Abstract
Axonal elongation and guidance are controlled by extracellular factors such as the neurotrophins. Indeed, nerve growth factor (NGF) seems to promote axon growth through binding to its p75NTR receptor and inactivating RhoA. Furthermore, the local inhibition of glycogen synthase kinase (GSK)-3β by NGF also favors microtubule polymerization and axon extension. Inactivation of GSK-3β may be due to the NGF/TrkA-mediated activation of phosphatidylinositol-3 kinase (PI-3 kinase), which increases the levels of phosphatydilinositol 3-phosphate [PI(3)P]. However, we show here that NGF may inactivate GSK-3β through an alternative mechanism. In cultured hippocampal neurons, the capacity of NGF to promote axon elongation is mostly mediated by p75NTR, and the activation of this pathway leads to the inactivation of GSK-3β. However, the signaling pathway triggered by NGF/p75NTR acts through casein kinase II (CK2). NGF/p75NTR-activated CK2 phosphorylates the phosphatase and tensin homologue deleted on chromosome 10 (PTEN), thus rendering this phosphatase inactive. Like activation of the PI-3 kinase, PTEN inactivation allows PI(3)P levels to increase, thus favoring GSK-3β inactivation and axon outgrowth. This newly disclosed mechanism may help to extend the repertoire of pharmacological agents that activate CK2 or that inhibit PTEN to stimulate axon regeneration after trauma or disease.
INTRODUCTION
Neurons are highly polarized cells that display a very varied structural and functional specialization. At the cellular level, neuronal polarity is principally based on the fact that axons send signals, whereas dendrites receive them. Indeed, this is essential to ensure the flow of information within the nervous system and between the nervous system and other tissues. Although all neurites initially have the potential to become an axon, only when one of the multiple neurites begins to grow rapidly does specialization commence. This fast-growing neurite will become the future axon, the other neurites will continue to develop as dendrites (Bradke and Dotti, 2000). Both the maintenance of axon identity and the promotion of axonal growth and guidance are controlled by extracellular cues. Such cues may be derived from either diffusible factors or from membrane-associated ligands. Thus, netrins, slit, semaphorins, ephrins, Wnts, sonic hedgehog, bone morphogenetic proteins, insulin and insulin-like growth factor (IGF)-I and neurotrophins all either promote or impair axonal outgrowth.
Some of the extracellular factors that promote axonal growth, such as insulin and IGF-I, Wnts, and neurotrophins, do so by inhibiting glycogen synthase kinase (GSK)-3β. Local inactivation of GSK-3β in an immature neurite suffices to specify an axon (Yoshimura et al., 2005). Furthermore, inactivation of GSK-3β causes dephosphorylation of certain microtubule binding proteins, such as collapsin response mediator protein 2 and adenomatous polyposis coli. In their nonphosphorylated state, these proteins bind to α- and β-tubulin with higher affinity, thereby favoring microtubule polymerization and axon extension (Yoshimura et al., 2005; Zhou et al., 2004; Zhou and Snider, 2005). One means to inactivate GSK-3β is through Ser9 phosphorylation by Akt/PKB (Cross et al., 1995). This kinase itself is activated upon its phosphorylation by the phosphoinositide-dependent kinase. Indeed, the whole process is dependent on the levels of the phosphatydilinositol 3-phosphate [PI(3)P] pool that is generated by activated phosphatidylinositol 3-kinase (PI-3 kinase) (Alessi et al., 1996; Stokoe et al., 1997; Stephens et al., 1998). It is therefore highly relevant that the Trk receptors activated by neurotrophins can themselves in turn activate PI-3 kinase (Segal, 2003; Vaillant et al., 1999; Zhou et al., 2004). Hence, it seems likely that neurotrophin/Trk activated PI-3 kinase is an upstream element in the inhibition of GSK-3β, which would constitute a molecular correlate for NGF-promoted axonal growth (Zhou et al., 2004).
In this communication, we show that NGF promotes axonal outgrowth in cultured hippocampal neurons by inhibiting GSK-3β after the activation of Akt/PKB via its p75NTR receptor. The interaction of NGF with p75NTR activates casein kinase II (CK2), which in turn inactivates the phosphatase and tensin homolog deleted on chromosome10 (PTEN), a phosphatase that antagonizes the PI-3 kinase pathway (Maehama and Dixon, 1998; Maehama and Dixon, 1999; Stiles et al., 2004). In this way, PI(3)P levels increase favoring the inhibition of GSK-β.
MATERIALS AND METHODS
Transfection Vectors
The HA-GSK-3β S9A/pcDNA3.1 vector that contains a constitutively active form of GSK-3β was generously provided by Dr. J. R. Woodgett (Ontario Cancer Institute, Princess Margaret Hospital, Toronto, Ontario, Canada). The pEGFP-N1 vector to express enhanced green fluorescent protein (EGFP) was obtained from Clontech (Palo Alto, CA).
Antibodies
The primary antibodies used in this study were anti-p75NTR rabbit polyclonal antiserum (Advances Targeting Systems, San Diego, CA) used at a dilution of 1:100; anti-phosphorylated neurofilament monoclonal antibody (mAb) SMI31 (Sternberger Monoclonals, Lutherville, MA) used at a dilution of 1:500; anti-hemagglutinin (HA) mAb 12CA5 (Roche Diagnostics, Indianapolis, IN) used at a dilution of 1:500; rabbit anti-green fluorescent protein (GFP) antiserum (Molecular Probes Europe, Leiden, Holland) used at a dilution of 1:500, anti-GSK-3β mAb (BD Transduction Laboratories, Lexington, KY) used at a dilution of 1:10,000; rabbit anti-phospho-GSK-3α/β Ser21/9 (Cell Signaling Technology, Beverly, MA) antiserum used at a dilution of 1:2500; rabbit anti-phospho-Akt Ser473 antiserum (Cell Signaling Technology) used at a dilution of 1:2500; a rabbit anti-Akt antiserum used at a dilution of 1:5000 dilution (Cell Signaling Technology); and a rabbit anti-phospho-PTEN Ser380 antiserum (Cell Signaling Technology) used at a dilution of 1:5000. The anti-unique β-tubulin mAb TUJ1 was used at a dilution of 1:2000 (Berkeley Antibody, Richmond, CA). All the secondary antibodies were obtained from Jackson ImmunoResearch Laboratories (West Grove, PA).
Chemicals
NGF was purified from mouse salivary glands and was a gift from Hermann Rohrer (Max Planck Institute for Brain Research, Frankfurt, Germany). 1-Oleyl-9-(cis)-2-lyso-sn-glycero-3 phosphate (LPA) was from Sigma-Aldrich (St. Louis, MO). The K252a inhibitor of membrane tyrosine kinases, the specific CK2 inhibitor 2-dimethylamino-4,5,6,7-tetrabromo-1H-benzimidazole (DMAT) (Pagano et al., 2004), and the PTEN inhibitor dipotassium bisperoxo(picolinato) oxovanadate (V) [bpV(pic)] (Schmid et al., 2004) were all purchased from Calbiochem (San Diego, CA).
Hippocampal Neuronal Cultures, Neuron Transfection, and Incubation Conditions
The hippocampus was dissected out from embryonic day 17 mouse embryos and dissociated to single cells after digestion with trypsin (Worthington Biochemicals, Freehold, NJ) and DNase I (Sigma-Aldrich) (Goslin and Banker, 1989). To determine axon length, neurons were plated on glass coverslips coated with poly-l-lysine (Sigma-Aldrich) at a density of 150–300 neurons/mm2, and they were cultured in Neurobasal A supplemented with B-27 and GlutaMAX I (Invitrogen, Crewe, United Kingdom). After 1–2 days in vitro (DIV), cultured neurons were transfected using the Effectene transfection reagent (QIAGEN, Hilden, Germany) and treated with either 100 ng/ml NGF, anti-p75-antibody (1:100), or 10 μM LPA for 16 h. The neurons were then processed for immunostaining.
In other experiments, neurons were plated on coated plastic dishes (see above) and after 7 DIV, the medium was replaced by fresh medium devoid of B27 supplement for 2 h before the cells were exposed to either 100 ng/ml NGF, anti-p75-antibody (1:100), 200 nM K252a, 200 nM bpV(pic), or 10 μM DMAT for 1 h. The cells were then lysed in lysis buffer (20 mM HEPES, pH 7.5, 100 mM NaCl, 100 mM NaF, 1 mM Na3VO4, 5 mM EDTA, 1% Triton X-100, 100 nM okadaic acid, and an anti-protease cocktail; Roche Diagnostics, Mannheim, Germany), and the cells were scraped and recovered from the culture dish. The suspension was maintained on ice for 10 min and then centrifuged at 15,000 × g for 15 min at 4°C. The supernatant was used for Western analysis and in kinase assays after estimating the protein content using the DC&RC method (Bio-Rad, Munich, Germany).
Western Blot Analysis
Equal amounts of protein (10–20 μg) were resolved by SDS-PAGE on 10% acrylamide gels after diluting the samples in loading buffer and heating them to 100°C for 3 min. After electrophoresis, the proteins were transferred to nitrocellulose membranes that were incubated in blocking buffer (Tris-buffered saline with 0.1% Tween 20 [Sigma-Aldrich], and 5% nonfat dry milk). The membranes were subsequently probed with primary antibodies and then incubated with the corresponding peroxidase-conjugated secondary antibodies. Immunoreactivity was visualized using an enhanced chemiluminescence detection system (GE Healthcare, Little Chalfont, Buckinghamshire, United Kingdom) and the intensity of the bands was evaluated by densitometric analysis using Quantity One software (Bio-Rad).
Endogenous GSK-3β and CK2 Activity in Cell Lysates
GSK-3β assays were performed essentially as described previously (Sayas et al., 1999). Cells were collected and homogenized in lysis buffer and the soluble fraction was used for the kinase assays. Protein samples (5 μg) were incubated in buffer (25 mM Tris, pH 7.5, 1 mM dithiothreitol, and 10 mM MgCl2) containing the specific substrate peptide 2B-SP at a final concentration of 0.75 mg/ml (Welsh et al., 1997), and in the presence of [γ-32P]ATP (50 μl final reaction volume). After 40 min at 30°C, the reaction was stopped by spotting aliquots onto P81 phosphocellulose paper and the radioactivity was measured by scintillation counting. The difference between the kinase activity in the presence or absence of 20 mM LiCl was considered as the measure of GSK-3 protein kinase activity.
CK2 activity was assayed using a casein kinase 2 assay kit (Upstate Biotechnology, Lake Placid, NY). The specific peptide R3D3SD3 (1 mM) was added to cleared lysate containing 2.5–5 μg of protein in the presence of [γ-32P]ATP and a PKA inhibitor cocktail (50 μl total reaction volume). After 15 min at 30°C, the reaction was stopped by adding 20 μl of 40% trichloroacetic acid, and the phosphorylated substrate was separated from the residual [γ-32P]ATP using P81 phosphocellulose paper. The radioactivity incorporated into the substrate was quantified by scintillation counting.
Image Acquisition and Morphometric Analysis of Immunolabeled Hippocampal Neurons
Labeled neurons were observed under a Zeiss Axiophot 2 microscope, using a plan-neofluor 40× oil objective with a numerical aperture of 1.3 (Carl Zeiss, Oberkochen, Germany). Images of ∼20 neurons per coverslip were acquired digitally (camera DP-10; Olympus, Tokyo, Japan) and imported to the final figures that were generated using CorelDraw 12 (Corel, Ottawa, Ontario, Canada). To measure axon length we used the ImageJ public domain software (developed at the National Institutes of Health, Bethesda, MD). Only neurons with a defined axon were evaluated, and axons were recognized by SMI31 antibody immunoreactivity.
RESULTS
NGF Induces Axonal Elongation through the Activation of p75NTR
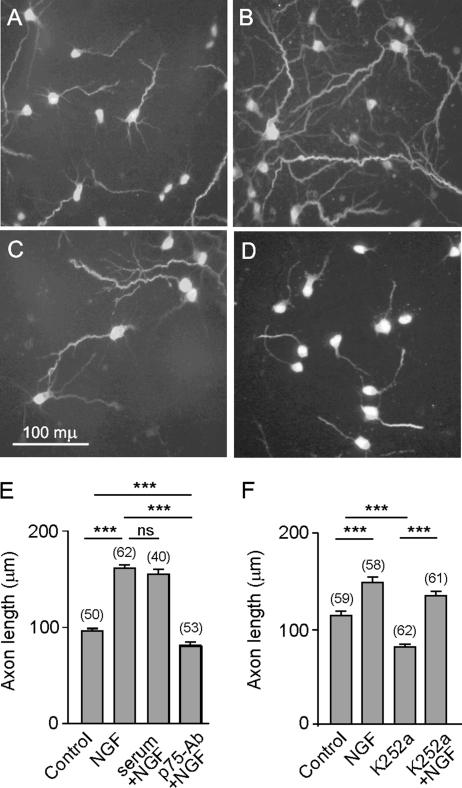
After 2 DIV, most hippocampal neurons from E17 mouse embryos are to a large extent polarized (Dotti et al., 1988; Goslin and Banker, 1989). By this stage, one of their processes has acquired axonal identity, usually the longest, and is recognized by the SMI31 antibody (Pennypacker et al., 1991) (Figure 1, A–D). In our experimental model, incubation of 2 DIV neurons with NGF for 16 h increased the length of axons by 35–80% (Figure 1, A, B, E, and F). Because TrkA mediates NGF-promoted axon elongation in other model systems (Kuruvilla et al., 2004; Goold and Gordon-Weeks, 2005), we assessed the role of p75NTR in NGF-promoted axon elongation in hippocampal neurons. To this end, the neurons were exposed to an anti-p75NTR antiserum before applying NGF to the cultures. This antiserum is raised against the extracellular domain of p75NTR, and it blocks the binding of NGF to the receptor (Huber and Chao, 1995). Although the antibody itself did not affect axon growth (our unpublished data), impairing NGF binding to p75NTR largely prevented axonal elongation (Figure 1, C and E). The presence of rabbit nonimmune serum did not prevent the axonal growth induced by NGF (Figure 1E). Furthermore, although the presence of K252a, a known Trk inhibitor, significantly reduced axon length, it did not prevent the axon growth promoted by NGF (Figure 1, D and F). Therefore, our results indicated that in hippocampal neurons, p75NTR mediates the axon growth promoted by NGF.
Figure 1.
NGF induced axonal elongation is mediated via p75NTR in hippocampal neurons. Cells were plated onto glass coverslips at a density of 150 neurons/mm2. After 2 DIV, NGF or NGF plus the p75-antibody or NGF plus 1% rabbit neutral serum was added to the cells and incubated for a further 16 h. In parallel experiments, the Trk inhibitor K252a was added to the cells in the absence or presence of NGF and incubated for a further 16 h. Digital fluorescence images of phosphorylated neurofilament immunostaining by SMI31 mAb in control cultures (A), 100 ng/ml NGF (B), 100 ng/ml NGF plus p75-antibody antiserum, diluted 1:100 (C); or 200 nM K252a (D). The means, SE, and number of the neurons observed (in brackets) for each treatment are shown (E and F). Note that NGF induced axon elongation and the application of the anti-p75NTR blocking antibody prevented such an effect, whereas the application of the neutral rabbit serum did not (E). In contrast, K252a did not prevent the axon growth promoted by NGF, although, by itself, decreased axon length (F). Here and in the following figures, the levels of significance (asterisks) were determined for the data sets connected by horizontal lines using an unpaired t test. *p < 0.05, **p < 0.01, and ***p < 0.001.
Role of GSK-3β in the NGF/p75NTR-induced Axon Growth
Previous studies have shown that p75NTR controls axonal elongation by activating or inactivating the RhoA GTPase, depending on the ligand that binds to the receptor (Wong et al., 2002; Yamashita et al., 2002; Yamashita and Tohyama, 2003; Gehler et al., 2004). However, in view of the evidence that NGF may promote axon elongation by inhibiting GSK-3β, we wanted to know whether an alternative signaling pathway to promote axon elongation might be triggered by p75NTR (Zhou et al., 2004; Jiang et al., 2005; Yoshimura et al., 2005). Therefore, we examined whether p75NTR might mediate axon elongation by inhibiting GSK-3β activity after NGF activation.
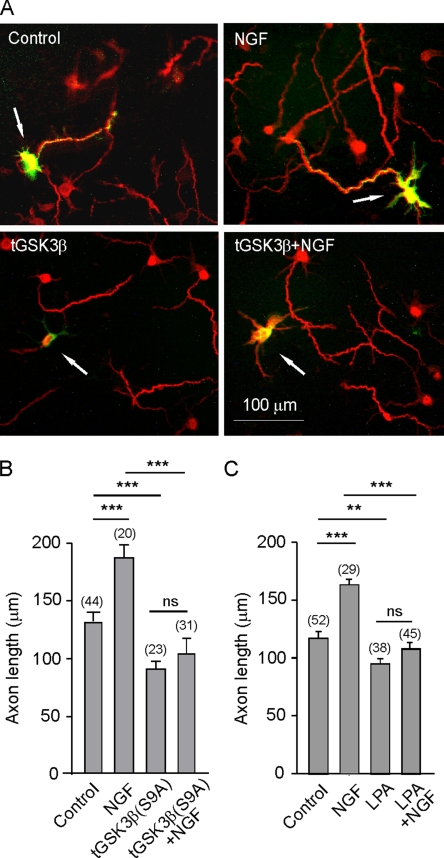
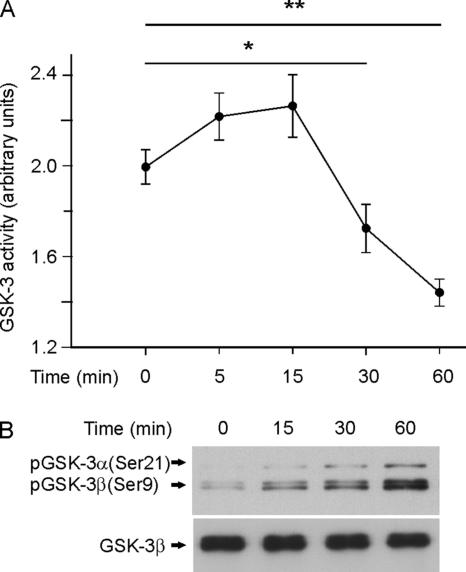
To determine whether the participation of p75NTR in axon elongation of hippocampal neurons involves GSK-3β, we transfected a constitutively active form of GSK-3β (GSK-3βS9A) into cultured neurons (Doble and Woodgett, 2003). These transfected neurons grew shorter axons, and the application of NGF did not produce any effect in terms of axonal growth (Figure 2, A and B). These results suggested that NGF does indeed induce axonal growth by inactivating GSK-3β. To further assess the role of NGF/p75NTR signaling in axon elongation, we measured the axonal length of cultured neurons in the presence of NGF and LPA, a known activator of GSK-3β (Sayas et al., 1999, 2002). LPA alone not only inhibited axonal growth but also prevented NGF from promoting axonal growth (Figure 2C), suggesting that this axon growth stimulated by NGF occurred through the inactivation of the GSK-3β. To examine this phenomenon more closely, we first carried out a time course of kinase activity in protein extracts of neurons after NGF application. Thus, a significant inactivation of GSK-3 activity was observed 30 min after treatment NGF (Figure 3A), which was maximal at 60 min. This inactivation of GSK-3 activity was paralleled by the NGF-induced phosphorylation of GSK-3β at its Ser9 residue, which is known to inhibit this enzyme (Figure 3, A and B).
Figure 2.
GSK-3β activation reduced axon length and prevented NGF-induced axonal growth. (A and B) Control cultures were transfected with EGFP, whereas in parallel cultures, neurons were cotransfected with vectors expressing EGFP and GSK-3β S9A, a constitutively active form of GSK-3β. Some cells were treated with NGF, and after 16 h, the cells were immunostained with the axon marker SMI31 and with anti-EGFP to detect the cotransfected proteins (arrows). Axon length was determined in EGFP-transfected and in EGFP plus GSK-3β S9A-cotransfected in the absence or in the presence of NGF (B). Note that NGF was incapable of increasing axon length in cells transfected with the constitutively active form of GSK-3β. (C) Cultures of hippocampal neurons were treated at 2 DIV with NGF, LPA (an activator of GSK-3β), or both during 16 h and then fixed and stained with the axon marker SMI31. Note that the axon growth in cells in which GSK-3β was stimulated was similar to that in those transfected with a constitutive enzyme.
Figure 3.
Time course of NGF-induced inactivation of GSK-3. (A) GSK-3 (α + β) activity was determined in cytosolic extracts from 7 DIV cultures of hippocampal neurons treated with 100 ng/ml NGF for the times reflected in the graph. (B) Western blots analysis of the same extracts with anti-phospho-GSK-3α/β (Ser21/9) (top) and anti-GSK-3β (bottom) antibodies. Note that the inactivation of GSK-3 correlates with the levels of Ser9 phosphorylation.
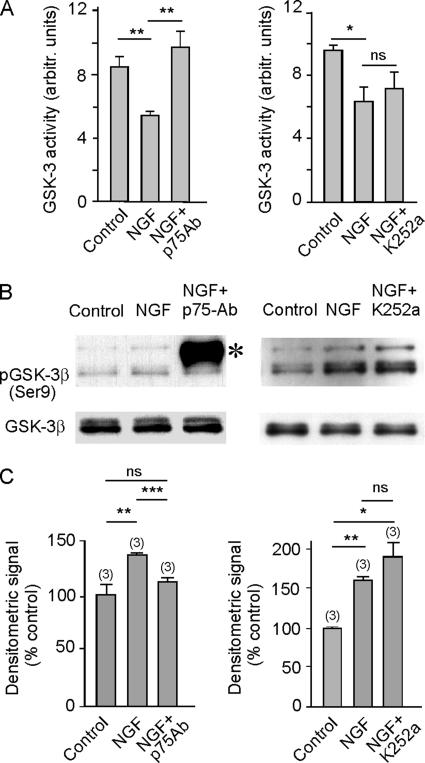
To determine through which receptor NGF acts when influencing axonal growth, GSK-3 activity was measured directly in the presence of NGF, NGF plus the anti-p75NTR blocking antibody, and NGF plus the Trk receptor inhibitor K252a. The decrease of GSK-3 activity induced by NGF was inhibited by the presence of the anti-p75 blocking antibody (Figure 4A), whereas K252a did not affect the influence exerted by NGF on enzyme activity. Furthermore, these changes in GSK-3 activity were paralleled by changes in Ser9 phosphorylation (Figure 4, B and C). Together, our results indicate that the activity of NGF in promoting axonal growth is triggered by the binding of this neurotrophin to the p75NTR receptor and that this effect is mediated by the inhibition of GSK-3β.
Figure 4.
NGF inactivates GSK-3β via p75NTR. (A) GSK-3 activity was determined in extracts from cultured hippocampal neurons that were incubated for 1 h in the presence or absence of 100 ng/ml NGF, 100 ng/ml NGF plus anti-p75NTR blocking antibody (1:100 diluted), or 100 ng/ml NGF plus K252a, a Trk inhibitor. Observe that the blockage of p75NTR prevented the NGF-induced inhibition of the kinase, whereas the inhibition of Trk receptors by K252a did not affect the inhibition of GSK-3 by NGF. (B) Western blot analysis of the same samples with anti-phospho-GSK-3α/β (Ser21/9) (top) and anti-GSK-3β (bottom) antibodies. The asterisk indicates the band corresponding to the heavy chain of the anti-p75 antibody. Note that in the presence of the p75-antibody, NGF did not induce Ser9 phosphorylation of the enzyme, whereas inhibition of the Trk receptor by K252a did not change the NGF-induced phosphorylation pattern. (C) Densitometric quantification of Western blots from three different experiments.
NGF Controls PTEN Activity through p75NTR
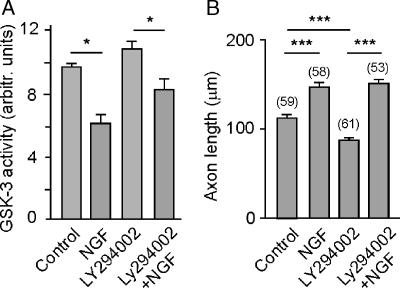
Binding of neurotrophins to their specific Trk receptors strong stimulates both the MAPK and the PI-3 kinase pathways (Segal, 2003). Of these, PI-3 kinase has been shown to be involved in axon morphogenesis and in the inactivation of GSK-3β (Segal, 2003; Zhou et al., 2004; Zhou and Snider, 2005). In cultured hippocampal neurons, we determined whether NGF could still inactivate GSK-3 when the PI-3 kinase was specifically inhibited by LY294002. When GSK-3 activity in neurons was measured directly in the presence of LY294002, NGF was still capable of inhibiting the activity of the kinase significantly (Figure 5A). These results suggest that NGF-induced inhibition of GSK-3β was at least partially independent of PI-3 kinase. We then studied the effects of NGF on axonal growth in short-term cultures of neurons treated with LY294002. Thus, as shown in Figure 5B, NGF induced axon growth under conditions of PI-3 kinase inhibition. Together, our results strongly suggest that NGF-induced axon elongation and GSK-3 inhibition are not dependent on PI-3 kinase activation. Therefore, we considered whether a phosphatase that acts on PI(3)P might be involved in the events induced by NGF activation of p75NTR, the inhibition of which may lead to GSK-3β inactivation and the subsequent elongation of the axon.
Figure 5.
NGF inactivates GSK-3β through a pathway different from PI-3 kinase activation. (A) GSK-3 activity was determined in extracts from cultured hippocampal neurons that were incubated for 1 h in the presence or absence of 100 ng/ml NGF, LY294002, a known inhibitor of PI-3 kinase, and 100 ng/ml NGF plus LY294002. Note that in the presence of the PI-3 kinase inhibitor, NGF was still capable of inhibiting GSK-3, suggesting an alternative pathway for NGF-induced GSK-3β inhibition. (B) NGF induced axon elongation is not prevented by inhibition of PI-3 kinase. Note that axon growth corresponds to the inhibition state of GSK-3.
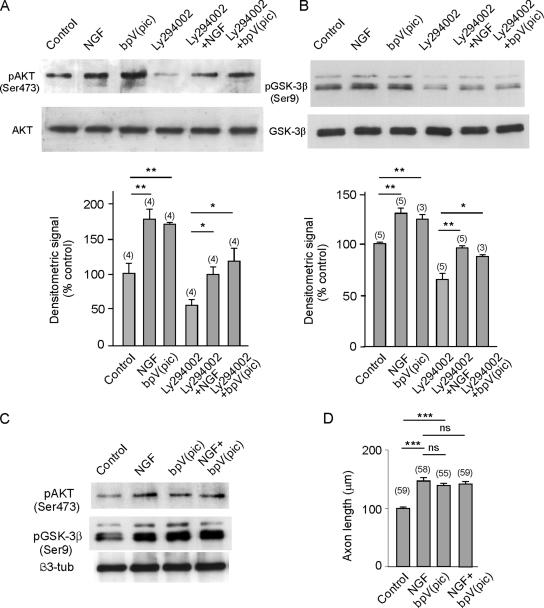
PTEN has been related with both GSK-3β activity and neuronal polarity (Jiang et al., 2005). To test the involvement of PTEN as an upstream element in GSK-3β signaling, cultured hippocampal neurons were treated with bpV(pic), which specifically inhibits PTEN at low concentrations (Schmid et al., 2004), and the phosphorylation of Akt/PKB (in Ser473) was determined by Western blotting. The phosphorylation of Akt/PKB reflects the levels of PI(3)P in the cytoplasm of the cultured neurons. Exposing the cultured neurons to bpV(pic) increased the phosphorylation of Akt/PKB to levels similar to those produced by NGF (Figure 6, A and C). As expected, LY294002 largely depressed the phosphorylation of the kinase, which was partially restored by applying NGF to the medium. Thus, the application of a pharmacological inhibitor of PTEN caused the same effect on Akt/PKB phosphorylation as the application of NGF, suggesting that this neurotrophin also inhibits the phosphatase. Indeed, under all experimental conditions the phosphorylation of Akt/PKB was paralleled by that of GSK-3β (Figure 6, A and B), suggesting that Akt/PKB phosphorylates and inhibits GSK-3β. Furthermore, these results indicate that the inhibition of GSK-3β by NGF may at least in part be due to the inhibition of PTEN by NGF. As shown above, both NGF and bpV(pic) induce phosphorylation of Akt and GSK-3β. Consistently, bpV(pic), like NGF, induced axon elongation (Figure 6F). Neither in inducing phosphorylation of Akt and GSK-3β nor in promoting axon growth had NGF and the phosphatase inhibitor additive effects (Figure 6, E and F).
Figure 6.
The PTEN phosphatase lies in the pathway leading to NGF-induced activation of Akt/PKB and inhibition of GSK-3β. Cultures of hippocampal neurons were treated with either 100 ng/ml NGF, bpV(pic) (a selective inhibitor of PTEN phosphatase), or LY296002 (an inhibitor of PI-3 kinase) in different combinations, and the cytosolic extracts were then analyzed for Akt, p-Akt (A), and quantified by densitometry. The same extracts were used to analyze GSK-3β and p-GSK-3β by Western blotting (B), and its densitometric quantification is shown in B. NGF and bpV(pic) were used in combination, and they did not show additive effects in terms of Akt and GSK-3β phosphorylation (C) and axon elongation (D).
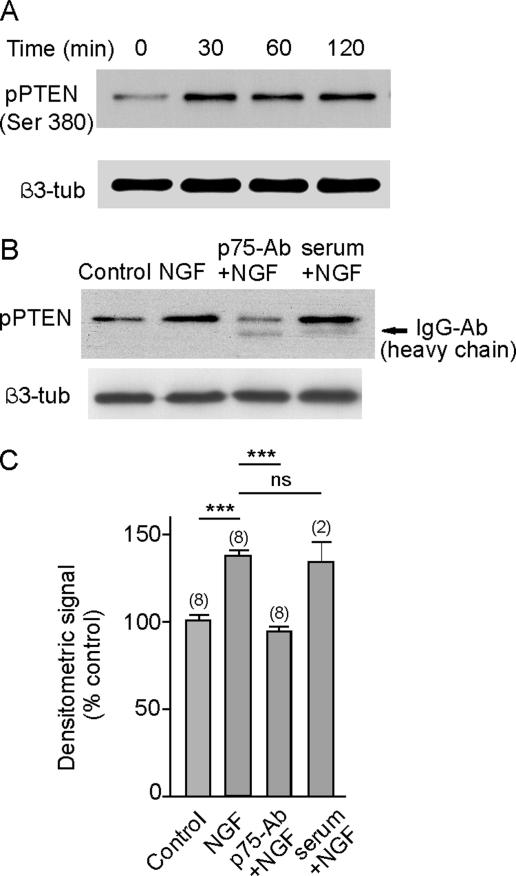
We further tested the relationship between NGF signaling and the activity of the PTEN phosphatase by applying NGF to cultured hippocampal neurons and assessing the phosphorylation of PTEN at Ser 380 (Figure 7A). Phosphorylation of this residue was observed after 30-min incubation in the presence of NGF, and it was maintained for at least 2 h. The increase of PTEN phosphorylation induced by NGF was mediated through p75NTR because prior addition of the anti-p75 blocking antibody prevented PTEN phosphorylation (Figure 7, B and C). As a control, the application of rabbit nonimmune serum did not prevent PTEN phosphorylation promoted by NGF (Figure 7, B and C). These results demonstrate the relationship between NGF/p75NTR and PTEN phosphorylation and in conjunction with the results described above, strongly suggest that this phosphorylation has an inhibitory effect on phosphatase activity.
Figure 7.
NGF induces phosphorylation of PTEN phosphatase. (A) Maximum phosphorylation was achieved 30 min after the application of NGF to the cultured hippocampal neurons. (B) Western blot analysis showing that prior application of the anti-p75 blocking antibody to cultured neurons prevented NGF-induced phosphorylation of PTEN phosphatase, whereas the addition of neutral rabbit serum did not. Quantitative results are the average of (n) different experiments, shown in C.
CK2 Mediates NGF-induced Axonal Growth
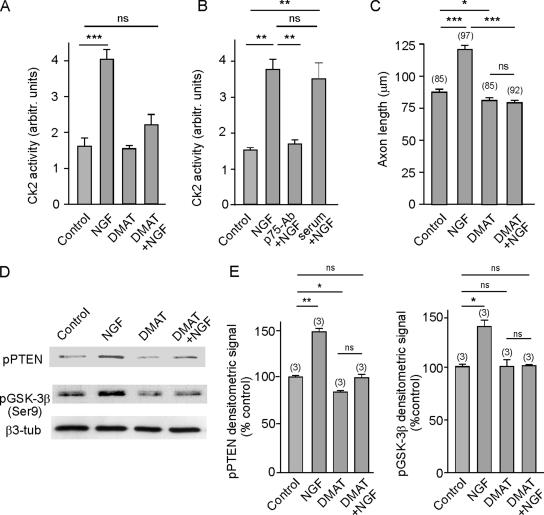
CK2 phosphorylates PTEN (Torres and Pulido, 2001), and because NGF and EGF stimulate CK2 activity in PC12 cells (Kleijn et al., 1998), we determined whether this kinase might be involved in NGF/p75NTR signaling. To test whether NGF might affect CK2 in hippocampal neurons, we assessed the activity of this kinase in cytosolic extracts from cultured hippocampal neurons previously incubated with either NGF and/or DMAT (a specific CK2 inhibitor; Pagano et al., 2004). Direct measurement of CK2 activity clearly showed that this kinase was activated by exposure to NGF and that this activation could be prevented by prior application of DMAT (Figure 8A). Furthermore, the NGF-induced activation of CK2 was also prevented in the presence of the anti-p75NTR blocking antibody but not by the presence of rabbit nonimmune serum (Figure 8B). Moreover, in our assay CK2 activity was abolished in the presence of heparin in the kinase reaction mixture, a known inhibitor of the CK2 (Hathaway et al., 1980). This served as a positive control for the assay and ensured that only genuine CK2 activity was measured (controls not shown). Moreover, a correlation between NGF-induced CK2 activity and NGF-induced axonal outgrowth was also found. Thus, Figure 8C shows that the inhibition of CK2 by DMAT counteracted the axonal growth induced by NGF.
Figure 8.
NGF-activated CK2 participates in NGF/p75NTR signaling, leading to PTEN and GSK-3β phosphorylation and subsequent axonal growth. (A) CK2 activity was determined from cytosolic extracts obtained from cultured hippocampal neurons. Cultures were incubated for 30 min in the presence or absence of NGF, DMAT (a specific CK2 inhibitor), or NGF plus DMAT. Observe that NGF augmented the kinase activity, and this increase was prevented by the presence of DMAT, highlighting the specificity of NGF-induced CK2 activity. (B) NGF activation of CK2 is mediated by p75NTR. Cultured neurons were incubated for 30 min either in the presence or absence of 100 ng/ml NGF or of NGF plus anti-p75-antibody blocking antibody. Note that there is a significant increase of CK2 activity upon exposure of the cells to NGF, and this effect is neutralized by the presence of the antibody. Also note that the inclusion of a rabbit neutral serum did not block NGF activity. (C) Inhibition of CK2 by DMAT prevented NGF-promoted axon elongation. (D) NGF-induced CK2 activation correlates with the NGF-induced phosphorylation of PTEN and Ser9 phosphorylation of GSK-3β. Staining for β3-tubulin was included as a loading control. Observe that NGF did not increase the Ser9 phosphorylation of GSK-3β when CK2 was inhibited by DMAT. (E) Quantification of the densitometric signals from D are the average of three independent experiments.
Both phosphorylation of the PTEN phosphatase at Ser380 (presumably inhibitory; see Discussion) and of GSK-3β at Ser9 (certainly inhibitory) were also assayed in parallel (Figure 8, D and E). Consistent with previous data, exposure to NGF augmented the phosphorylation of both enzymes. In contrast, the phosphorylation of both enzymes diminished in the presence of the CK2 inhibitor DMAT, both in neurons grown in the presence or the absence of NGF. Together, our results reveal a new pathway for NGF/p75NTR that controls the activity of the PTEN phosphatase through CK2 activation, this phosphatase lying upstream of GSK-3β in this signaling cascade.
DISCUSSION
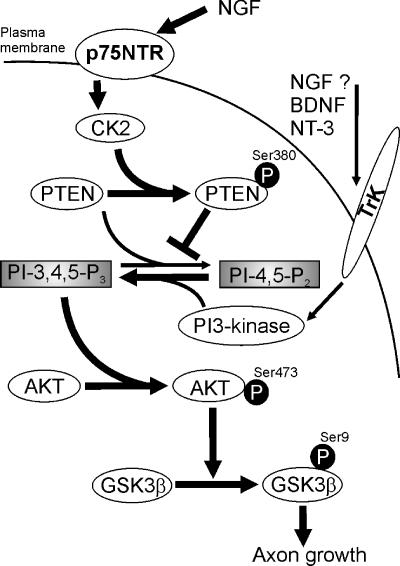
We have shown here that via p75NTR, NGF stimulates axonal elongation in cultured hippocampal neurons by inhibiting GSK-3β activity. More importantly, in our experimental system the NGF/p75NTR induced inhibition of GSK-3β was not entirely mediated through the activation of PI-3 kinase but rather by NGF/p75NTR-induced inhibition of PTEN phosphatase. Thus, it seems most likely that the inhibition of the PTEN phosphatase increases the levels of phosphoinositol-3 phosphate to a state necessary to activate Akt/PKB and therefore to phosphorylate GSK-3β. Our data reveal a new signaling pathway by which NGF/p75 NTR inhibits GSK-3β, thus promoting axon elongation (Figure 9).
Figure 9.
Diagram depicting the mechanism by which NGF may inhibit GSK-3β and promote axon growth. The binding of NGF to p75 activate CK2 via an unknown process. Activated CK2 phosphorylates PTEN phosphatase, thus rendering it inactive and unable to hydrolyze PI3P. A high concentration of this secondary messenger phosphorylates and activates Akt/PKB, which phosphorylates and inhibits GSK-3β. The better known pathway to increase PI(3)P concentrations consists of stimulating its synthesis by activating PI-3 kinase. Such activation is carried out by ligand activated protein kinase receptors such Trks, although it is unclear whether NGF uses this pathway in hippocampal neurons.
The Role of p75NTR in NGF-promoted Inhibition of GSK-3β and Axon Elongation
p75NTR has been shown to control axon growth in a variety of model systems. However, all these studies have pointed to RhoA as the mediator of NGF/p75NTR signaling. Indeed, this small GTPase can be both activated or deactivated by p75NTR, depending on the ligand that is bound to this receptor, and on the other membrane proteins to which the neurotrophin receptor may be associated (Wong et al., 2002; Yamashita et al., 2002; Yamashita and Tohyama, 2003; (Gehler et al., 2004). By binding to p75NTR, NGF inhibits the capacity of the receptor to activate RhoA, thus favoring axon growth (Wong et al., 2002; Yamashita et al., 2002; Yamashita and Tohyama, 2003). However, neurotrophin signaling may also be mediated by the activation of the PI-3 kinase and/or the integrin-linked kinase (Mills et al., 2003; Edwards et al., 2005). Although in most of these studies, the NGF receptor mediating such activity was not determined, it might be assumed that TrkA mediates the activation of PI-3 kinase and Akt/PKB (reviewed in Segal, 2003). Our results suggest a novel mechanism by which NGF might inhibit GSK-3β and promote the subsequent growth of axons, mediated only by p75NTR.
In our experimental system, NGF stimulated axonal growth via p75NTR, and we clearly demonstrated that the inhibition of GSK-3β promoted by NGF was mediated by p75NTR. Indeed, the presence of the Trk inhibitor K252a did not prevent the NGF-induced inhibition of GSK-3β. Although both TrkA and p75NTR mRNA can be found in the pyramidal cell layer of the hippocampus (Salama-Cohen et al., 2006), it seems that only the low-affinity receptor is involved in GSK-3β inhibition and axon elongation. Therefore, our results show that p75NTR mediates both the inhibition of GSK-3β and axon elongation.
From NGF/p75NTR to GSK-3β Inhibition: An Alternative Pathway
Measurements of GSK3-β activity revealed that the inhibition of PI-3 kinase by LY294002 did not prevent NGF from inhibiting GSK-3β, at least not totally (Figure 5). Because initial studies revealed that NGF was capable of provoking the phosphorylation of Akt/PKB, even in the presence of LY294002, we contemplated the possibility that the levels of phosphatidylinositol-3P might increase upon exposure to NGF through the inhibition of a lipid phosphatase. Previous experiments discarded SHIP I and -II phosphatases as enzyme targets for NGF; thus, we examined the possibility that NGF/p75NTR regulates the activity of PTEN, thereby reversing the function of PI-3 kinase by dephosphorylating phosphatidylinositol 3,4,5-triphosphate (Maehama and Dixon, 1998; Maehama and Dixon, 1999). Indeed, PTEN phosphatase has been implicated in determining neuronal polarity and it acts upstream of GSK-3β (Jiang et al., 2005). Furthermore, PTEN overexpression in hippocampal neurons inhibits axon formation (Shi et al., 2003) without affecting dendrite formation. Conversely, transfection of PTEN small interfering RNA impairs the accumulation of PTEN protein in hippocampal neurons by >50% and increases the number of axons that are produced at the expense of dendrites (Jiang et al., 2005). The phosphorylation status of PTEN exerts a strong influence on its regulation. Not only is it involved in protein stabilization but also it controls the subcellular location of PTEN and/or its association with regulatory molecules (Vazquez et al., 2001; Valiente et al., 2005). Phosphorylation of the PTEN tail suppresses the activity of PTEN by interfering with electrostatic membrane binding of PTEN, thereby controlling its recruitment into the PTEN-associated complex (Vazquez et al., 2001).
Our results are consistent with the phosphorylation of PTEN at Ser380 being associated with its inactivation. On the one hand, NGF/p75NTR promotes PTEN phosphorylation, while on the other hand, the pharmacological inhibition of PTEN produces the same effect as NGF application, namely, phosphorylation (activation) of Akt/PKB and phosphorylation (inhibition) of GSK-3β. Furthermore, a decrease in the phosphorylation of either Akt/PKB or GSK-3β is to a large extent reverted in a similar manner either by inhibiting PTEN activity with bpV(pic) or by exposing the neurons to NGF. Therefore, our data indicate that in the pathway used by NGF/p75NTR to activate Akt/PKB and to inhibit GSK-3β, PTEN phosphatase lies upstream of these kinases.
NGF/p75NTR Stimulates CK2 Activity
We also investigated the pathway by which NGF phosphorylates PTEN phosphatase via p75NTR. One kinase known to phosphorylate PTEN is the protein kinase CK2 (Torres and Pulido, 2001). The depletion of CK2 in neuroblastoma cells with antisense oligodeoxyribonucleotides blocks neuritogenesis. This effect is accompanied by the site-specific dephosphorylation of microtubule-associated protein MAP1B in parallel with the release of MAP1B from microtubules. It has therefore been proposed that phosphorylation by CK2 may be required for correct MAP1B function in promoting the assembly of the microtubules that constitute the cytoskeletal scaffolding of growing axon-like neurites (Ulloa et al., 1993). Furthermore, it has been reported that the activation of CK2 by NGF in PC12 cells parallels neurite extension (Kleijn et al., 1998). For the first time, we show here that NGF largely stimulates CK2 activity in cultured hippocampal neurons, an effect that seems to be specific because it was completely reversed by DMAT, a specific CK2 inhibitor. Moreover, the activation of CK2 by NGF seems to be mediated by p75NTR, in accordance with other results. Interestingly, the pharmacological inhibition of CK2 also prevented the NGF-stimulated phosphorylation of both PTEN and GSK-3β.
Together, these results reveal a novel pathway by which NGF activates Akt/PKB, which by inhibiting GSK-3β activity, primes axon elongation. Via p75NTR, NGF activates CK2, which in turn phosphorylates and inhibits PTEN phosphatase. This inhibition results in an increase in PI(3)P levels, which stimulates Akt/PKB that subsequently phosphorylates and inhibits GSK-3β, a necessary step for axon growth.
ACKNOWLEDGMENTS
We gratefully acknowledge helpful discussions with Rosemarie Grantyn (Charité-University Medicine Berlin, Berlin, Germany). We are also indebted to J. R. Woodgett for the HA-GSK3β S9A/pcDNA3.1 plasmid. We thank Maia Chved and Laura Castillo for technical assistance. This work was supported by Grant PB08477 from Ministerio de Ciencia y Tecnología (to A.R.T.); Grant PX-0124 from Comunidad de Madrid; Grant 00/003–00 from the Fundació La Caixa, Barcelona; and the Red de Centros de Investigaciones de Enfermedades Neurológicas.
Abbreviations used:
- bpV(pic)
dipotassium bisperoxo(picolinato) oxovanadate (V)
- DMAT
2-dimethylamino-4,5,6,7-tetrabromo-1H-benzimidazole.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E05-12-1144) on May 12, 2006.
REFERENCES
- Alessi D. R., Andjelkovic M., Caudwell B., Cron P., Morrice N., Cohen P., Hemmings B. A. Mechanism of activation of protein kinase B by insulin and IGF-1. EMBO J. 1996;15:6541–6551. [PMC free article] [PubMed] [Google Scholar]
- Bradke F., Dotti C. G. Establishment of neuronal polarity: lessons from cultured hippocampal neurons. Curr. Opin. Neurobiol. 2000;10:574–581. doi: 10.1016/s0959-4388(00)00124-0. [DOI] [PubMed] [Google Scholar]
- Cross D.A.E., Alessi D. R., Cohen P., Andjelkovich M., Hemmings B. A. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 1995;378:785–789. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- Doble B. W., Woodgett J. R. GSK-3, tricks of the trade for a multi-tasking kinase. J. Cell Sci. 2003;116:1175–1186. doi: 10.1242/jcs.00384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dotti C. G., Sullivan C. A., Banker G. A. The establishment of polarity by hippocampal neurons in culture. J. Neurosci. 1988;8:1454–1468. doi: 10.1523/JNEUROSCI.08-04-01454.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards L. A., Thiessen B., Dragowska W. H., Daynard T., Bally M. B., Dedhar S. Inhibition of ILK in PTEN-mutant human glioblastomas inhibits PKB/Akt activation, induces apoptosis, and delays tumor growth. Oncogene. 2005;24:3596–3605. doi: 10.1038/sj.onc.1208427. [DOI] [PubMed] [Google Scholar]
- Gehler S., Gallo G., Veien E., Letourneau P. C. p75 Neurotrophin receptor signaling regulates growth cone filopodial dynamics through modulating RhoA activity. J. Neurosci. 2004;24:4363–4372. doi: 10.1523/JNEUROSCI.0404-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goold R. G., Gordon-Weeks P. R. The MAP kinase pathway is upstream of the activation of GSK3beta that enables it to phosphorylate MAP1B and contributes to the stimulation of axon growth. Mol. Cell Neurosci. 2005;28:524–534. doi: 10.1016/j.mcn.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Goslin K., Banker G. Experimental observations on the development of polarity by hippocampal neurons in culture. J. Cell Biol. 1989;108:1507–1516. doi: 10.1083/jcb.108.4.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hathaway G. M., Lubben T. H., Traugh J. A. Inhibition of casein kinase II by heparin. J. Biol. Chem. 1980;255:8038–8041. [PubMed] [Google Scholar]
- Huber L. J., Chao M. V. A Potential Interaction of P75 and Trka Ngf Receptors revealed by affinity cross-linking and immunoprecipitation. J. Neurosci. Res. 1995;40:557–563. doi: 10.1002/jnr.490400415. [DOI] [PubMed] [Google Scholar]
- Jiang H., Guo W., Liang X., Rao Y. Both the establishment and the maintenance of neuronal polarity require active mechanisms: critical roles of GSK-3beta and its upstream regulators. Cell. 2005;120:123–135. doi: 10.1016/j.cell.2004.12.033. [DOI] [PubMed] [Google Scholar]
- Kleijn M., Welsh G. I., Scheper G. C., Voorma H. O., Proud C. G., Thomas A. A. Nerve and epidermal growth factor induce protein synthesis and eIF2B activation in PC12 cells. J. Biol. Chem. 1998;273:5536–5541. doi: 10.1074/jbc.273.10.5536. [DOI] [PubMed] [Google Scholar]
- Kuruvilla R., Zweifel L. S., Glebova N. O., Lonze B. E., Valdez G., Ye H. H., Ginty D. D. A neurotrophin signaling cascade coordinates sympathetic neuron development through differential control of TrkA trafficking and retrograde signaling. Cell. 2004;118:243–255. doi: 10.1016/j.cell.2004.06.021. [DOI] [PubMed] [Google Scholar]
- Maehama T., Dixon J. E. The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3,4,5-trisphosphate. J. Biol. Chem. 1998;273:13375–13378. doi: 10.1074/jbc.273.22.13375. [DOI] [PubMed] [Google Scholar]
- Maehama T., Dixon J. E. PTEN: a tumour suppressor that functions as a phospholipid phosphatase. Trends Cell Biol. 1999;9:125–128. doi: 10.1016/s0962-8924(99)01519-6. [DOI] [PubMed] [Google Scholar]
- Mills J., Digicaylioglu M., Legg A. T., Young C. E., Young S. S., Barr A. M., Fletcher L., O’Connor T. P., Dedhar S. Role of integrin-linked kinase in nerve growth factor-stimulated neurite outgrowth. J. Neurosci. 2003;23:1638–1648. doi: 10.1523/JNEUROSCI.23-05-01638.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagano M. A., Meggio F., Ruzzene M., Andrzejewska M., Kazimierczuk Z., Pinna L. A. 2-Dimethylamino-4,5,6,7-tetrabromo-1H-benzimidazole: a novel powerful and selective inhibitor of protein kinase CK2. Biochem. Biophys. Res. Commun. 2004;321:1040–1044. doi: 10.1016/j.bbrc.2004.07.067. [DOI] [PubMed] [Google Scholar]
- Pennypacker K., Fischer I., Levitt P. Early in vitro genesis and differentiation of axons and dendrites by hippocampal neurons analyzed quantitatively with neurofilament-H and microtubule-associated protein 2 antibodies. Exp. Neurol. 1991;111:25–35. doi: 10.1016/0014-4886(91)90047-g. [DOI] [PubMed] [Google Scholar]
- Salama-Cohen P., Arevalo M. A., Grantyn R., Rodriguez-Tebar A. Notch and NGF/p75 control dendrite morphology and the balance of excitatory/inhibitory synaptic input to hippocampal neurones through neurogenin 3. J. Neurochem. 2006;97:1269–1278. doi: 10.1111/j.1471-4159.2006.03783.x. [DOI] [PubMed] [Google Scholar]
- Sayas C. L., Avila J., Wandosell F. Glycogen synthase kinase-3 is activated in neuronal cells by G alpha(12) and G alpha(13) by Rho-independent and Rho-dependent mechanisms. J. Neurosci. 2002;22:6863–6875. doi: 10.1523/JNEUROSCI.22-16-06863.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayas C. L., Moreno-Flores M. T., Avila J., Wandosell F. The neurite retraction induced by lysophosphatidic acid increases Alzheimer’s disease-like Tau phosphorylation. J. Biol. Chem. 1999;274:37046–37052. doi: 10.1074/jbc.274.52.37046. [DOI] [PubMed] [Google Scholar]
- Schmid A. C., Byrne R. D., Vilar R., Woscholski R. Bisperoxovanadium compounds are potent PTEN inhibitors. FEBS Lett. 2004;566:35–38. doi: 10.1016/j.febslet.2004.03.102. [DOI] [PubMed] [Google Scholar]
- Segal R. A. Selectivity in neurotrophin signaling: theme and variations8. Annu. Rev. Neurosci. 2003;26:299–330. doi: 10.1146/annurev.neuro.26.041002.131421. [DOI] [PubMed] [Google Scholar]
- Shi S. H., Jan L. Y., Jan Y. N. Hippocampal neuronal polarity specified by spatially localized mPar3/mPar6 and PI 3-kinase activity. Cell. 2003;112:63–75. doi: 10.1016/s0092-8674(02)01249-7. [DOI] [PubMed] [Google Scholar]
- Stephens L. Protein kinase B kinases that mediate phosphatidylinositol 3,4,5-trisphosphate-dependent activation of protein kinase. Science. 1998;279:710–714. doi: 10.1126/science.279.5351.710. [DOI] [PubMed] [Google Scholar]
- Stiles B., Groszer M., Wang S. Y., Jiao J., Wu H. PTENless means more. Dev. Biol. 2004;273:175–184. doi: 10.1016/j.ydbio.2004.06.008. [DOI] [PubMed] [Google Scholar]
- Stokoe D., Stephens L. R., Copeland T., Gaffney P.R.J., Reese C. B., Painter G. F., Holmes A. B., McCormick F., Hawkins P. T. Dual role of phosphatidylinositol-3,4,5-trisphosphate in the activation of protein kinase B. Science. 1997;277:567–570. doi: 10.1126/science.277.5325.567. [DOI] [PubMed] [Google Scholar]
- Torres J., Pulido R. The tumor suppressor PTEN is phosphorylated by the protein kinase CK2 at its C terminus. Implications for PTEN stability to proteasome-mediated degradation. J. Biol. Chem. 2001;276:993–998. doi: 10.1074/jbc.M009134200. [DOI] [PubMed] [Google Scholar]
- Ulloa L., Diaz-Nido J., Avila J. Depletion of casein kinase II by antisense oligonucleotide prevents neuritogenesis in neuroblastoma cells. EMBO J. 1993;12:1633–1640. doi: 10.1002/j.1460-2075.1993.tb05808.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaillant A. R., Mazzoni I., Tudan C., Boudreau M., Kaplan D. R., Miller F. D. Depolarization and neurotrophins converge on the phosphatidylinositol 3-kinase-Akt pathway to synergistically regulate neuronal survival. J. Cell Biol. 1999;146:955–966. doi: 10.1083/jcb.146.5.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valiente M., Andres-Pons A., Gomar B., Torres J., Gil A., Tapparel C., Antonarakis S. E., Pulido R. Binding of PTEN to specific PDZ domains contributes to PTEN protein stability and phosphorylation by microtubule-associated serine/threonine kinases. J. Biol. Chem. 2005;280:28936–28943. doi: 10.1074/jbc.M504761200. [DOI] [PubMed] [Google Scholar]
- Vazquez F., Grossman S. R., Takahashi Y., Rokas M. V., Nakamura N., Sellers W. R. Phosphorylation of the PTEN tail acts as an inhibitory switch by preventing its recruitment into a protein complex1. J. Biol. Chem. 2001;276:48627–48630. doi: 10.1074/jbc.C100556200. [DOI] [PubMed] [Google Scholar]
- Welsh G. I., Patel J. C., Proud C. G. Peptide substrates suitable for assaying glycogen synthase kinase-3 in crude cell extracts. Anal. Biochem. 1997;244:16–21. doi: 10.1006/abio.1996.9838. [DOI] [PubMed] [Google Scholar]
- Wong S. T., Henley J. R., Kanning K. C., Huang K. H., Bothwell M., Poo M. A p75(NTR) and Nogo receptor complex mediates repulsive signaling by myelin-associated glycoprotein. Nat. Neurosci. 2002;5:1302–1308. doi: 10.1038/nn975. [DOI] [PubMed] [Google Scholar]
- Yamashita T., Higuchi H., Tohyama M. The p75 receptor transduces the signal from myelin-associated glycoprotein to Rho. J. Cell Biol. 2002;157:565–570. doi: 10.1083/jcb.200202010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita T., Tohyama M. The p75 receptor acts as a displacement factor that releases Rho from Rho-GDI. Nat. Neurosci. 2003;6:461–467. doi: 10.1038/nn1045. [DOI] [PubMed] [Google Scholar]
- Yoshimura T., Kawano Y., Arimura N., Kawabata S., Kikuchi A., Kaibuchi K. GSK-3beta regulates phosphorylation of CRMP-2 and neuronal polarity. Cell. 2005;120:137–149. doi: 10.1016/j.cell.2004.11.012. [DOI] [PubMed] [Google Scholar]
- Zhou F. Q., Snider W. D. Cell biology. GSK-3beta and microtubule assembly in axons. Science. 2005;308:211–214. doi: 10.1126/science.1110301. [DOI] [PubMed] [Google Scholar]
- Zhou F. Q., Zhou J., Dedhar S., Wu Y. H., Snider W. D. NGF-induced axon growth is mediated by localized inactivation of GSK-3beta and functions of the microtubule plus end binding protein APC. Neuron. 2004;42:897–912. doi: 10.1016/j.neuron.2004.05.011. [DOI] [PubMed] [Google Scholar]