Abstract
Purpose
To evaluate brain activity associated with sexual arousal, fully conscious male marmoset monkeys were imaged during presentation of odors that naturally elicit high levels of sexual activity and sexual motivation.
Material and Methods
Male monkeys were lightly anesthetized, secured in a head and body restrainer with a built-in birdcage resonator and positioned in a 9.4-Tesla spectrometer. When fully conscious, monkeys were presented with the odors of a novel receptive female or an ovariectomized monkey. Both odors were presented during an imaging trial and the presentation of odors was counterbalanced. Significant changes in both positive and negative BOLD signal were mapped and averaged.
Results
Periovulatory odors significantly increased positive BOLD signal in several cortical areas: the striatum, hippocampus, septum, periaqueductal gray, and cerebellum, in comparison with odors from ovariectomized monkeys. Conversely, negative BOLD signal was significantly increased in the temporal cortex, cingulate cortex, putamen, hippocampus, substantia nigra, medial preoptic area, and cerebellum with presentation of odors from ovariectomized marmosets as compared to periovulatory odors. A common neural circuit comprising the temporal and cingulate cortices, putamen, hippocampus, medial preoptic area, and cerebellum shared both the positive BOLD response to periovulatory odors and the negative BOLD response to odors of ovariectomized females.
Conclusion
These data suggest the odor-driven enhancement and suppression of sexual arousal affect neuronal activity in many of the same general brain areas. These areas included not only those associated with sexual activity, but also areas involved in emotional processing and reward.
Keywords: BOLD technique, approach/avoidance, magnetic resonance imaging, functional imaging, common marmoset, cerebellum, neural circuit, sexual motivation, suppressed sexual activity
ODORS ARE IMPORTANT in modulating sexual behavior in a variety of species. However, few studies have connected the behavioral response and the brain activation to the odor stimulation. Attractiveness of a female can be transmitted by chemosensory, visual, and auditory cues; ensuring an increase in male sexual behaviors at a time when females are most fertile (1). Chemical or olfactory cues derived from female scent secretions are known to provide cues of the female’s reproductive status and are important in mediating male sexual behavior in marmosets and tamarins (family Callitrichidae) (2). In response, males have increased sexual behaviors such as tongue-flicks, mounts, and ejaculation (3). While male common marmosets respond with sexual behavior to other sensory cues from female marmosets, such as visual, olfactory cues are important for coordinating male sexual activity with female endocrine status (1). Additionally, scent odors from periovulatory marmoset females are preferred over luteal phase scents from the same female (4).
Lesions of the medial preoptic area/anterior hypothalamic continuum (MPOA/AH) have been shown to produce deficiencies in male sexual behavior (5) with decreases in precopulatory and copulatory behaviors, mounting, and anogenital investigation of females. The preoptic area of the hypothalamus has input from the olfactory and vomeronasal systems as well as other sensory systems and induces the release of hormones. Using functional magnetic resonance imaging (fMRI), we corroborated the work of Lloyd and Dixson (5) in demonstrating activation of the MPOA/AH (6). Awake male marmosets presented with the scent marks of unfamiliar females showed enhanced signal intensity in the MPOA/AH to the ovulatory scent versus the ovariectomized (OVX) scent. Although both odors elicited activation versus vehicle control, there was greater activation for the ovulatory scent in the awake males. This imaging study provided evidence that olfactory signals activate the MPOA/AH sexual arousal system in the male common marmoset.
Brain imaging has many advantages over lesion studies. Awake male marmosets can be imaged repeatedly and remain intact. In addition to examining functionality of specific brain areas, the entire brain can be imaged to provide information on the integration of arousal and inhibitory processes of the brain in responding to sexually relevant cues. Other neural systems interacting with sexual behavior can be imaged to examine positive affect areas of the brain as well as inhibitory processes. Scent detection is a good starting point for examining brain activity since odor cues elicit distinct behavioral actions. Marmosets, as in all callitrichidae species, show distinct hormonal and behavioral responses to relevant chemical sensory cues, and are therefore a good model for examining behavior responses and brain activation.
The present study extends our previous analysis to other brain areas. We imaged four male marmosets while presenting periovulatory, ovariectomized female scents and vehicle controls to assess key neural structures involved in stimulus processing for positive affective (reward) sexual chemosensory signals.
MATERIALS AND METHODS
MR studies were conducted on four adult male common marmoset monkeys (400–450 gm) that were family reared and socially housed at the Wisconsin Primate Research Center. Two of the males were sexually experienced and living with a reproductive female. The other two males (brothers) were sexually naïve and lived together. All subjects were extensively habituated to a specially designed MR restraint device (Insight Neuroimaging Systems, LLC, Worcester, MA) and to the imaging procedure in a simulated environment. During MR sessions, animals were first lightly sedated with ketamine HCl (Ketaset, 2 mg) plus medetomidine (Domitor, 0.02 mg) and then placed in the MR head and body restraint. Once securely restrained, anesthesia was reversed with atipamezole (Antiseden, 0.1 mg). Prior to imaging, animals were tested for their sensitivity and recovery to this anesthetic procedure. Animals recovering from anesthesia were judged to be fully conscious when they could demonstrate normal perceptual and motor function (i.e., demonstrate normal movement and visually follow a food reward). The recovery time for all animals was less than 30 minutes.
Anatomical imaging began at least 45 minutes after anesthesia reversal, followed by functional imaging. A 40-mm diameter birdcage resonator was built into the head restraint and used in both transmit and receive modes. All images were acquired using a 9.4-T/31-cm horizontal magnet (Magnex, Abingdon, UK) interfaced to a Unity INOVA console (Varian, Palo Alto, CA) at the University of Minnesota. High-resolution anatomical data sets were acquired (fast spin echo, TR = 2 seconds; echo train length = 8; echo train spacing = 9.5 msec; field of view = 4 × 4 cm; data matrix = 256 × 256; slice thickness = 2 mm) at the beginning and end of each imaging session. Functional images were acquired using a gradient-echo (FLASH) sequence (TR = 115 msec; TE = 11 msec; flip angle = 15 degrees; field of view = 4 × 4 cm; data matrix = 128 × 128; slice thickness = 2 mm, approximately 300 micron resolution).
Motion artifact was addressed in three ways: 1) subtraction of anatomical data across the two to three hour imaging session, 2) qualitative analysis of time series movies looking for voxel displacement, and 3) analysis of raw data time series for course spikes. The time series movies correlated with course spike activity. The multiple data sets collected from the six imaging sessions showed very little motion artifact using these criteria. On the rare occasion there would be a course spike usually caused by movement of the mouth such as in swallowing. The data for these images were excluded. Indeed, in pilot studies we used ketamine as an anesthetic and discovered during recovery in the magnet greater than usual motion artifact due to excessive salivation and swallowing, something we do not see with the Domitor/Antiseden regimen.
Female scent marks were collected and pooled from either periovulatory (determined through measurements of serum progesterone) or ovariectomized females. Females were presented with a ground glass stopper and allowed to rub their anogenital gland on the stopper resulting in a mixture of scent secretion and urine. The stopper was removed from the cage and rinsed with 300 μL of an ethanol:water (50:50) solution. The solution was degassed prior to use to minimize oxidation of the scent chemicals. After collection, the scent samples were stored at −70°C and thawed immediately prior to presentation. The vehicle, ethanol:water, was stored exactly as the scent secretions. The fully conscious males were imaged during presentation of scent marks from periovulatory, OVX females, and vehicle. The males had no previous exposure to any of the females providing odors. BOLD fMRI data sets of seven slices each were collected continuously at 15-second intervals. Following seven minutes of baseline data acquisition (28 seven-slice volume), periovulatory, OVX, or vehicle control scents applied to a wooden disc were positioned 9 cm from the nose of the monkey for seven minutes, followed by another 10 minutes of data acquisition after removal of the odor.
Four monkeys were imaged in this study. Two of the four animals were imaged twice, six months apart. The order of scent presentation was reversed to determine if presenting OVX before or after ovulate odors in an imaging session affected the response. There was no order effect. The final data analysis came from six imaging sessions, two from the same animals. All of these data have been collapsed into Table 1. The boundaries delineating the region of interest (ROI) for all of the areas listed in Table 1 area are shown in Figs. 1–5. The images showing the ROIs were registered to comparable coronal images taken from the marmoset atlas of Saavedra and Massuchelli (7). The area best fitting the description in the atlas was manually drawn around the ROI prior to any overlay of activity.
Table 1.
Comparing Positive and Negative BOLD Signal*
| Positive BOLD signal
|
Negative BOLD signal
|
Between
|
Within
|
|||||
|---|---|---|---|---|---|---|---|---|
| Area | Ovulate | OVX | Ovulate | OVX | Positive | Negative | Ovulate | Ovx |
| Prefrontal cortex (16) | 9.4 ± .87 | 6.5 ± .82 | 9.8 ± 1.0 | 10.4 ± 1.0 | .05 | NS | NS | .01 |
| Temporal cortex (52) | 10.0 ± .80 | 5.7 ± .49 | 8.2 ± .86 | 12.5 ± 1.0 | .001 | .001 | NS | .01 |
| Somatosensory (12) | 10.2 ± 1.9 | 5.7 ± 1.5 | 5.4 ± 1.0 | 7.6 ± 1.5 | .05 | NS | .05 | NS |
| Insular cortex (24) | 4.4 ± .54 | 1.6 ± .39 | 3.4 ± .67 | 5.2 ± .76 | .001 | NS | NS | .001 |
| Cingulate cortex (30) | 8.9 ± .88 | 5.4 ± .61 | 7.8 ± .98 | 10.8 ± 1.1 | .001 | .05 | NS | .001 |
| Caudate (22) | 4.6 ± .67 | 2.4 ± .43 | 2.9 ± .66 | 4.4 ± .61 | .01 | NS | NS | .01 |
| Putamen (22) | 4.7 ± .72 | 1.9 ± .44 | 3.5 ± .68 | 6.3 ± .76 | .005 | .05 | NS | .001 |
| Substantia nigra (12) | 2.8 ± .67 | 2.0 ± .49 | 1.7 ± .50 | 3.5 ± .89 | NS | .01 | NS | NS |
| Dorsal thalamus (12) | 2.1 ± .34 | 2.5 ± .74 | 2.4 ± .54 | 3.1 ± .53 | NS | NS | NS | NS |
| Midline thalamus (12) | 2.2 ± .54 | 2.0 ± .77 | 2.1 ± .79 | 3.8 ± 1.3 | NS | NS | NS | NS |
| Amygdala (20) | 5.0 ± .99 | 4.4 ± .90 | 4.9 ± 1.0 | 8.2 ± 1.9 | NS | NS | NS | NS |
| Hippocampus (24) | 11.5 ± 1.6 | 6.9 ± 1.0 | 9.1 ± 1.5 | 13.7 ± 1.9 | .001 | .01 | NS | .01 |
| Septum (6) | 6.3 ± 1.5 | 2.3 ± .76 | 3.0 ± .93 | 6.3 ± 1.4 | .05 | NS | NS | .05 |
| Olfact. tubercle (12) | 3.2 ± .83 | 3.0 ± .89 | 2.9 ± .76 | 1.6 ± .35 | NS | NS | NS | NS |
| Medial preoptic (6) | 9.5 ± 2.8 | 4.0 ± 1.4 | 2.6 ± 1.7 | 7.0 ± .68 | .05 | .05 | .05 | NS |
| Anterior hypo. (6) | 12.0 ± .33 | 3.1 ± .74 | 5.1 ± 2.2 | 6.6 ± .55 | .05 | NS | NS | .01 |
| Periaqueductal (6) | 4.1 ± .79 | 2.0 ± .51 | 3.1 ± .74 | 5.8 ± 2.5 | .05 | NS | .05 | NS |
| Raphe nucleus (7) | 1.4 ± .36 | 4.4 ± .97 | 2.8 ± 1.1 | 1.1 ± .50 | .05 | NS | NS | .01 |
| Cerebellum (6) | 40.0 ± 5.1 | 18.0 ± 1.0 | 41 ± 9.8 | 65 ± 13 | .05 | .05 | NS | .01 |
| Sup. colliculus (12) | 2.4 ± .65 | 1.6 ± .33 | 2.9 ± .62 | 3.4 ± .7 | NS | NS | NS | .05 |
| Inf. Colliculus (12) | 3.2 ± .39 | 2.2 ± .78 | 3.0 ± .52 | 5.2 ± .76 | NS | NS | NS | .05 |
Mean ± SEM number of voxels activated relative to baseline in response to ovulatory odors and odors of ovariectomized (OVX) females in selected regions of interest. P-values for differences between responses to ovulate odors and OVX odors in both positive and negative BOLD signals are indicated under “Between.” Differences between positive and negative BOLD responses within each stimulus are shown under “Within.”
Figure 1.
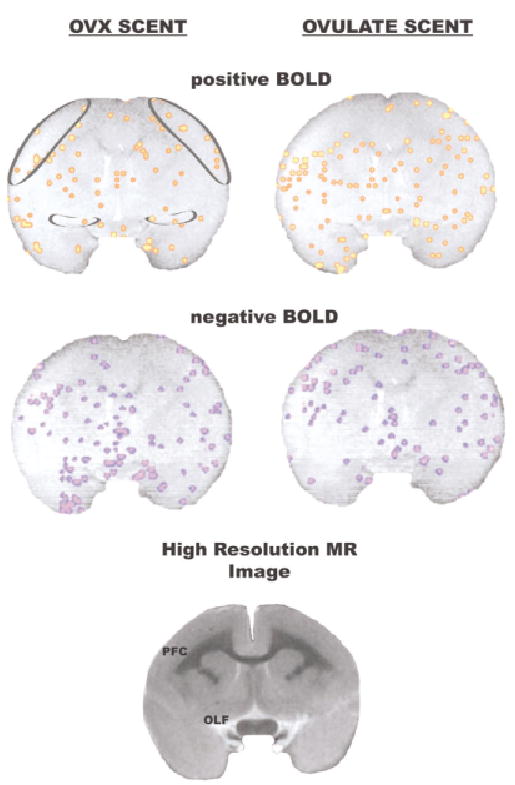
Activational maps at the level of the prefrontal cortex. Significant changes in positive and negative BOLD signal are shown for presentation of OVX scent and ovulatory scent. Rather than show positive and negative BOLD for each scent as a single composite, they are presented individually one above the other, respectively. Regions of interest are shown in the circumscribed areas in the upper right coronal section. These images are taken from a single animal. A high-resolution image taken from a formalin-fixed marmoset brain is presented to show neuroanatomical detail. Abbreviations: PFC = prefrontal cortex, OLF = olfactory tubercles.
Figure 5.
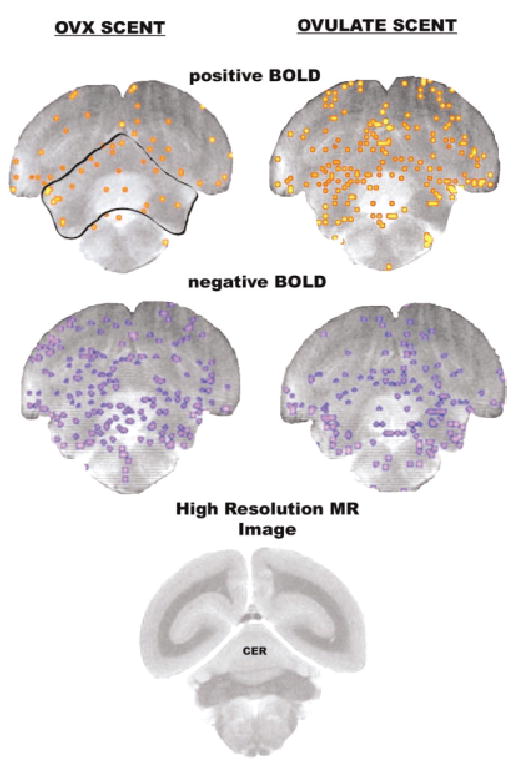
Activational maps at the level of the cerebellum. Images taken from a single animal. See Fig. 1 for detailed description. Abbreviations: CER = cerebellum.
In Table 1, the numbers in parentheses represent the number of ROIs measured over the six imaging sessions. For example, the neuroanatomical area defining the prefrontal cortex was identified in eight slices from the six imaging sessions. The prefrontal cortex is bilateral so there were 16 measures. The bilateral temporal cortex was identified in 26 slices (see Figs. 2–4) so there are 52 measures. Brain areas like the septum and MPOA were outlined as unilateral structures appearing in only one brain slice so the number of measures is six. These ROIs were analyzed for changes in BOLD signal intensity using STIMULATE software (8). Statistical comparisons of vehicle and baseline showed no differences for any of the ROIs, so subsequent analyses involved using only baseline and stimulus analyses. Statistical comparisons between baseline periods to olfactory stimulus periods was carried out using the Student’s paired t-test to generate an activation map for each control-stimulus data set. Those voxels (three-dimensional pixels) whose percent change ([average response over the stimulation period–average response over the baseline period)/(average response over the baseline period]) were identified as significant at a 95% confidence level were evaluated. Voxels satisfying these established criteria were overlaid on their respective anatomical data set. Changes in BOLD signal were also presented as time course data. Each data acquisition period of 15 seconds represents the average of all significantly activated positive or negative voxels within the ROI.
Figure 2.
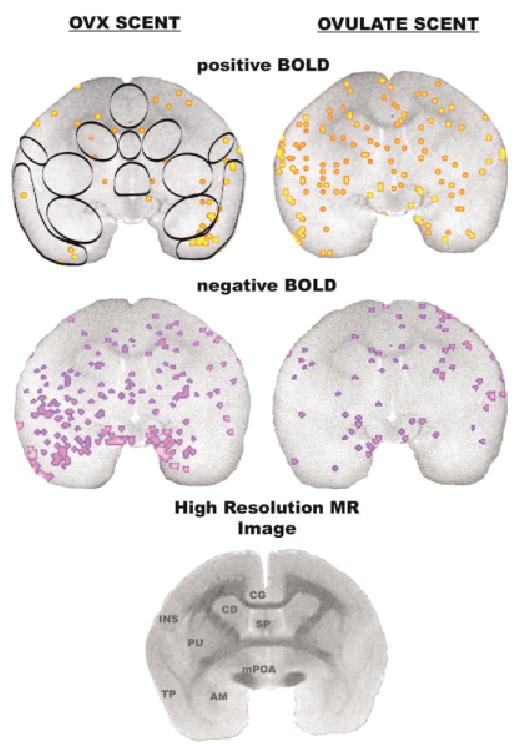
Activational maps at the level of the amygdala. Images were taken from a single animal. See Fig. 1 for detailed description. Abbreviations: INS = insular cortex, CG = cingulate cortex, CD = caudate, SP = septum, PU = putamen, TP = temporal cortex, AM = amygdala, mPOA = medial preoptic area.
Figure 4.
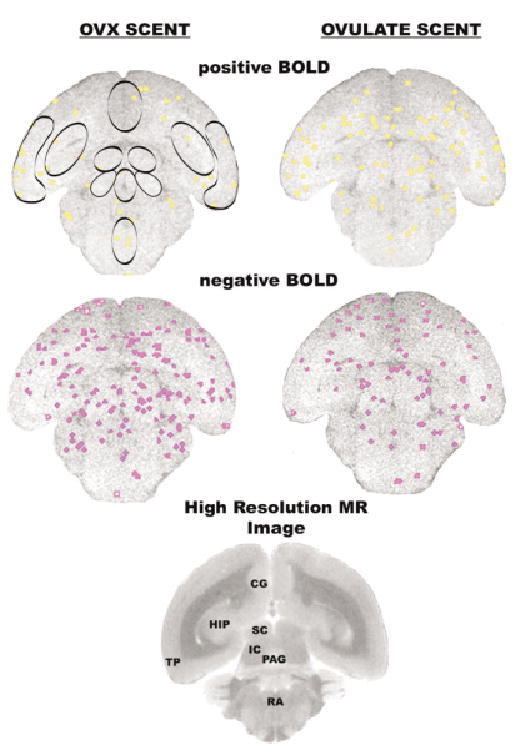
Activational maps at the level of the midbrain. Images taken from a single animal. See Fig. 1 for detailed description. Abbreviations: SC = superior colliculus, IC = inferior colliculus, PAG = periaqueductal gray, RA = raphe.
For pilot testing, one male was tested in his home cage a week following imaging with the same stimulus scent marks. Each individual scent (vehicle, periovulatory, OVX) was placed on a wooden disc in the cage of the male and his paired female for 10 minutes. Latency to first sniff and frequency to sniff, scent mark, touch disc, attempt to mount female, sniff or lick female, and lick the disc were recorded.
RESULTS
We found no difference between baseline data acquisition and the vehicle control in all brain areas examined. However, multiple brain areas showed changes in BOLD signal intensity in response to periovulatory or OVX odors. Table 1 reports the number of voxels (mean + SEM) that showed increased BOLD signal intensity (positive BOLD) and decreased signal intensity (negative BOLD) as compared to baseline in twenty-one brain areas for each odor. Within Table 1 are comparisons between the two odors (periovulatory [ovulate] and OVX) for both positive BOLD (columns 1 and 2) and negative BOLD (columns 3 and 4). Column 5 shows significance levels of positive BOLD between periovulatory and OVX odors, and column 6 shows the same comparisons for negative BOLD. Column 7 shows the significance levels for comparisons of positive versus negative BOLD for periovulatory odors and column 8 presents the same comparison for OVX odors. Activational maps showing significant positive and negative BOLD signals and the position and dimension of each of the brain areas or ROI reported in Table 1 are shown in Figs. 1–5. The neuroanatomical locations of the activational maps show anterior to posterior coronal sections starting from the prefrontal cortex and ending at the cerebellum. Data from each of the four monkeys studied are represented in these activational maps. For example, all of the maps in Fig. 2 come from one animal while all of the maps from Fig. 3 come from a different animal. The activational maps in Fig. 1 and Fig. 5 are from the same monkey.
Figure 3.
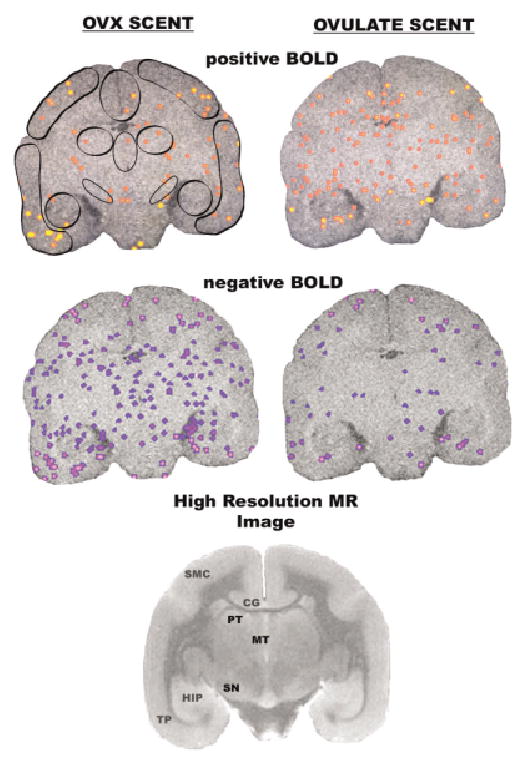
Activational maps at the level of the thalamus. Images taken from a single animal. See Fig. 1 for detailed description. Abbreviations: SMC = sensory motor cortex, PT = posterior thalamus, MT = medial thalamus, HIP = hippocampus, SN = substantia nigra.
A total of 14 of 21 ROIs showed increased positive BOLD to periovulatory versus OVX odors. These included many areas of the cerebral cortex, e.g., prefrontal, temporal, somatosensory, insula, and cingulate. This was also true of the dorsal striatum, e.g., caudate and putamen, the hippocampus, septum, medial preoptic area, anterior hypothalamus, periaqueductal gray, and cerebellum. The only site that showed a greater increase in BOLD signal in response to the OVX scent as compared to periovulatory scent was the raphe nucleus. Seven of the 21 ROIs also showed a significant change in negative BOLD signal between periovulatory and OVX odors; however, the direction of the response was opposite with a greater negative BOLD response to OVX compared with periovulatory odors. The temporal cortex, cingulate cortex, putamen, hippocampus, medial preoptic area, and cerebellum showed increased BOLD signal to periovulatory odors but decreased BOLD signal to OVX odors. This negative “mirror image” can be seen in each series of activational maps shown in Figs. 1–5 by comparing the OVX negative BOLD data with the ovulate positive BOLD data. Time course data showing the change in positive and negative BOLD signal in response to ovulate and OVX odors, respectively, for the temporal and cingulate cortices, putamen, and medial preoptic area are shown in Fig. 6. Note that the activation curves for positive BOLD to periovulatory odors and negative BOLD to OVX odors do not immediately converge to baseline after stimulus removal. The effects of stimulation on brain activity continue for several minutes beyond removal of the stimulus.
Figure 6.
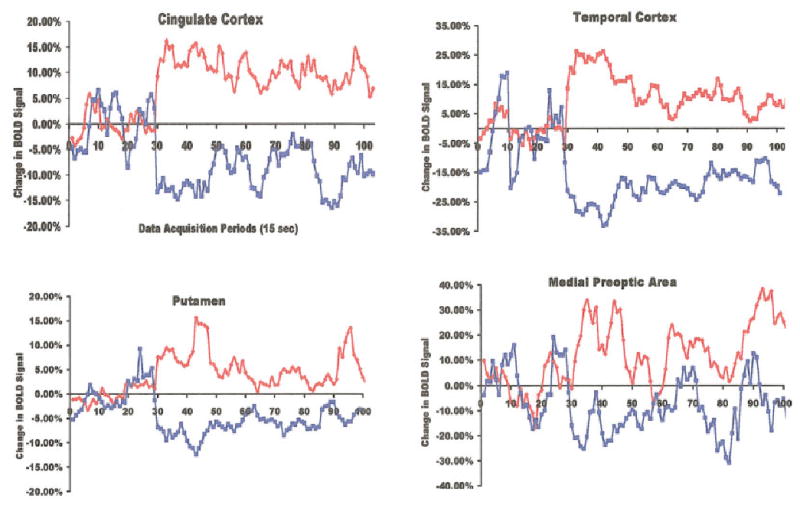
Change in BOLD signal intensity over time. The average percent change in BOLD signal intensity in response to OVX scent and ovulatory scent in the cingulate cortex, temporal cortex, putamen, and medial preoptic area are shown plotted over time. Scents were given at the 30th data acquisition period. Red denotes data from ovulatory scent and blue OVX scent.
As shown in Table 1, there is no absolute increase or decrease in BOLD signal within an individual brain area during odor presentation; instead, there are some voxels showing a positive BOLD signal and others showing a negative BOLD signal for each ROI. For example, in the temporal cortex the ROI has a combined 850 voxels taken from three bilateral brain sections (Figs. 2–4). When presented with periovulatory odors 10.0 ± 0.80 of the voxels, or less than 2%, show an increase in BOLD signal while about the same number 8.2 ± 0.86 show a decrease in signal. Indeed, this pattern where approximately the same number of voxels were positive as negative for any given ROI in response to periovulatory odors was true for eighteen of the twenty-one brain areas reported. Only the somatosensory cortex, medial preoptic area, and the periaqueductal gray showed significantly (P < 0.05) fewer negative voxels during periovulatory odors as compared to positive voxels.
In contrast, animals presented with OVX odors had a robust increase in number of negative BOLD voxels above positive BOLD voxels in 13 of the 21 brain regions. The eight brain areas that did not show this pattern of negative BOLD signal fall into two groups. The first group covers five brain areas that did not show significant positive BOLD to periovulatory scent above OVX scent and include the substantia nigra, dorsal and midline thalamus, amygdala, and olfactory tubercles. The second group was also unique because it includes the only three brain areas that showed significantly more positive BOLD voxels than negative BOLD voxels for periovulatory scent, i.e., the somatosensory cortex, medial preoptic area, and periaqueductal gray.
Pilot testing the periovulatory and OVX odors showed a differential increase in the male’s attention to his own female (mounting the female, sniffing and licking the female) compared to the vehicle control, as expected from previous studies. Males interacted with the wooden disc more often and licked only when the scent was ovulatory.
DISCUSSION
We previously reported that periovulatory odors, an important sexual stimulus for male marmosets, produced activation of the MPOA and anterior hypothalamus two areas critical for male precopulatory and copulatory behavior (6). Here we demonstrate both activation and deactivation of multiple brain areas in response to periovulatory and OVX odors compared to vehicle control odors. Many areas showing BOLD activation to sexually arousing stimuli in marmosets overlap with comparable areas in humans (using both PET and fMRI), although the stimuli are quite different (odors vs. visual and auditory stimuli). Both humans and marmosets show activation in the cingulate cortex, temporal cortex, insula, caudate, hypothalamus, and cerebellum (9–12,14).
The most notable difference between humans and marmosets is the absence of amygdala activation in marmosets. The amygdaloid complex appears critical in sexual arousal and motivation. Marmosets showed both significant activation and deactivation of the amygdala to both periovulatory and OVX odors compared to vehicle control, but no significant difference between odors. The most plausible explanation is inadequate spatial resolution. The amygdaloid complex has over 20 discrete nuclear areas, and only a few may be involved in processing sexually arousing stimuli. We included the entire amygdala in our ROI (see Fig. 2), so potentially significant changes in both positive and negative BOLD to each stimulus may have occurred in different individual nuclei.
Interestingly, the cerebellum showed robust activation in both marmosets and humans to sexually arousing stimuli. PET and fMRI studies report increased cerebellar activity in response to positive and negative emotional stimuli (15,16). The cerebellum has extensive neural connections with the somatosensory cortex and association areas, i.e., prefrontal, temporal, and cingulate cortices, as well as the hypothalamus (17). Beauregard et al (13) suggested the cerebellum might be involved in the somatic experience associated with sexual arousal.
The brain areas activated by sexually provocative cues in marmosets and humans represent not only a common circuit for sexual arousal and motivation, but also are part of a larger appetitive or approach motivational system (18). Physiological and behavioral data on marmoset reproductive activity support this notion since odors from ovulating marmosets induce approach behavior in males. Presentation of sensory stimuli with positive affective valence activated the prefrontal cortex, temporal cortex, somatosensory cortex, insula, cingulate, and amygdala in healthy human subjects (16,19,20).
One of the most interesting results was the “mirror image” of brain activity in marmosets exposed to periovulatory versus OVX odors. Several areas associated with arousal and sexual motivation: temporal cortex, MPOA, cingulate, insula, caudate, and cerebellum, showed significant deactivation in response to the OVX odor relative to vehicle control. OVX odors may have a more negative emotional valence, inhibiting appetitive motivation. However, marmoset males copulate with ovariectomized females when housed together as a bonded pair (21). Possibly the olfactory cues of reproductive status are most important to a male evaluating his response to an unfamiliar female, whereas with a pair-bonded mate learned responses to other features override the olfactory cues. If this is true, then we should find different responses to cues from an unfamiliar female versus learned cues from a mate. This provides a way to separate the biological and experiential aspects of sexual response. Imaging data from non-human primates has heuristic value in interpreting and understanding brain images of human sexual responses.
In human PET studies, changes in brain activity to negatively valenced emotions produce increased activation in left inferior frontal, left anterior cingulate, and left temporal pole regions (22). With all negative emotional stimuli, the left temporal cortex is activated. We found little lateralization of odor-induced brain activity in male marmosets except the left temporal cortex. OVX odors increased activity in the left temporal cortex, suggesting that this area, as in humans, may be involved in processing negative emotional stimuli.
Both periovulatory and OVX odors produced both positive and negative BOLD signal in many of the same brain areas suggesting that positive and negative motivational systems reside in the same areas. Lane et al (16) used PET in female subjects presented with pleasant and unpleasant stimuli and discovered a consistent overlap for each motivational system in the medial insular cortex and prefrontal cortex. In marmosets, positive stimuli (periovulatory odors), and presumably negative stimuli (OVX odors), consistently increased BOLD signal in the same brain areas even though there was significantly more activation with the periovulatory odors in most areas. Only three of 21 brain areas showed significant differences in positive versus negative BOLD to periovulatory odors.
These data suggest a common neuroanatomical circuit for sexual arousal and sexual motivation that may be affected by other regions weighted for context-dependent approach and withdrawal responses. The periaqueductal gray (PAG) and MPOA are two areas where the number of positive BOLD voxels significantly exceeded the number of negative BOLD voxels during presentation of periovulatory odors. These sites might be selectively weighted toward approach behavior in the context of sexual arousal and motivation. The MPOA is critical for the organization and initiation of male sexual behavior (23). Activation of the MPOA facilitates sexual behavior, while lesions prevent copulatory behavior in every species studied. In rats the MPOA has dense reciprocal connections to the PAG (24). In turn, the PAG has inputs to the nucleus paragigantocellularis (nPGi) whose connections to the spinal cord control male reproductive reflexes (25). This MPOA–PAG–nPGi neural circuit may be essential in activation of male sexual reflexes (25).
Conversely, the raphe nucleus was the only site showing a significant decrease in positive BOLD voxels in response to periovulatory odors and the only site with a significant increase in positive bold voxels to OVX odors. The raphe may be weighing toward avoidance behavior in the context of sexual arousal and motivation. The raphe is the primary source of serotoninergic innervation of the forebrain and is considered part of a behavioral inhibition system. Electrical stimulation of the raphe elicits freezing behavior (26), and blocking raphe activity reduces the inhibitory effect of conditioned aversive stimuli on bar pressing (27). Disrupting serotoninergic neurotransmission from the raphe promotes feeding (28) and enhances male sexual activity (29). Periovulatory odors may suppress raphe activity, disinhibiting sexual behavior, whereas OVX odors may enhance raphe activity, suppressing sexual approach behavior.
The robust negative BOLD signal observed is interpreted as brain deactivation. Use of the phrase “inhibition of brain activity” would be incorrect, because synaptic inhibition in the form of inhibitory postsynaptic potentials is an active process accompanied by enhanced oxidative metabolism. Brain deactivation suggests reduced synaptic and neuronal activity without specifying a biophysical mechanism. None of the human fMRI studies examining sexual arousal in males presented data on negative BOLD signal. However, Bartels and Zeki (30), imaging romantic love in young women, discussed widespread deactivation in the prefrontal, parietal, and temporal cortices concomitant with enhanced BOLD signal in the insula, cingulate, caudate, and putamen. The occurrence of negative BOLD signal is often recognized as brief “dips” and “troughs” in blood flow at the beginning and end of a stimulus, but not normally associated with brain deactivation (31–35). However, examples of robust negative BOLD signal have been found in the visual cortex, presumably reflecting suppression of neuronal and synaptic activity (36,37). For example, activation of the visual cortex in children and adults was studied using BOLD and arterial water spin labeling to assess regional Cerebral Blood Flow (rCBF). Adults showed both an increase in BOLD and rCBF to visual stimulation. However, children showed a decrease in BOLD concomitant with a decrease in rCBF (37).
This study shows that fMRI is a feasible method for identifying neuroanatomical circuits involved in sexual arousal in conscious marmoset monkeys. Data from marmosets appear to translate to human sexual arousal and motivation since many of the neuroanatomical areas identified previously in human imaging studies overlap with those reported here. The circuitry is part of a general appetitive circuit for positively valenced emotional stimuli. The virtue of the marmoset model is the ability to control precisely the developmental and social history and to carry out conditioning studies not possible in humans.
Acknowledgments
We thank Dr. Kamil Ugurbil for facilitating these studies at the Center for Magnetic Resonance Research and Dr. Seong-Gi Kim and his staff for their guidance and technical assistance with the imaging protocols.
Footnotes
Contract grant sponsor: National Institute of Mental Health, Program in Behavioral and Integrative Neuroscience; Contract grant number: MH58700; Contract grant sponsor: NIH; Contract grant sponsor: Wisconsin Primate Research Center; Contract grant number: RR00167.
References
- 1.Dixson AF. Primate sexuality: comparative studies of the prosimians, monkeys, apes, and human beings. Oxford: Oxford University Press, 1998. p. 546
- 2.Dixson AF, Lunn SF. Post-partum changes in hormones and sexual behavior in captive groups of marmosets (Callithrix jacchus) Physiol Behav. 1987;41:577–583. doi: 10.1016/0031-9384(87)90314-3. [DOI] [PubMed] [Google Scholar]
- 3.Kendrick KM, Dixson AF. The effect of the ovarian cycle on the sexual behaviour of the common marmoset (Callithrix jacchus) Physiol Behav. 1983;30:735–742. doi: 10.1016/0031-9384(83)90171-3. [DOI] [PubMed] [Google Scholar]
- 4.Smith TE, Abbott DH. Behavioral discrimination between circumgenital odor from peri-ovulatory dominant and anovulatory female common marmosets (Callithrix jacchus) Am J Primatol. 1998;46:26–284. doi: 10.1002/(SICI)1098-2345(1998)46:4<265::AID-AJP1>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 5.Lloyd SAC, Dixson AF. Effects of hypothalamic lesions upon the sexual and social behaviour of the male common marmoset (Callithrix jacchus) Brain Res. 1988;463:317–329. doi: 10.1016/0006-8993(88)90405-2. [DOI] [PubMed] [Google Scholar]
- 6.Ferris CF, Snowdon CT, King JA, et al. Functional imaging of brain activity in conscious monkeys responding to sexually arousing cues. Neuroreport. 2001;12:2231–2236. doi: 10.1097/00001756-200107200-00037. [DOI] [PubMed] [Google Scholar]
- 7.Saavedra JP, Mazzucheli AL. A stereotaxic atlas of the brain of the marmoset (Hapale jacchus) J Hirnforsch. 1969;11:105–122. [PubMed] [Google Scholar]
- 8.Strupp JP. Stimulate: a GUI based fMRI analysis software package. Neuroimage. 1996;3:S607. [Google Scholar]
- 9.Rauch SL, Shin LM, Dougherty DD, et al. Neural activation during sexual and competitive arousal in healthy men. Psychiatry Res. 1999;30:1–10. doi: 10.1016/s0925-4927(99)00020-7. [DOI] [PubMed] [Google Scholar]
- 10.Stolerru A, Gregoire M-C, Gereard D, et al. Neuroanatomical correlates of visually evoked sexual arousal in human males. Arch Sexual Behav. 1999;28:1–21. doi: 10.1023/a:1018733420467. [DOI] [PubMed] [Google Scholar]
- 11.Park K, Seo JJ, Kang HK, Ryu SB, Kim HJ, Jeong GW. A new potential of blood oxygenation level dependent (BOLD) functional MRI for evaluating cerebral centers of penile erection. Int J Impot Res. 2001;13:73–81. doi: 10.1038/sj.ijir.3900649. [DOI] [PubMed] [Google Scholar]
- 12.Arnow BA, Desmond JE, Banner LL, et al. Brain activation and sexual arousal in healthy, heterosexual males. Brain. 2002;125:1014–1023. doi: 10.1093/brain/awf108. [DOI] [PubMed] [Google Scholar]
- 13.Beauregard M, Levesque J, Bourgouin P. Neural correlates of conscious self-regulation of emotion. J Neurosci. 2001;21:RC165. doi: 10.1523/JNEUROSCI.21-18-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karama S, Lecours AR, Leroux JM, et al. Areas of brain activation in males and females during viewing of erotic film excerpts. Hum Brain Mapp. 2002;16:1–13. doi: 10.1002/hbm.10014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.George MS, Ketter TA, Parekh PI, Horwitz B, Herscovitch P, Post RM. Brain activity during transient sadness and happiness in healthy women. Am J Psychiatry. 1995;152:341–351. doi: 10.1176/ajp.152.3.341. [DOI] [PubMed] [Google Scholar]
- 16.Lane RD, Reiman EM, Ahern GL, Schwartz GE, Davidson RJ. Neuroanatomical correlates of happiness, sadness, and disgust. Am J Psychiatry. 1997;54:926–933. doi: 10.1176/ajp.154.7.926. [DOI] [PubMed] [Google Scholar]
- 17.Schmahmann JD, Pandya DN. The cerebrocerebellar system. Int Rev Neurobiol. 1997;41:31–60. doi: 10.1016/s0074-7742(08)60346-3. [DOI] [PubMed] [Google Scholar]
- 18.Robbins TW, Everitt BJ. Neurobehavioural mechanisms of reward and motivation. Curr Opin Neurobiol. 1996;6:228–236. doi: 10.1016/s0959-4388(96)80077-8. [DOI] [PubMed] [Google Scholar]
- 19.Francis S, Rolls ET, Bowtell R, et al. The representation of pleasant touch in the brain and its relationship with taste and olfactory areas. Neuroreport. 1999;10:453–459. doi: 10.1097/00001756-199902250-00003. [DOI] [PubMed] [Google Scholar]
- 20.Gordon CM, Dougherty D, Rauch SL, et al. Neuroanatomy of human appetitive function: A positron emission tomography investigation. Int J Eat Disord. 2000;27:163–171. doi: 10.1002/(sici)1098-108x(200003)27:2<163::aid-eat4>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 21.Kendrick KM, Dixson AF. Ovariectomy does not abolish proceptive behaviour cyclicity in the common marmoset (Callithrix jacchus) J Endocrinol. 1984;101:155–162. doi: 10.1677/joe.0.1010155. [DOI] [PubMed] [Google Scholar]
- 22.Kimbrell TA, George MS, Parekh PI, et al. Regional brain activity during transient self-induced anxiety and anger in healthy adults. Biol Psychiatry. 1999;46:454–465. doi: 10.1016/s0006-3223(99)00103-1. [DOI] [PubMed] [Google Scholar]
- 23.Edwards DA, Einhorn LC. Preoptic and midbrain control of sexual motivation. Physiol Behav. 1986;37:329–335. doi: 10.1016/0031-9384(86)90242-8. [DOI] [PubMed] [Google Scholar]
- 24.Rizvi TA, Ennis M, Shipley MT. Reciprocal connections between the medial preoptic area and the midbrain periaqueductal gray in rat: a WGA-HRP and PHA-L study. J Comp Neurol. 1992;315:1–15. doi: 10.1002/cne.903150102. [DOI] [PubMed] [Google Scholar]
- 25.Murphy AZ, Hoffman GE. Distribution of gonadal steroid receptor-containing neurons in the preoptic-periaqueductal gray-brainstem pathway: A potential circuit for initiation of male sexual behavior. J Comp Neurol. 2001;438:191–212. doi: 10.1002/cne.1309. [DOI] [PubMed] [Google Scholar]
- 26.Graeff FG, Silveira Filho NG. Behavioral inhibition induced by electrical stimulation of the median raphe nucleus on the rat. Physiol Behav. 1978;21:477–484. doi: 10.1016/0031-9384(78)90116-6. [DOI] [PubMed] [Google Scholar]
- 27.Thiébot MH, Jobert A, Soubrié P. Chlordiazepoxide and GABA injected into the raphe dorsalis release the conditioned behavioural suppression induced in rats by a conflict procedure without nociceptive component. Neuropharmacology. 1980;19:633–641. doi: 10.1016/0028-3908(80)90037-4. [DOI] [PubMed] [Google Scholar]
- 28.Flectcher PJ, Tampakeras M, Yeomans JS. Median raphe injections of 8-OH-DPAT lower frequency thresholds for lateral hypothalamic self-stimulation. Pharmacol Biochem Behav. 1995;52:65–71. doi: 10.1016/0091-3057(94)00441-k. [DOI] [PubMed] [Google Scholar]
- 29.Hillegaart V, Ahlenius S, Larsson K. Effects of local application of 5-HT into the median and dorsal raphe nuclei on male rat sexual and motor behavior. Behav Brain Res. 1991;33:279–286. doi: 10.1016/s0166-4328(89)80122-6. [DOI] [PubMed] [Google Scholar]
- 30.Bartels A, Zeki S. The neural basis of romantic love. Neuroreport. 2000;27:3829–3834. doi: 10.1097/00001756-200011270-00046. [DOI] [PubMed] [Google Scholar]
- 31.Menon RS, Ogawa S, Strupp JS, Andersen P, Ugurbil K. BOLD-based functional MRI at 4 Tesla includes a capillary bed contribution: echo-planar imaging correlates with previous optical imaging using intrinsic signals. Magn Reson Med. 1995;33:453–459. doi: 10.1002/mrm.1910330323. [DOI] [PubMed] [Google Scholar]
- 32.Buxton RB, Wong EC, Frank LR. Dynamics of blood flow and oxygenation changes during brain activation: the balloon model. Magn Reson Med. 1998;39:855–864. doi: 10.1002/mrm.1910390602. [DOI] [PubMed] [Google Scholar]
- 33.Duong TQ, Kim DS, Ugurbil K, Kim SG. Spatiotemporal dynamics of the BOLD fMRI signals: toward mapping submillimeter cortical columns using the early negative response. Magn Reson Med. 2000;44:231–242. doi: 10.1002/1522-2594(200008)44:2<231::aid-mrm10>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 34.Mandeville JB, Marota JJA, Ayata C, Moskowitz MA, Weisskoff RM, Rosen BR. MRI measurement of the temporal evolution of relative CMRO(2) during rat forepaw stimulation. Magn Reson Med. 1999;42:944–951. doi: 10.1002/(sici)1522-2594(199911)42:5<944::aid-mrm15>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 35.Krüger G, Kastrup A, Takahashi A, Glover GH. Simultaneous monitoring of dynamic changes in cerebral blood flow and oxygenation during sustained activation of the human visual cortex. Neuroreport. 1999;10:2939–2943. doi: 10.1097/00001756-199909290-00012. [DOI] [PubMed] [Google Scholar]
- 36.Czisch M, Wetter TC, Kaufmann C, Pollmacher T, Holsboer F, Auer DP. Altered processing of acoustic stimuli during sleep: reduced auditory activation and visual deactivation detected by a combined fMRI/EEG study. Neuroimaging. 2002;16:251–258. doi: 10.1006/nimg.2002.1071. [DOI] [PubMed] [Google Scholar]
- 37.Born AP, Rostrup E, Miranda MJ, Larsson HB, Lou HC. Visual cortex reactivity in sedated children examined with perfusion MRI (FAIR) Magn Reson Imaging. 2002;20:199–205. doi: 10.1016/s0730-725x(02)00469-1. [DOI] [PubMed] [Google Scholar]


