Abstract
Background: Acute organophosphorus (OP) pesticide poisoning is a major clinical problem in the developing world. Textbooks ascribe most deaths to respiratory failure occurring in one of two distinct clinical syndromes - acute cholinergic respiratory failure or the intermediate syndrome. The delayed failure appears to be due to respiratory muscle weakness, but its pathophysiology is not yet clear.
Aim: To describe the clinical patterns of OP-induced respiratory failure and to determine whether the two syndromes are clinically distinct.
Design: Prospective cohort study of 376 patients with confirmed OP poisoning.
Methods: Patients were observed throughout their admission to three Sri Lankan hospitals. Exposure was confirmed by butyrylcholinesterase and blood OP assays.
Results: Ninety of 376 patients (24%) required intubation, 52 (58%) within 2 hrs of admission while unconscious with cholinergic features. Twenty-nine (32%) were well on admission but then required intubation after 24 hrs while conscious and without cholinergic features. These two syndromes were not clinically distinct and had much overlap. In particular, some patients who required intubation on arrival subsequently recovered conscious but could not be extubated, requiring ventilation for up to 6 days.
Discussion: Respiratory failure did not occur as two discrete clinical syndromes within distinct time frames. Instead, the pattern of failure was variable and overlapped in some patients. There seemed to be two underlying mechanisms - an early acute mixed central and peripheral respiratory failure, and a late peripheral respiratory failure - rather than two defined clinical syndromes.
Introduction
Acute organophosphorus (OP) pesticide self-poisoning is a major global problem.1-3Although pesticide poisoned patients make high demands on intensive care facilities in industrialized countries,4,5 it is in the developing world that practically all deaths occur.6-8
Many deaths occur within hours of pesticide ingestion during the acute cholinergic crisis,9,10 either before or soon after reaching medical care.11,12 Most result from acute respiratory failure due to central respiratory depression, respiratory muscle weakness, and/or direct pulmonary effects (bronchospasm and bronchorrhoea).9 Complications of pre-hospital respiratory arrest and unconsciousness, such as aspiration and anoxic brain damage, cause further deaths during the inpatient stay.
Deaths also occur after patients have been stabilised and treated with atropine.13-15Some are due to cardiovascular failure 12 but others are due to sudden respiratory arrests occurring hours to days after admission. Unfortunately, in the resource poor hospitals that admit most patients, facilities do not exist for poisoned patients to be routinely admitted to an intensive care unit (ICU) for close observation.
Wadia described the neurological features of OP poisoning, including the respiratory failure. He differentiated these features into those that occurred within 24 hrs (type I paralysis) and those that occurred after 24 hrs (type II paralysis)13 Senanayake and Karaliedde subsequently reported a series of patients with a syndrome of delayed neuromuscular weakness and respiratory failure, calling it the ‘intermediate syndrome’.14 The late neurological syndrome was defined as “paralytic signs that appear about 24hrs after admission and after atropine has already been given in large doses” 13 or “muscle weakness with an acute onset within 24 to 96 hours after the poisoning affecting conscious patients without fasciculations or other cholinergic manifestations with or without respiratory muscle failure”.14
Textbooks routinely describe respiratory failure associated with the acute cholinergic crisis or intermediate syndrome as two distinct clinical syndromes.10,16-19 However,while studying OP poisoned patients in rural Sri Lanka, we have noted patients whose respiratory failure did not fit into such distinct syndromes. We therefore set up a prospective observational study of respiratory failure in patients with confirmed OP poisoning to better describe its clinical features as a first step to improved understanding of its pathophysiology.
Methods
A large prospective cohort study of acute self-poisoned patients was established in three hospitals in the North Central and North Western provinces of Sri Lanka during 2002. A randomized controlled trial (RCT) of multiple dose (MDAC; six 50g doses q6h) and single dose (SDAC; 50g) regimens of superactivated charcoal (Carbomix, Norit, NL; ISRCTN02920054) was nested into this cohort. Ethics review committee approval for the study was obtained from Colombo and Oxford.
Patients with a history of self-poisoning were seen on admission and data recorded prospectively from 31 March 2002 in Anuradhapura and 4 June 2002 in Polonnaruwa, until 31 December 2003, and in Kurunegala for two months at the end of 2002. The poison ingested was provisionally identified from the patient's or relatives' histories, bottles brought in to hospital, or doctor's comments in transfer letters. A plasma sample was taken from patients consenting to enter the RCT for confirmatory identification of the ingested OP. The patients were a subset of a previously published cohort 12 - intubated patients with positive blood samples for both OP and BuChE inhibition presenting before 31st December 2003.
Patients remained under the care of the hospitals' consultant physicians. Management protocols were agreed between the ward doctors and study team. Decisions about intubation and transfer to ICU were made by the medical team independently of study doctors. All decisions were made on the basis of the patient's clinical condition and did not reflect the particular OP ingested, as per usual hospital practice.
Patients were assessed on admission for cholinergic features (in particular: sweating, pinpoint pupils, urinary and fecal incontinence, bronchorrhoea, bronchospasm, and hypotension) and treated following a standard protocol.20 Resuscitation was performed with intubation as required and provision of oxygen. Atropine was given rapidly with IV fluids in doubling doses from an initial dose of 1-3mg until the heart rate was >80/min, bronchospasm and bronchorrhoea had resolved, and systolic blood pressure >80mmHg. Once this was attained, an atropine infusion was set up to keep the patient atropinised but not toxic with frequent adjustments of dose. All symptomatic patients requiring atropine received pralidoxime chloride 1g IV q6h for one to three days, as per the standard protocol for Sri Lankan hospitals.21
A ward round was performed twice a day to assess the patients' condition. Patients were also seen frequently after admission while adjustments of the atropine infusion rate were required and then at least every 3 hours by study doctors. Patients with any indications of respiratory failure were treated and the event recorded.
Seriously ill patients, as judged by the ward's medical staff, were transferred to the ICU when a bed became available. Each hospital had 2-8 ICU beds with ventilators for medical patients; many were filled with OP poisoned patients and there was always difficulty in obtaining a bed. The lack of ventilators resulted in intubation and sedation being performed at the last possible moment and the patients being ambu- bagged until a ventilator became available. Early transfer of ill patients to an ICU for close observation, as would occur in hospital with more ICU beds, was usually not possible, resulting in some deaths. In this study, therefore, the need for intubation was considered to be synonymous with respiratory failure.
Intubation was primarily motivated by respiratory failure and not GCS. Criteria for intubation were tidal volume less than 180ml/breath using a Wright's respirometer, respiratory rate less than 10 breaths/minute, abdominal breathing, or failure of a less invasive measures airway to maintain airway patency. Arterial blood gases were not available to guide therapy.
Toxicological analysis
Admission plasma samples (taken a median time post-ingestion of 3-4 hours) were assayed for butyrylcholinesterase (BuChE; EC 3.1.1.8) activity (an activity <50% of the normal mean was taken to indicate substantial exposure) and concentration of chlorpyrifos, dimethoate, fenthion, or quinalphos OP. Plasma was separated, frozen at -20C, and sent to Munich for analysis. BuChE activity was assessed as described 22.OPs in plasma were quantified by reversed phase HPLC after n-hexane extraction along with an internal standard (chlorpyrifos, fenthion, and quinalphos) or deproteinisation with trichloroacetic acid (dimethoate) with UV detection at 288 and 200 nm, respectively.
Statistics
The primary data analysis was performed in GraphPad Prism (version 4). Clinical characteristics were summarised using counts (percentages) for categorical variables and the median (interquartile range [IQR]) for non-normally distributed continuous variables.
Results
From the 31 March 2002 until 31 December 2003, 4341 patients were reviewed on admission. 806 (18.6%) reported ingestion of OP pesticides. 644 reported ingestion of one of four OPs - chlorpyrifos, dimethoate, fenthion or quinalphos - for which HPLC assays were available to confirm exposure.
Plasma samples were tested for BuChE activity and OP in 455 patients recruited to the RCT. This analysis demonstrated BuChE inhibition - indicative of substantial exposure - and identified the ingested OP in 376 patients (chlorpyrifos 216; dimethoate 99; fenthion 46; quinalphos 15). The pattern of respiratory failure in these patients with confirmed OP exposure was then assessed.
Pattern of respiratory failure
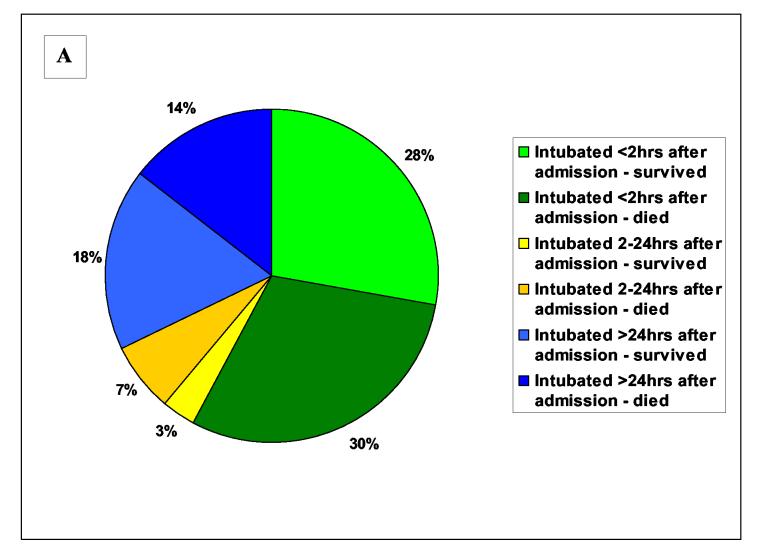
Of these 376 patients, 90 (24%) required intubation and ventilatory support during their hospital admission. Forty six (51%) of the intubated patients died.
Careful observation of patients requiring intubation revealed two major patterns of respiratory failure: i) an early form in unconscious patients with cholinergic features (patient video 1 - see website) and ii) a delayed form in conscious patients who often had few cholinergic signs (patient video 2 - see website).
Early intubation
The majority of patients requiring intubation (52/90; 58%) were intubated on, or soon after, admission because of severe cholinergic features (figures 1 & 2). Twenty seven of these patients (27/52, 52%) died before hospital discharge (figure 2). In patients requiring early intubation, atropine usually treated bronchospasm and bronchorrhoea but did not affect either GCS or respiratory rate. The effect of pralidoxime was not apparent.
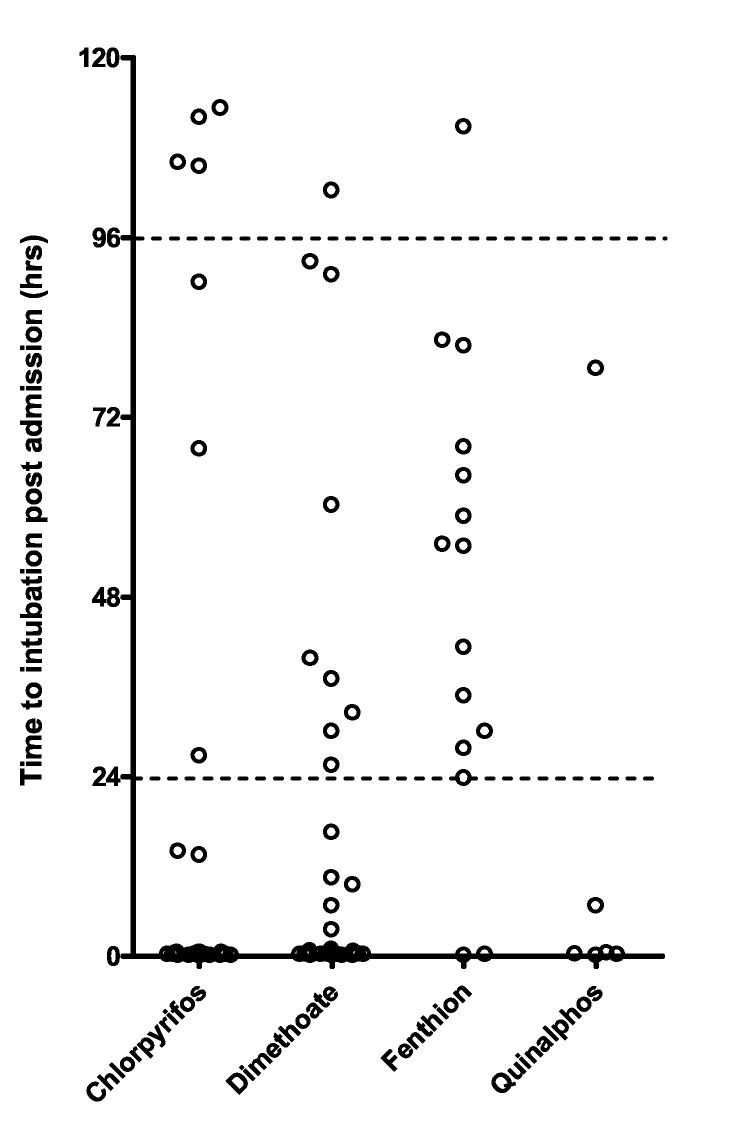
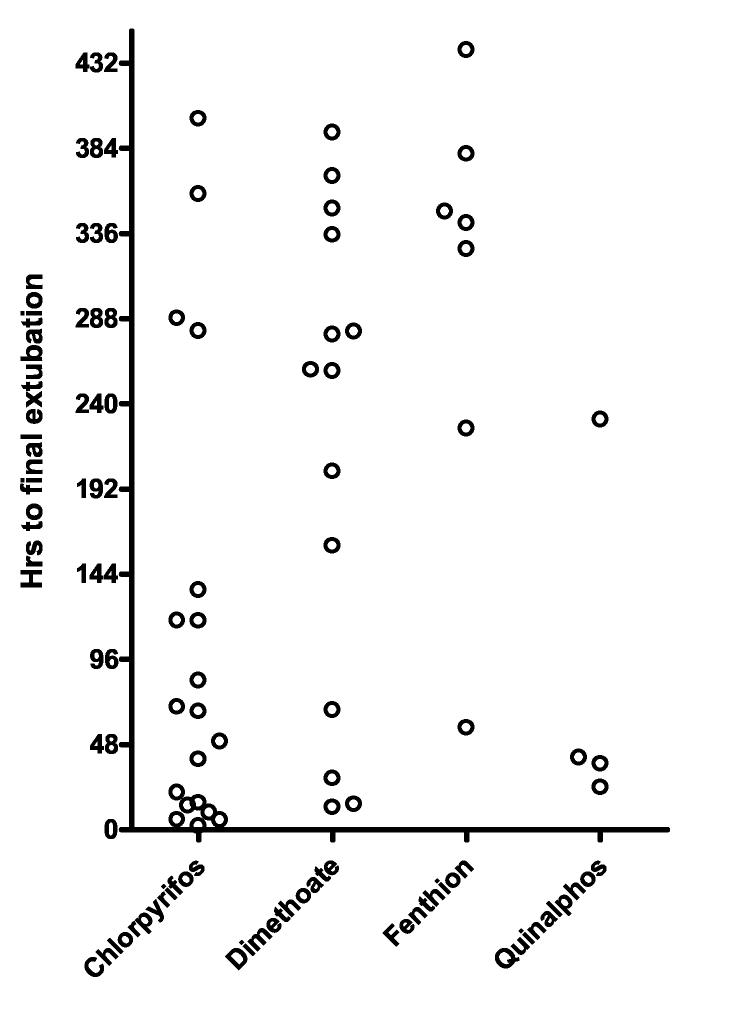
Figure 1.
Time to A) first intubation and B) final extubation according to OP ingested. There were marked differences in time to intubation and to extubation between pesticides. The dotted lines in A indicate the 24 to 96 hr period during which late respiratory failure is classically said to occur. Some patients developed sudden respiratory failure both before and after this time period, up to 115 hours post- intubation. A large proportion of patients with chlorpyrifos, dimethoate, and quinalphos poisoning were intubated around admission (see figure 2).
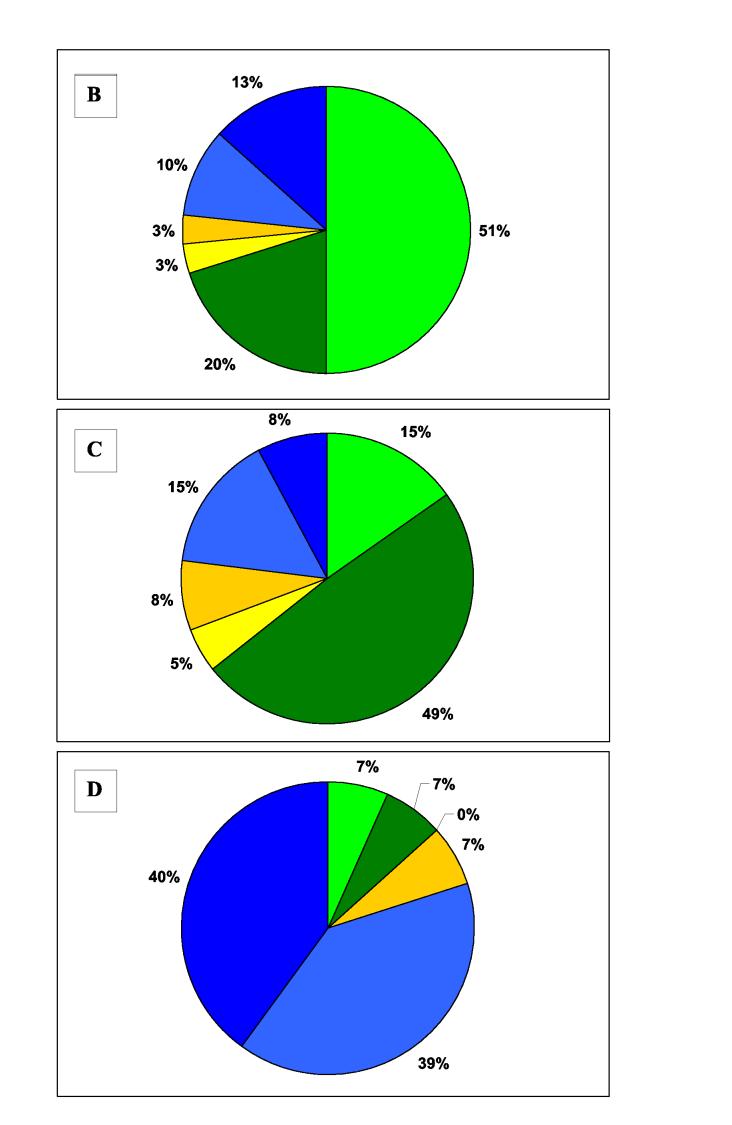
Figure 2.
Timing of respiratory failure and outcome Pie charts showing the timing of respiratory failure according to outcome for A) all intubated patients, and patients poisoned by B) chlorpyrifos, C) dimethoate and D) fenthion. There were too few quinalphos poisoned patients to be represented in this way. Green colouring is for intubations within 2 hrs of admission, blue for intubations after 24 hrs, and yellow for intubations at intermediate times. Dark colouring indicates patients who died after intubation.
Five died within eight hours of intubation - each patient was severely ill on admission, was never stabilised, and did not regain consciousness before death. Thirteen died 12 to 48 hrs after intubation. Most had ingested dimethoate (12/13, 92%) and all but one remained unconscious until death.
Nine patients died later during their inpatient stay, from 3 to 18 days post intubation, mostly from pneumonia that developed soon after admission and probably resulted from pre-hospital aspiration in some cases. Five patients intermittently regained consciousness, while still requiring ventilation, before they died.
Twenty five patients survived to discharge. Although most (67%) were extubated within 48hrs, some were extubated after periods of up to 9 days. Four of this latter group regained consciousness but could not be extubated for a further 1 to 7 days. During this time, despite reductions in muscle power, the patients could move their limbs and eyes to communicate but they could not be weaned from the ventilator (patient video 3 - see website).
Four patients redeveloped respiratory failure over the three days following extubation and required reintubation and ventilation for 3 to 15 days. Three patients had a GCS of 15/15 at the time of reintubation; the fourth had a sudden respiratory arrest and required cardiopulmonary resuscitation before intubation. The patients were conscious for 3 to 13 days before extubatation.
Late intubation
Twenty nine patients (32%) required intubation for the first time more than 24 hrs after admission. Respiratory failure occurred a median of 64 hrs (interquartile range [IQR] 36 to 92) post-admission.
Sixteen patients (55%) survived to extubation and hospital discharge. The patients were intubated for a median of 219 hrs (IQR 154 to 276). One patient was extubated after 123 hrs but, 96 hrs later, required reintubation for a further 98 hrs. Eight patients were fully conscious throughout the time they were intubated (up to 14 days); a further seven patients regained consciousness 2 to 10 days before they could be extubated.
Of the thirteen patients who died, four had an unexpected respiratory arrest on the ward and died rapidly following unsuccessful cardiopulmonary resuscitation. One patient, who had been intubated for 342 hrs, died 102 hrs after extubation from a sudden cardiorespiratory arrest. The other eight patients died while being ventilated. Four of these eight patients had long periods spent fully conscious while being ventilated before they died.
Intermediate timing of intubation
Nine patients required intubation between 2 and 24 hrs after admission. Five were unconscious and were similar to the patients with early respiratory failure. Four patients were conscious at the time of intubation and were therefore similar to patients with the late form of respiratory failure. Four patients died while intubated. The others were extubated 2 to 14 days after intubation; two subsequently died.
Relationship of time to intubation to hours intubated
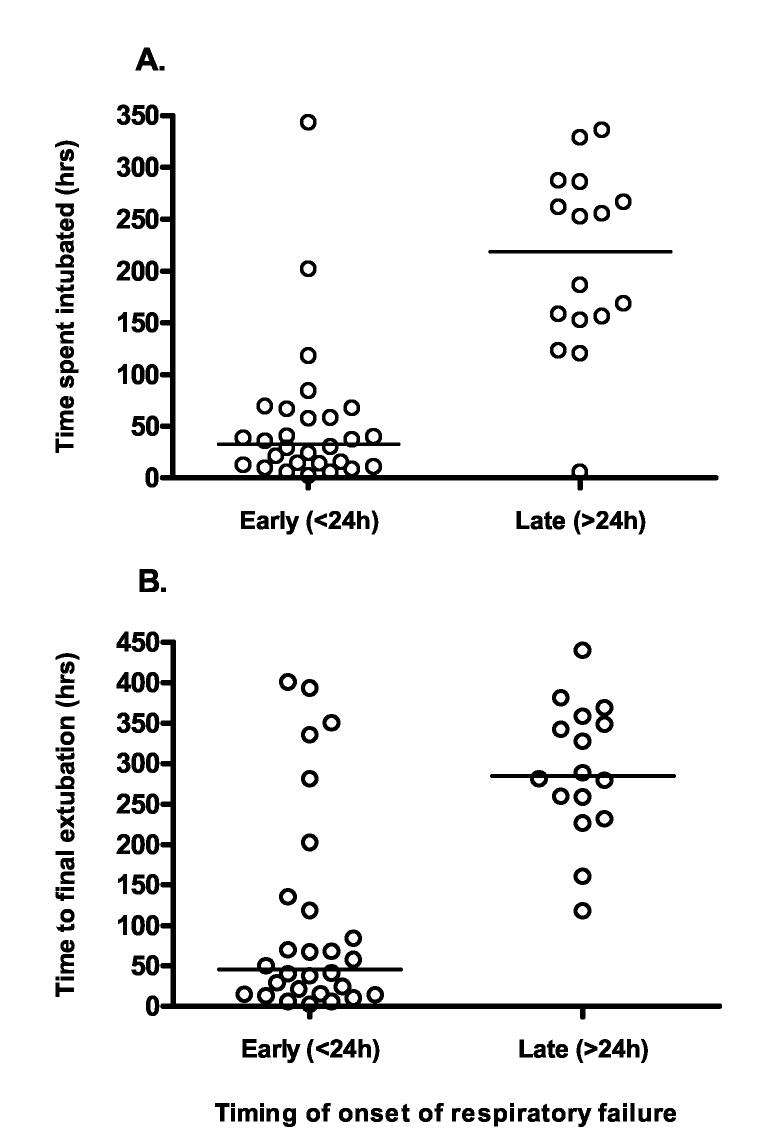
We analysed whether the timing of intubation affected the length of time that survivors required ventilatory support. Patients intubated more than 24 hrs after admission required intubation for significantly longer than patients intubated within 24 hrs (median time to first extubation 33 vs. 219 hrs, P<0.0001; median time to final extubation: 45 vs. 284 hrs; P<0.0001, figure 3).
Figure 3.
Comparison of time to first intubation with need for ventilation Patients intubated within 24hrs of admission had a shorter time to A) first extubation and B) final extubation compared to patients intubated after 24hrs. The bars show the median time.
Patterns of respiratory failure for particular OPs
We noticed marked variation in the pattern of respiratory failure between OPs (figure 1 & 2). The majority of intubated patients with chlorpyrifos and dimethoate poisoning were intubated on admission. However, these dimethoate poisoned patients had a much worse prognosis (figure 2) - 19/25 dimethoate poisoned patients died compared to 6/21 chlorpyrifos poisoned patients (76% vs. 29%, relative risk 2.7 (95%CI 1.3 to 5.4]). The majority of intubations following fenthion poisoning occurred late, after 24 hrs. There were few quinalphos poisoned patients in the cohort but 4 of 6 were intubated on admission.
Discussion
In this large cohort study of patients with confirmed OP poisoning, we noted two common forms of respiratory failure: an early form that occurred on or soon after admission and a delayed form that occurred from several hours to five days after admission. The early form occurred in unconscious patients with marked cholinergic features while the delayed form occurred in conscious patients with no cholinergic features and selected paralysis.
The syndromes are similar to the two distinct and non-overlapping syndromes of OP- induced respiratory failure described in textbooks - respiratory failure during the acute cholinergic crisis and the delayed intermediate syndrome. However, we noted many patients who did not fall cleanly into these two categories. In particular, early respiratory failure sometimes merged seamlessly with late respiratory failure such that some patients intubated on admission regained consciousness only to find themselves requiring ventilatory support for several weeks. It may not be as easy as suggested by textbooks 10,16-19 to clearly distinguish either clinically or temporally the two clinical syndromes.
Early respiratory failure during the acute cholinergic crisis
Early respiratory failure occurred in unconscious patients. In all but 9% of patients it occurred within 2hrs of admission, which was a median of 3 to 4 hrs post-ingestion. As long as complications did not occur, it usually lasted for no more than 48 hrs. Respiratory failure lasting longer than this was normally associated with return of consciousness, unless patients were septic from pneumonia or other infections. Many intubated patients poisoned by chlorpyrifos, dimethoate or quinalphos had respiratory failure of this pattern.
The mechanism of early respiratory failure during the acute cholinergic crisis in humans is unclear but is likely to involve three components: depression of central respiratory drive from the respiratory centre in the ventrolateral medulla, respiratory muscles weakness, and direct pulmonary effects (bronchospasm, bronchorrhoea).9,10,23
Animal studies have suggested that the dominant component varies between OP and between species: for example, bronchospasm dominates in cats while central respiratory depression dominates in monkeys and rabbits.23,24 Bronchospasm and bronchorrhoea are probably important initially in untreated patients. However, once these features have been reversed with atropine,9,10 and oxygenation is satisfactory, these components are unlikely to be responsible for the respiratory failure seen in unconscious patients.
Definitive proof of whether central respiratory centre or neuromuscular junction (NMJ) dysfunction predominates in human poisoning awaits studies using non- invasive methods to measure phrenic nerve activity.23 However, we have seen marked improvement in respiratory function and conscious level in a few Sri Lankan patients administered diazepam rapidly before intubation (Eddleston, unpublished). This suggests that disordered central activity may disrupt central respiratory function and that reducing this disorder with benzodiazepines improves function and respiration. Rat studies have already shown such an effect of diazepam.25 If confirmed in further human studies, this will support the view that the central component predominates in the early OP induced respiratory failure in humans.
Type II paralysis or intermediate syndrome
Many patients required intubation more than 24 hrs after admission. Many were conscious up until intubation, suggesting a non-central cause. This pattern of delayed respiratory failure often fulfilled the original criteria for type II paralysis 13 or intermediate syndrome.14
However, there were some differences from the original descriptions in our patients. Although described as occurring from 24 to 96 hrs after admission, 10 of 33 (30%) patients requiring intubation for the first time while conscious were intubated either before 24 hrs (4/33, 12%) and after 96 hrs (6/33; 18%). Wadia's original definition also indicated that patients required large amounts of atropine before developing this form of respiratory failure. However, in our series, some fenthion-poisoned patients required little if any atropine before developing delayed respiratory failure.
Type II paralysis or intermediate syndrome was particularly common in fenthion poisoning - 12 of 15 (80%) patients were intubated after 24 hrs, compared to less than 30% of patients ingesting the other OPs. In some patients, it occurred several days after a very mild or even absent cholinergic crisis. Fenthion is a highly fat soluble (log P = 4.3 26) and AChE inhibition occurs slowly.12 It may distribute into the fat in its thion form and then slowly leak out to be converted to the oxon, causing persistent AChE inhibition over days and delayed respiratory failure. Fat solubility alone cannot be the answer, however, since chlorpyrifos is also highly soluble (log P = 5.1 26) but this rapidly activated thion 12 relatively rarely causes delayed respiratory failure.
Late or peripheral respiratory failure
Not all delayed respiratory failure fitted the original clinical descriptions by Wadia and Senanayake as being separated in time from the acute cholinergic crisis. For example, delayed respiratory failure sometimes occurred at the same time as recurrent cholinergic poisoning in fenthion poisoning.
Other patients regained consciousness from their severe acute cholinergic crisis, which had required intubation, into a form of respiratory failure that probably did not involve the CNS. Of the 34 patients still being ventilated 4 days after intubation, 24 (71%) were conscious. Among these conscious patients, it was not possible to distinguish the seven patients who had been intubated within 24 hrs of admission, and had developed a peripheral failure, from the 17 who had been intubated for type II paralysis/intermediate syndrome (patient video 3 - see website).
The distinguishing feature of all late respiratory failure was its apparent peripheral dysfunction. After a few days, all patients who survived were conscious and many could at least weakly move their arms, legs, and cranial nerve-innervated muscles while being quite unable to breath. It seems unlikely that localised central dysfunction affecting only the central respiratory centre would cause this respiratory failure.
Some authors have proposed that the peripheral dysfunction results from sustained overstimulation of the NMJ by high synaptic concentrations of ACh.14,27 Themolecular mechanism is not known, although it may well involve down regulation of receptors in response to sustained stimulation.28,29 There is no consensus concerning whether the dysfunction is pre- and/or post-synaptic; however, failure of the muscarinic antagonist atropine to prevent peripheral respiratory failure might favour involvement of pre- or post-synaptic nicotinic receptors over pre-synaptic muscarinic receptors.
Some researchers believe that the late respiratory failure is due to direct OP toxicity to skeletal muscle.30 However, there is currently little evidence to support such a mechanism. Although animal models show muscle necrosis in OP poisoning,31,32 the extent of muscle damage does not match the degree of weakness. Furthermore, small studies of patients with intermediate syndrome have failed to find either significant muscle damage or raised muscle enzymes in blood.33,34
The report of raised blood creatinine kinase (CK) in OP patients with respiratory failure for many days 30 is confounded by a lack of controls with similar levels of inactivity and long-term bed rest. It is possible that paralysed patients with Guillain- Barré disease or Bungarus envenoming would have had similar rises in CK. In addition, although fasciculations cause muscle damage in animals 32, their occurrence in the study patients was not recorded. Overall, there is little evidence that direct muscle damage causes the peripheral respiratory failure.
Relationship of the peripheral respiratory failure to oxime therapy
The delayed respiratory failure may be due to insufficient or delayed oxime therapy.35-37 The patients in our cohort often received only one day of intermittent pralidoxime treatment, significantly less than currently recommended and clearly not ideal.37,38 We have noted AChE re-inhibition in chlorpyrifos and quinalphos poisoned patients after 24 hrs despite initial good reactivation.39 This late re-inhibition may account for some chlorpyrifos and quinalphos patients requiring intubation after 24hrs.
A post-hoc analysis of a study from Vellore suggested that early administration of a loading 1g dose of pralidoxime prevented late respiratory failure.40 It is possible that higher doses of pralidoxime will more effectively reactivate AChE and then prevent both early and late respiratory failure in all forms of OP poisoning. Proof may come from analysis of the RCTs of high dose pralidoxime now underway in India and Sri Lanka.
Incidence of respiratory failure
We have found marked differences between OPs in both the form (this paper) and frequency 12 of respiratory failure they cause. Many previous studies have reported the incidence for OP-induced respiratory failure or type II paralysis/intermediate syndrome.13,34,41-47 However, only Wadia's study examined relatively large numbers of patients poisoned by a single identified OP (200 patients with diazinon poisoning 13). It is difficult to state a general incidence for OP-induced respiratory failure since it will vary according to the locally common OPs, the facilities available, and whether all patients are studied or just those that are admitted to ICU.
This paper clearly demonstrates how difficult OP-induced respiratory failure is to manage since it demands high levels of resources that are simply not available in the majority of hospitals seeing OP poisoned patients. Some patients died soon after being extubated; others died from sudden unanticipated respiratory arrests. Many patients were intubated for days and weeks, using up valuable ICU beds and preventing the admission of other patients. Tragically, some patients died after they had been ventilated for several weeks, during which they were often conscious, from pneumonia or other complications of long term ventilation.
Conclusion
We have not found it possible to divide up OP pesticide-induced respiratory failure into two non-overlapping clinical syndromes. Instead, it seems that respiratory failure results from two pathophysiologies that can occur separately or together in a patient. Research is required to identify the mechanisms of both early and late respiratory failure and to translate this knowledge into prevention.
More ICU beds and more resources would have reduced the number of patients who died in this study. But this is not going to be practical in the foreseeable future in the developing world. While we wait for research to identify methods of preventing respiratory failure, it is probably worth identifying the OPs that cause most respiratory failure and banning them. It may simply be too difficult to treat in a resource poor country.
Finally, while it may not be possible to clearly distinguish the respiratory syndromes of OP poisoning, it still seems valuable to keep active the concept of type II paralysis or intermediate syndrome. It is essential that doctors are aware that patients who are apparently well may have sudden respiratory arrests for many days after admission.
Acknowledgements
We thank the directors, consultant physicians, medical and nursing staff of the study hospitals for their support, Andrew Dawson, Pradeepa Jayawardene and John Newsom-Davis for critical review, Renate Heilmair, Bodo Pfeiffer and Elisabeth Topoll for technical assistance, and the Ox-Col study staff for their immensely valuable work. ME is a Wellcome Trust Career Development Fellow; this work was funded by grant GR063560MA from Wellcome's Tropical Interest Group to ME. The South Asian Clinical Toxicology Research Collaboration (SACTRC) is funded by Wellcome Trust/ National Health and Medical Research Council International Collaborative Research Grant 071669MA.
Footnotes
Contributions
ME designed and set up the cohort, designed this study, did the analysis and wrote the first draft of the paper. FM ran one of the trial centres and with ME extracted and checked patient data for analysis. JOJD saw patients with respiratory failure and helped develop the concepts described here. PE and FW performed analyses on blood samples and discussed key concepts. MHRS is a senior Sri Lankan co-ordinator of the Ox-Col Collaboration. NAB helped design the cohort study and contributed to the analysis. All authors had a role in improving the study design and in reviewing and editing the final version of the manuscript. ME had full access to the data in the study and final responsibility for the decision to submit for publication.
References
- 1.World Health Organization . Public health impact of pesticides used in agriculture. 0 edn WHO; Geneva: 1990. [Google Scholar]
- 2.van der Hoek W, Konradsen F, Athukorala K, Wanigadewa T. Pesticide poisoning: a major health problem in Sri Lanka. Soc Sci Med. 1998;46:495–504. doi: 10.1016/s0277-9536(97)00193-7. [DOI] [PubMed] [Google Scholar]
- 3.Langley R, Sumner D. Pesticide mortality in the United States, 1979-1998. Vet Hum Toxicol. 2002;44:101–5. [PubMed] [Google Scholar]
- 4.Thiermann H, Szinicz L, Eyer F, Worek F, Eyer P, Felgenhauer N, Zilker T. Modern strategies in therapy of organophosphate poisoning. Toxicol Lett. 1999;107:233–9. doi: 10.1016/s0378-4274(99)00052-1. [DOI] [PubMed] [Google Scholar]
- 5.Bruyndonckx RB, Meulemans AI, Sabbe MB, Kumar AA, Delooz HH. Fatal intentional poisoning cases admitted to the University Hospitals of Leuven, Belgium, from 1993 to 1996. Eur J Emerg Med. 2002;9:238–43. doi: 10.1097/00063110-200209000-00006. [DOI] [PubMed] [Google Scholar]
- 6.Jeyaratnam J. Acute pesticide poisoning: a major global health problem. Wld Hlth Statist Quart. 1990;43:139–44. [PubMed] [Google Scholar]
- 7.Gunnell D, Eddleston M. Suicide by intentional ingestion of pesticides: a continuing tragedy in developing countries. Int J Epidemiol. 2003;32:902–9. doi: 10.1093/ije/dyg307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eddleston M, Phillips MR. Self poisoning with pesticides. BMJ. 2004;328:42–4. doi: 10.1136/bmj.328.7430.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ballantyne B, Marrs TC. Clinical and experimental toxicology of organophosphates and carbamates. 0 edn Butterworth heinemann; Oxford: 1992. Overview of the biological and clinical aspects of organophosphates and carbamates; pp. 3–14. [Google Scholar]
- 10.Lotti M. Handbook of pesticide toxicology. 2 edn Vol. 2. Academic Press; San Diego: 2001. Clinical toxicology of anticholinesterase agents in humans; pp. 1043–85. [Google Scholar]
- 11.Srinivas Rao CH, Venkateswarlu V, Surender T, Eddleston M, Buckley NA. Insecticide poisoning in south India - opportunities for prevention and improved medical management. Trop Med Int Health. 2005;10:581–8. doi: 10.1111/j.1365-3156.2005.01412.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eddleston M, Eyer P, Worek F, Mohamed F, Senarathna L, von Meyer L, Juszczak E, Hittarage A, Azhar S, Dissanayake W, Sheriff MHR, Szinicz L, Dawson AH, Buckley NA. Differences between organophosphorus insecticides in human self-poisoning: a prospective cohort study. Lancet. 2005;366:1452–9. doi: 10.1016/S0140-6736(05)67598-8. [DOI] [PubMed] [Google Scholar]
- 13.Wadia RS, Sadagopan C, Amin RB, Sardesai HV. Neurological manifestations of organophosphate insecticide poisoning. J Neurol Neurosurg Psych. 1974;37:841–7. doi: 10.1136/jnnp.37.7.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Senanayake N, Karalliedde L. Neurotoxic effects of organophosphate insecticides: an intermediate syndrome. N Engl J Med. 1987;316:761–3. doi: 10.1056/NEJM198703263161301. [DOI] [PubMed] [Google Scholar]
- 15.Basnyat B. Organophosphate poisoning: the importance of the intermediate syndrome. Journal of the Institute of Medicine. 2000;22:248–50. [Google Scholar]
- 16.Reigart JR, Roberts JR. Recognition and management of pesticide poisonings. 5 edn Office of Pesticide Programs, U.S. Environmental Protection Agency; Washington DC: 1999. Organophosphate insecticides; pp. 34–47. [Google Scholar]
- 17.Aaron CK. Clinical toxicology. W.B.Saunders Company; Philadelphia: 2001. Organophosphates and carbamates; pp. 819–28. [Google Scholar]
- 18.Clark RF. Goldfrank's Toxicological Emergencies. 7 edn McGraw-Hill Professional; New York: 2002. Insecticides: organic phosphorus compounds and carbamates; pp. 1346–60. [Google Scholar]
- 19.Erdman AR. Medical toxicology. 3 edn Lippincott Williams & Wilkins; Philadelphia: 2004. Insecticides; pp. 1475–96. [Google Scholar]
- 20.Eddleston M, Dawson A, Karalliedde L, Dissanayake W, Hittarage A, Azher S, Buckley NA. Early management after self-poisoning with an organophosphorus or carbamate pesticide - a treatment protocol for junior doctors. http://ccforum.com/content/8/6/R391. Crit Care. 2004;8:R391–R397. doi: 10.1186/cc2953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fernando R. Management of acute poisoning. 2 edn National Poisons Information Centre; Colombo, Sri Lanka: 1998. [Google Scholar]
- 22.Worek F, Mast U, Kiderlen D, Diepold C, Eyer P. Improved determination of acetylcholinesterase activity in human whole blood. Clin Chim Acta. 1999;288:73–90. doi: 10.1016/s0009-8981(99)00144-8. [DOI] [PubMed] [Google Scholar]
- 23.de Candole CA, Douglas WW, Lovatt Evans C, Holmes R, Spencer KEV, Torrance RW, Wilson KM. The failure of respiration in death by anticholinesterase poisoning. Brit J Pharmacol. 1953;8:466–75. doi: 10.1111/j.1476-5381.1953.tb01350.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rickett DL, Glenn JF, Beers ET. Central respiratory effects versus neuromuscular actions of nerve agents. Neurotoxicol. 1986;7:225–36. [PubMed] [Google Scholar]
- 25.Dickson EW, Bird SB, Gaspari RJ, Boyer EW, Ferris CF. Diazepam inhibits organophosphate-induced central respiratory depression. Acad Emerg Med. 2003;10:1303–6. doi: 10.1111/j.1553-2712.2003.tb00001.x. [DOI] [PubMed] [Google Scholar]
- 26.Tomlin C. The pesticide manual. 13 edn BCPC; London: 2003. [Google Scholar]
- 27.de Bleecker JL. The intermediate syndrome in organophosphate poisoning: an overview of experimental and clinical observations. J Toxicol Clin Toxicol. 1995;33:683. doi: 10.3109/15563659509010628. [DOI] [PubMed] [Google Scholar]
- 28.Pitcher JA, Freedman NJ, Lefkowitz RJ. G protein-coupled receptor kinases. Annu Rev Biochem. 1998;67:653–92. doi: 10.1146/annurev.biochem.67.1.653. [DOI] [PubMed] [Google Scholar]
- 29.Gainetdinov RR, Premont RT, Bohn LM, Lefkowitz RJ, Caron MG. Desensitization of G protein-coupled receptors and neuronal functions. Annu Rev Neurosci. 2004;27:107–44. doi: 10.1146/annurev.neuro.27.070203.144206. [DOI] [PubMed] [Google Scholar]
- 30.John M, Oommen A, Zachariah A. Muscle injury in organophosphorous poisoning and its role in the development of intermediate syndrome. Neurotoxicol. 2003;24:43–53. doi: 10.1016/s0161-813x(02)00111-0. [DOI] [PubMed] [Google Scholar]
- 31.Laskowski MB, Dettbarn W-D. The pharmacology of experimental myopathies. Annu Rev Pharmacol Toxicol. 1977;17:387–409. doi: 10.1146/annurev.pa.17.040177.002131. [DOI] [PubMed] [Google Scholar]
- 32.Yang TP, Morrow J, Wu A, Roberts LJ. Dettbarn W-D. Diisopropylphosphorofluoridate-induced muscle hyperactivity associated with enhanced lipid peroxidation in vitro. Biochem Pharmacol. 1996;52:357–61. doi: 10.1016/0006-2952(96)00214-6. [DOI] [PubMed] [Google Scholar]
- 33.de Bleecker J, van der Neucker K, Colardyn F. Intermediate syndrome in organophosphorus poisoning. A prospective study. Crit Care Med. 1993;21:1706–11. doi: 10.1097/00003246-199311000-00020. [DOI] [PubMed] [Google Scholar]
- 34.He F, Xu H, Qin F, Xu L, Huang J, He X. Intermediate myasthenia syndrome following acute organophosphate poisoning - an analysis of 21 cases. Hum Exp Toxicol. 1998;17:40–5. doi: 10.1177/096032719801700107. [DOI] [PubMed] [Google Scholar]
- 35.Benson B, Tolo D, McIntire M. Is the intermediate syndrome in organophosphate poisoning the result of insufficient oxime therapy. J Toxicol Clin Toxicol. 1992;30:347. [Google Scholar]
- 36.Johnson MK, Vale JA, Marrs TC, Meredith TJ. Pralidoxime for organophosphorus poisoning [letter] Lancet. 1992;340:64. doi: 10.1016/0140-6736(92)92487-z. [DOI] [PubMed] [Google Scholar]
- 37.Johnson MK, Jacobsen D, Meredith TJ, Eyer P, Heath AJW, Ligtenstein DA, Marrs TC, Szinicz L, Vale JA, Haines JA. Evaluation of antidotes for poisoning by organophosphorus pesticides. Emergency Medicine. 2000;12:22–37. [Google Scholar]
- 38.Eyer P. The role of oximes in the management of organophosphorus pesticide poisoning. Toxicol Rev. 2003;22:165–90. doi: 10.2165/00139709-200322030-00004. [DOI] [PubMed] [Google Scholar]
- 39.Eyer P, Kiderlen D, Meischner V, Szinicz L, Thiermann H, Worek F, Eyer F, Felgenhauer N, Pfab R, Zilker T, Eddleston M, Senarathna L, Sheriff MHR, Buckley N. The current status of oximes in the treatment of OP poisoning - comparing two regimes. J.Toxicol.Clin.Toxicol. 41:441–3. [Google Scholar]
- 40.Samuel J, Thomas K, Jeyaseelan L, Peter JV, Cherian AM. Incidence of intermediate syndrome in organophosphorus poisoning. J Assoc Physicians India. 1995;43:321–3. [PubMed] [Google Scholar]
- 41.Wadia RS, Bhirud RH, Gulavani AV, Amin RB. Neurological manifestations of three organophosphate poisons. Indian J Med Res. 1977;66:460–8. [PubMed] [Google Scholar]
- 42.Kamat SR, Heera SS, Potdar PV, Shah SV, Bhambure NM, Mahashur AA. Bombay experience in intensive respiratory care over 6 years. J Postgrad Med. 1989;35:123–34. [PubMed] [Google Scholar]
- 43.Tsao TCY, Juang YC, Lan RS, Shieh WB, Lee CH. Respiratory failure of acute organophosphate and carbamate poisoning. Chest. 1990;98:631–6. doi: 10.1378/chest.98.3.631. [DOI] [PubMed] [Google Scholar]
- 44.Goswamy R, Chaudhuri A, Mahashur AA. Study of respiratory failure in organophosphate and carbamate poisoning. Heart & Lung. 1994;23:466–72. [PubMed] [Google Scholar]
- 45.Goel A, Joseph S, Dutta TK. Organophosphate poisoning: predicting the need for ventilatory support. J Assoc Physicians India. 1998;46:786–90. [PubMed] [Google Scholar]
- 46.Poojara L, Vasudevan D, Arun Kumar AS, Kamat V. Organophosphate poisoning: diagnosis of intermediate syndrome. Indian J Crit Care Med. 2003;7:94–102. [Google Scholar]
- 47.Godhwani S, Godhwani S, Tulsiani KL. Management of organic insecticide poisoning in intensive care unit (ICU) Indian J Anaesth. 2004;48:2. [Google Scholar]