 278: 1457–1462 [DOI] [PubMed] [Google Scholar]
- Yoder BK, Hou X, Guay-Woodford LM (2002) The polycystic kidney disease proteins, polycystin-1, polycystin-2, polaris, and cystin, are co-localized in renal cilia. J Am Soc Nephrol 13: 2508–2516 [DOI] [PubMed] [Google Scholar]


