Abstract
Focal adhesion kinase (FAK) is phosphorylated on tyrosine and serine residues after cell activation. In the present work, we investigated the relationship between tyrosine and serine phosphorylation of FAK in promoting endothelial cell migration in response to vascular endothelial growth factor (VEGF). We found that VEGF induces the activation of the Rho-dependent kinase (ROCK) downstream from vascular endothelial growth factor receptor (VEGFR) 2. In turn, activated ROCK directly phosphorylates FAK on Ser732. Proline-rich tyrosine kinase-2 (Pyk2) is also activated in response to VEGF. Its activation requires the clustering of integrin αvβ3 and triggers directly the phosphorylation of Tyr407 within FAK, an event necessary for cell migration. Interestingly, ROCK-mediated phosphorylation of Ser732 is essential for Pyk2-dependent phosphorylation of Tyr407, because the latter is abrogated in cells expressing a FAK mutant that is nonphosphorylatable on Ser732. We suggest that VEGF elicits the activation of the VEGFR2–ROCK pathway, leading to phosphorylation of Ser732 within FAK. In turn, phosphorylation of Ser732 would change the conformation of FAK, making it accessible to Pyk2 activated in response to its association with integrin β3. Then, activated Pyk2 triggers the phosphorylation of FAK on Tyr407, promoting cell migration.
INTRODUCTION
Cell migration requires hierarchical and coordinate signaling events that converge on appropriate actin remodeling. It regulates several physiological and pathological processes. In particular, actin-driven cell motility is centrally involved in ensuring endothelial cell migration in response to angiogenic agents such as vascular endothelial growth factor (VEGF) (Rousseau et al., 2000a). VEGF is a potent angiogenic agent that initiates endothelial cell migration after its binding to the tyrosine kinase receptor vascular endothelial growth factor receptor (VEGFR) 2 (Bernatchez et al., 1999; Rousseau et al., 2000b). VEGFR2-mediated endothelial cell migration is associated with activation of several kinase pathways, including stress-activated protein kinase 2/p38 MAP kinase, phosphatidylinositol-3 kinase, and focal adhesion kinase (FAK) (Abedi and Zachary, 1997; Rousseau et al., 1997; Matsumoto and Claesson-Welsh, 2001; Lamalice et al., 2004). These pathways act complementarily to transmit signals that trigger the actin remodeling that underlies cell migration. Notably, VEGF-induced activation of FAK regulates the proper turnover of focal adhesions that is required to allow the dynamic adhesion and de-adhesion processes inherent to cell migration (Le Bœuf et al., 2004). Intriguingly, despite the role of FAK as a biosensor and integrator of cell migration, much remains to be known concerning the mechanisms by which it is activated by growth factors.
FAK is a nonreceptor protein kinase that is found in integrin-enriched focal contacts (Guan et al., 1991; Kornberg et al., 1992; Mitra et al., 2005). The protein is very well conserved between species in which it plays similar functions (Parsons, 2003; Mitra et al., 2005). It comprises a central catalytic domain located between an N-terminal region containing a protein four.1, ezrin, radixin, moesin (FERM) domain that interacts with integrin and growth factor receptors, and a C-terminal region that contains the focal adhesion-targeting (FAT) domain. This latter domain is implicated in interactions with structural and signaling proteins (Parsons, 2003; Mitra et al., 2005). FAK possesses six tyrosyl residues that are differentially phosphorylated by diverse agonists and that are implicated in transmitting different signals and effects. Two of these tyrosyl residues (Tyr397 and Tyr407) are located at the boundary between the N-terminal and kinase domains, two are located in the catalytic domain (Tyr576 and Tyr577), and two are located in the C-terminal region (Tyr861 and Tyr925) (Cornillon et al., 2003; Parsons, 2003; Mitra et al., 2005). Tyr397, a highly conserved site between species, is an autophosphorylation site that recruits Src homology-2 domain-containing proteins, including members of Src family kinases, phospholipase C-γ, GRB7, and the p85 subunit of phosphatidylinositol-3 kinase (Mitra et al., 2005). It seems that Src kinases are first recruited to Tyr397 and that they are involved in transphosphorylating other tyrosyl residues within FAK. For example, Src kinases-induced transphosphorylation of Tyr576 and Tyr577 confers maximal activation of FAK and signaling in response to adhesion (Calalb et al., 1995; Owen et al., 1999). Moreover, Tyr576 and Tyr861 are both phosphorylated in a Src-dependent manner in response to VEGF (Holmqvist et al., 2004; Le Bœuf et al., 2004). In contrast, Tyr407 is phosphorylated in a Src-independent manner after exposure to VEGF (Le Bœuf et al., 2004). The phosphorylation of this site by VEGF is associated with the recruitment of heat-shock protein of 90 kDa (HSP90) to the C-terminal portion of VEGFR2 and with the activation of the RhoA–Rho-dependent kinase (ROCK) cascade. Then, recruitment of vinculin and paxillin to focal adhesions occurs (Le Bœuf et al., 2004). Interestingly, FAK possess four serine sites located in the C-terminal tail: Ser722, Ser732, Ser843, and Ser910 (Parsons, 2003). The role of these sites is still ill defined. Nevertheless, it has been shown that Ser732 is implicated in cell migration and can be phosphorylated by Cdk5, a serine/threonine kinase implicated in neuron migration (Xie and Tsai, 2004). Nothing is known concerning the functional interplay between phosphotyrosine and phosphoserine sites within FAK.
Proline-rich tyrosine kinase-2 (Pyk2), also called cell adhesion kinase-β or related adhesion focal tyrosine kinase, is another nonreceptor protein kinase closely related to FAK. It is found in high amounts in endothelial cells in which it modulates cell adhesion and migration (Dikic et al., 1998; Wang et al., 2003; Mitra et al., 2005). The homology between Pyk2 and FAK is strong, being characterized by 60% sequence identity in the central kinase domain, conservation of the proline-rich regions, and identical positions of four tyrosine phosphorylation sites (Mitra et al., 2005). Tyr402, -579, -580, and -881 within Pyk2 correspond to Tyr397, -576, -577, and -925 within FAK, respectively (Mitra et al., 2005). The phosphorylation of these sites within Pyk2 triggers the activation of signaling pathways that are common to those activated by corresponding tyrosyl residues within FAK (Schlaepfer et al., 1999). Nevertheless, the differential binding activities of the FERM and FAT domains within FAK and Pyk2 contribute to limit the functional redundancy between the two kinases (Mitra et al., 2005). Interestingly, in leukocytes adhering to vitronectin, ligation of integrin αvβ3 induces phosphorylation of Pyk2 on its autophosphorylation site Tyr402 and its association with phosphorylated Tyr747 within integrin β3 (Butler and Blystone, 2005). This finding is of particular interest in the context of VEGF, because VEGFR2 should interact with integrin αvβ3 to induce a productive signaling to both p38 and FAK (Soldi et al., 1999; Byzova et al., 2000; Masson-Gadais et al., 2003). Overall, these findings suggest that FAK and Pyk2 may jointly participate to transmit the VEGF signal downstream from the VEGFR2-αvβ3 integrin complex.
In the present study, we investigated the mechanisms that regulate FAK phosphorylation and migratory functions in response of VEGF in endothelial cells. We investigated both the interplay between serine and tyrosine phosphorylation and the interaction between FAK and Pyk2. We report that exposure of endothelial cells to VEGF drives the activation of the RhoA-dependent kinase ROCK downstream from VEGFR2. Then, activated ROCK phosphorylates FAK on Ser732, which is essential for phosphorylation of Tyr407 and for cell migration. We further show that Pyk2 is activated by VEGF-induced clustering of integrin αvβ3 and is responsible for the phosphorylation of Tyr407.
MATERIALS AND METHODS
Reagents
VEGF-A165, endothelial cell growth supplement (ECGS), and geldanamycin were purchased from Sigma-Aldrich (St. Louis, MO). Y27632 and SU6656 were obtained from Calbiochem (San Diego, CA). Peptides DEL-1 (CEISEAYRGDTFIGYVCK) and FAK-Tyr407 (EIIDEEDTY407TMPSTRD) were synthesized by Le Centre de Synthèse de Peptides de l’Est du Québec (Québec, Canada). Constitutively active form of ROCKI and Pyk2 as well as the synthetic peptide [GG(EEEEY)10EE] biotin conjugate at the N terminus were obtained from Upstate Biotechnology (Charlottesville, VA). Porcine myosin light chain (MLC) was from Sigma-Aldrich.
Cells
Human umbilical vein endothelial cells (HUVECs) were isolated by collagenase digestion of umbilical veins from undamaged sections of fresh cords (Huot et al., 1997). After isolation, cells were plated on gelatin-coated 75-cm2 culture dishes in MXV medium (199 medium containing 20% heat-inactivated fetal bovine serum [FBS], 60 μg/ml ECGS, glutamine, heparin, and antibiotics). Subcultures were obtained by trypsination and were used at passages <4. Treatments were done on HUVECs cultivated on gelatin and made quiescent by incubation for 16–20 h in ECGS-free medium containing 5% FBS. Bovine aortic endothelial cells (BAECs) were isolated from a bovine aortic segment (Neagoe et al., 2005). The cells were obtained by gentle scraping of the superficial internal layer of the aortic segment. BAECs were collected in DMEM supplemented with 10% characterized FBS (Hyclone Laboratories, Logan, UT) and were grown on gelatin (Neagoe et al., 2005). Mouse embryo fibroblasts (MEFs) FAK−/− cells (Ilic et al., 1995) were cultivated in DMEM supplemented with 10% FBS and β-mercaptoethanol (3.5 μl/500 ml medium). All cultures were maintained at 37°C in a humidified atmosphere containing 5% CO2.
Antibodies
The anti-FAK antibody used for Western blotting was purchased from BD Biosciences (Missisauga, Ontario, Canada). The anti-Src (Src2) antibody is a rabbit polyclonal antibody obtained from Santa Cruz Biotechnology (Santa Cruz, CA). The mouse monoclonal anti-vinculin (clone hVIN-1) and anti-tubulin (clone B5–1-2) antibodies were from Sigma-Aldrich. The anti-integrin β3 antibody used in immunoprecipitation experiments is a mouse polyclonal antibody (GPIIIa), whereas the anti-β3 antibody used for Western blotting is a rabbit antibody (CD61). They were obtained from Chemicon International (Temecula, CA). The phospho-specific rabbit polyclonal antibodies against phosphorylated human, chicken, and mouse FAK were from BioSource International (Camarillo, CA), except those against pTyr397, which were obtained from Upstate Biotechnology. The specificity of these antibodies for their phosphorylated residues is supported by competition studies showing that only the phosphopeptide corresponding to the given specific site blocks the antibody signal (BioSource International product analysis sheet). Nevertheless, we found that the anti-Ser732 and anti-Tyr407 detect two bands in total extracts (Figure 1, A and B; our unpublished data). However, both antibodies, respectively, detect only one band for Ser732 or for Tyr407 in immunoprecipitated extracts (Figure 3; our unpublished data). These specific Ser732 or Tyr407 bands correspond to the strongest band in Figures 1 and 2 and were used for quantification. The phospho-specific rabbit polyclonal antibody against Pyk2 (pY402) was from BD Biosciences. The hemagglutinin (HA) antibody (clone 12Ca5) was from Roche Diagnostics (Indianapolis, IN), and the antibody against Myc (clone 9E10) was from Covance (Berkeley, CA). The anti-mouse-IgG-horseradish peroxidase (HRP) and anti-rabbit-IgG-HRP antibodies were from The Jackson Laboratory (Bar Harbor, ME). The phospho-specific rabbit polyclonal antibody against phosphorylated MLC was from Cell Signaling Technology (Beverly, MA). Porcine anti-GAPDH was purchased from Novus Biologicals (Littleton, CO).
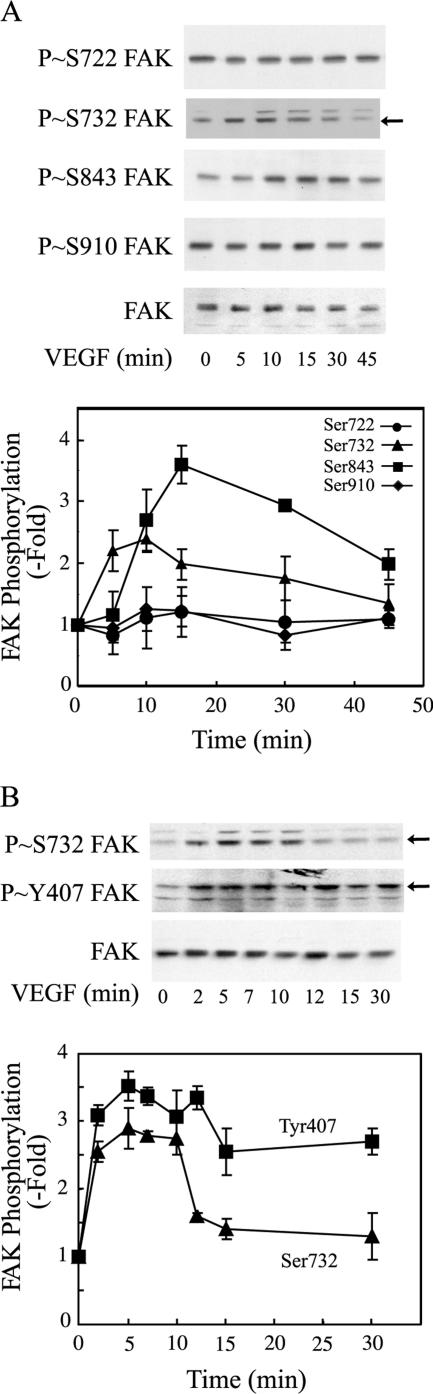
Figure 1.
VEGF induces phosphorylation of FAK on serine residues. (A) Quiescent HUVECs were treated or not with 5 ng/ml VEGF for increasing periods. Proteins were extracted and were separated by SDS-PAGE and transferred to a nitrocellulose membrane. The membrane was processed for immunodetection of phospho-FAK Ser722, Ser732, Ser843, Ser910, and total FAK. (B) Quiescent HUVECs were treated or not with 5 ng/ml VEGF for increasing periods. Proteins were extracted as in A and were processed for immunodetection of phospho-FAK Ser732, Tyr407, and total FAK. Representative blots are shown at the top and quantification of the blots (means ± SD) of three separate experiments is shown at the bottom. Arrows denote the bands that specifically correspond to phospho-Ser732 and phospho-Tyr407 (see Materials and Methods) and that were used for quantification.
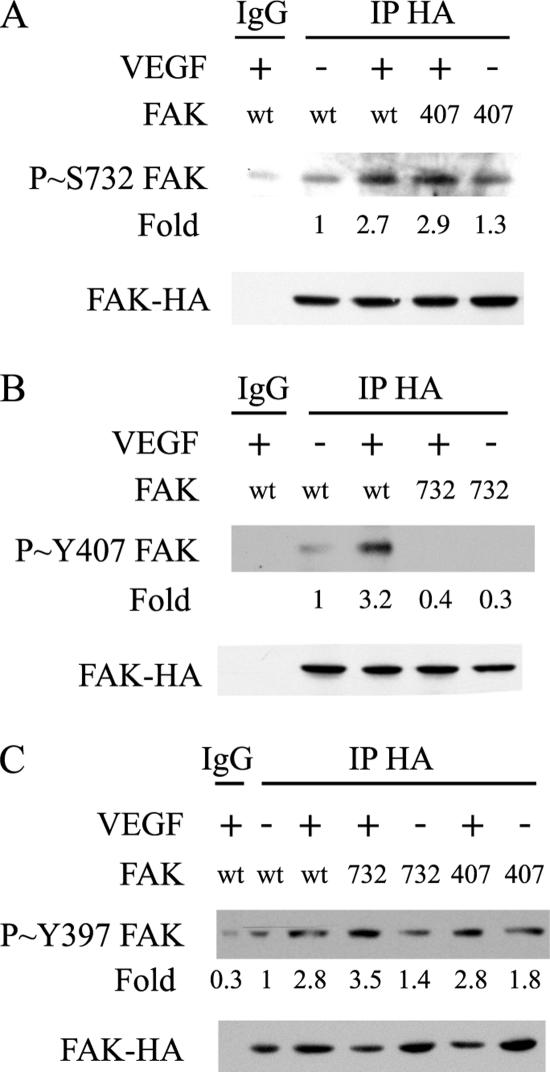
Figure 3.
VEGF-induced phosphorylation of FAK on Tyr407, but not on Tyr397, requires the phosphorylation of FAK on Ser732. (A) Quiescent HUVECs transiently expressing wild-type HA-tagged FAK (wt) or HA-tagged FAK mutant Tyr407Phe (407) were treated or not with 5 ng/ml VEGF for 10 min. After extraction, the HA-tagged proteins were immunoprecipitated using anti-HA mouse antibody. Control immunoprecipitation was done similarly using purified mouse IgG. Proteins were separated by SDS-PAGE and transferred to a nitrocellulose membrane. The membrane was processed for immunodetection of phospho-FAK Ser732 (top) and total FAK-HA (bottom). Representative blots of three separate experiments are shown. (B) Quiescent HUVEC transiently expressing HA-tagged FAK (wt) or HA-tagged FAK-Ser732Ala (732) were treated or not with 5 ng/ml VEGF for 10 min. Cell extracts were prepared as described in A, and the membrane was processed for immunodetection of phospho-FAK Tyr407 (top) and total FAK-HA (bottom). Representative blots of two separate experiments are shown. (C) Quiescent HUVECs transiently expressing wild-type HA-tagged FAK (wt), HA-tagged FAK mutants Ser732Ala (732), or Tyr407Phe (407) were treated or not with 5 ng/ml VEGF for 10 min. After extraction, the HA-tagged proteins were immunoprecipitated using anti-HA mouse antibody and processed as described in A. The membrane was processed for immunodetection of phospho-FAK Tyr397 (top) and total FAK-HA (bottom).
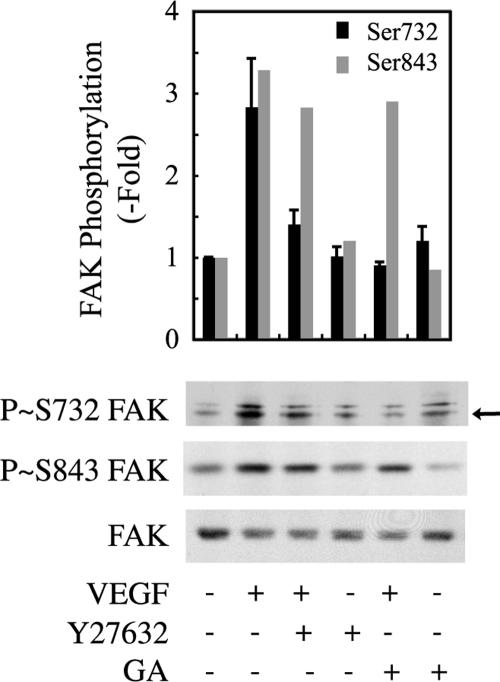
Figure 2.
VEGF-induced phosphorylation of FAK on Ser732 but not on Ser843 is inhibited by geldanamycin (GA) or Y27632. Quiescent HUVECs were pretreated for 60 min with 1 μg/ml GA or vehicle (0.25% dimethyl sulfoxide [DMSO]) or for 120 min with 25 μM Y27632, followed by VEGF treatment (5 ng/ml for 10 min). Cell extracts were prepared, and proteins were separated by SDS-PAGE and transferred to a nitrocellulose membrane. The membrane was processed for immunodetection of phospho-FAK Ser732 (top), phospho-FAK, Ser843 (middle), and total FAK (bottom). Data points represent means ± SD of three experiments. Representative blots are shown. The arrow denotes the band that specifically corresponded to Ser732 (see Materials and Methods) and that was used for quantification.
Plasmids, Small Interfering RNA (siRNA), and Adenovirus
HA-FAK cDNA constructs were provided by Dr. Jun-Lin Guan (Department of Molecular Medicine, Cornell University, Ithaca, NY); green fluorescent protein (GFP)-FAK, GFP-FAK Ser732Ala, and HA-FAK Ser732Ala were from Dr. Li-Huei Tsai (Harvard Medical School, Boston, MA; Xie et al., 2003). FAK-related nonkinase domain (FRNK)-glutathione S-transferase (GST) construct was designed by inserting the corresponding fragments derived from BamH1-EcoR1 digested into the vector pGEX-6P-3 (Amersham-General Healthcare, Baie d’Urfé, Québec, Canada). Mutant FAK Tyr407Phe in pKH3 was obtained by site-directed mutagenesis (Le Bœuf et al., 2004). Wild-type ROCK and constitutively active ROCK Δ3 were from Dr. Shuh Narumiya (Department of Pharmacology, Kyoto University, Kyoto, Japan; Ishizaki et al., 1997). Adenoviruses containing wild-type Pyk2 and Tyr402Phe mutant were described previously (Melendez et al., 2004). FAK siRNA was purchased from Dharmacon (Lafayette, CO) and was designed to target the mRNA of human FAK (GenBank accession no. NM_005607). The target sequence is as follows: sense, 5′-GAAGUUGGGUUGUCUAGAAUU-3′ and antisense, 5′-PUUCUAGACAACCCAACUUCUU-3′. The SMART pool siRNA duplexes designed to target Pyk2 (NM_004103) and ROCK1 (NM_005406) mRNAs were also purchased from Dharmacon. Control siRNA to target GAPDH mRNA was a gift from Dr. Darren E. Richard (Laval University, Québec, Canada).
Gene and siRNA Transfer
Gene transfer in HUVECs was done by electroporation (Le Bœuf et al., 2004) and adenoviral-mediated infection. Briefly, electroporation with an Eppendorf Multiporator was done on 1 × 106 cells in 200 mOsM electroporation buffer (Eppendorf, Boulder, CO) using 3 μg of DNA and 4-mm cuvettes. For electroporation, cotransfection of an enhanced green fluorescent protein (EGFP) construct allows evaluating transfection efficiency as 30% after determination of the percentage of cells expressing EGFP. For gene transfer by infection, subconfluent HUVEC cultures were infected for 24 h with adenoviral vectors expressing wild-type Pyk2, Pyk2-Tyr402Phe, or EGFP. After 24-h infection, the medium was aspirated and replaced with serum-free medium for 16 h. Then, cells were treated with VEGF, and lysates were prepared for biochemical studies. Immunofluorescence detection of cells infected with Pyk2 revealed that >90% of the cells expressed the proteins. BAECs were transfected using polyethylenimine (PEI) high-molecular-weight from Sigma-Aldrich. Plasmids (20 μg DNA/2 × 106 cells) were mixed with 500 μl of 150 mM NaCl and 10 μl of 0.43% PEI for 15 min at room temperature. Cells were incubated with DNAs during 4 h, and medium was replaced by fresh medium. Cells were overlaid with complete medium, and assays were done 48 h after transfection. Cotransfection of a GFP construct allows evaluating transfection efficiency as 40%. For transient transfection, MEF FAK−/− cells (5 × 105 cells/60-mm Petri dishes) were lipofected using 11 μg of DNA with a ratio of 3:1 with Tfx-50 (Promega, Madison, WI) for 90 min in the absence of serum. Cells were then overlaid with complete medium, and assays were done 48 h after transfection. Sixteen hours before the experiments, cells were incubated in serum-free medium. Cotransfection of a GFP construct allows evaluating transfection efficiency between 35 and 45% after determination of the percentage of cells that express GFP under the fluorescence microscope. siRNAs were introduced into the cells by electroporation, as for plasmids, except that 40–360 pmol of siRNA were used. The cells were used 48 h later.
Immunoprecipitation and Western Blotting
HA-FAK and integrin β3 were immunoprecipitated as described previously (Le Bœuf et al., 2004). After treatments, the cells were washed with phosphate-buffered saline (PBS). Then, they were extracted in B buffer containing 150 mM NaCl, 50 mM Tris-HCl, pH 7.5, 1% Triton X-100, 0.1% sodium deoxycholate, 2 mM EDTA, 2 mM EGTA, 1 mM Na3VO4, 1 mM benzamide, 1 μM leupeptin, 50 mM NaF, and 1 mM phenylmethylsulfonyl fluoride (PMSF). Further steps were done at 4°C. Cells were centrifuged at 16,000 × g for 10 min. Proteins were quantified by the Bradford assay, and an equal quantity of proteins was diluted in B buffer before being precleared for 60 min with protein A- or protein G-Sepharose. Supernatants were incubated on ice for 90 min with appropriate antibodies. Then, 10 μl of 50% (vol/vol) protein G-Sepharose (Amersham-General Healthcare) was added, and the incubation was extended for 30 min on ice. Antigen-antibody complexes were washed four times with B buffer before adding SDS-PAGE loading buffer. Proteins were separated through SDS-PAGE, and the gels were transferred onto nitrocellulose membranes for Western blotting. The immunoprecipitations with the HA-agarose conjugate were done similarly, except that proteins were directly incubated overnight with the conjugated antibody. After reacting nitrocellulose membranes with primary antibody, antigen–antibody complexes were detected with an anti-IgG coupled to HRP antibody and were revealed using an ECL kit (Amersham-General Healthcare). For stripping, nitrocellulose membranes were first washed in 1× Tris-buffered saline (TBS) containing 0.1% Tween. Then, they were incubated for 30 min at 68°C in fresh stripping buffer and were washed again in TBS containing 0.1% Tween. Quantification of the immunoreactive bands was done by densitometric scanning using the NIH Image software (http://rsb.info.nih.gov/nih-image/).
Immunocomplex Kinase Assay of ROCK
In these assays, we used BAECs, because they allow for expression of higher levels of exogenous proteins than HUVECs. Myc-tagged-ROCK constructs were transfected in BAECs with PEI and treated or not with 10 ng/ml VEGF. Proteins were extracted with immunoprecipitation assay-base buffer (50 mM HEPES, pH 7.4, 150 mM NaCl, 1,5 mM MgCl2, 1 mM EGTA, 1% Triton, 10% glycerol, 50 mM NaF, 10 mM tetrasodium diphosphate decahydrate [NaPPi], 1 mM Na3VO4, 1 mM benzamidine, 1 mM PMSF, and 1 mM leupeptin). Proteins were centrifuged, and supernatants were added to HNTG buffer (50 mM HEPES, pH 7.4, 150 mM NaCl, 1 mM EGTA, 0.1% Triton, 10 mM NaPPi, and 1 mM Na3VO4). Myc-tagged proteins were immunoprecipitated using anti-Myc antibody during 2 h at 4°C, and 20 μl of 50% (vol/vol) protein G-Sepharose (Amersham-General Healthcare) was added for 1 h to the incubation mixture (250 mM HEPES, pH 7.4, 50 mM MgCl2, 250 mM NaCl, 7.5 mM dithiothreitol, 0.15% Brj35, and 0.5 mM ATP). ROCK kinase activity was assayed by the addition of 10 or 4 μg of GST-FRNK or 4 μg of MLC as positive control to the kinase buffer at 30°C. The reaction was stopped after 20 min by the addition of SDS-loading buffer. Proteins were run through SDS-PAGE and transferred onto nitrocellulose membranes. Phosphorylation of Ser732 was evaluated in Western blotting using a specific antibody against FAK phospho-Ser732.
In Vitro Kinase Assays of ROCK and Pyk2
Direct in vitro kinase assay of ROCK1 was performed by adding constitutively active form of ROCKI to 4 μg of FRNK or 4 μg of MLC for 20 min at 30°C. For Pyk2, the assay was done similarly by adding constitutively active form of Pyk2 to the FAK-Tyr407 peptide EIIDEEDTY407TMPSTRD (2 μg) or the synthetic peptide [GG(EEEEY)10EE] biotin conjugate, used as a positive control (2 μg) for 20 min at 30°C. The reaction was stopped after 20 min by the addition of SDS-loading buffer. Thereafter, the proteins were run through SDS-PAGE and transferred onto nitrocellulose membranes. Phosphorylation of Ser732 or MLC was evaluated in Western blotting using specific antibodies against phospho-Ser732 within FRNK or phospho-Ser19 within MLC. Phosphorylation of Tyr407 within the FAK peptide EIIDEEDTY407TMPSTRD or Tyr within the [GG(EEEEY)10EE] peptide was evaluated using specific antibodies against phospho-Tyr407 and anti-phospho-Tyr4G10 mouse antibody, respectively.
Immunofluorescence
HUVECs were plated on gelatin-coated Lab-Tek chambers (Lab-Tek, Naperville, IL). After treatments, cells were fixed with 3.7% formaldehyde and permeabilized with 0.1% saponin in PBS, pH 7.5. F-actin was detected using fluorescein isothiocyanate-conjugated phalloidin (33.3 μg/ml) diluted 1:50 in PBS. Vinculin was detected using hVIN-1 monoclonal antibody. Vinculin antigen–antibody complexes were detected with biotin-labeled anti-mouse IgG and were revealed with Texas Red-conjugated streptavidin. GFP-FAK constructs were electroporated, and immunofluorescence was realized to detect GFP. The cells were examined by confocal microscopy (Huot et al., 1998). The percentage of cells showing recruitment of vinculin to the ventral focal adhesions was established after counting 400–500 cells.
Cell Migration Assays
Cell migration was evaluated by means of Boyden chamber migration assays as described previously (Rousseau et al., 2000b). MEF FAK−/− cells were transfected or not with increasing concentrations of FAK wild-type cDNA along with a constant concentration of VEGFR2 (4 μg). EGFP was used as a control of transfection, and an empty vector was used to keep the cDNA concentration constant. Forty hours later, cells were incubated in serum-free medium. Sixteen hours later, cells were harvested with trypsin, counted, centrifuged, and resuspended at 0.5 × 106 cells/ml in migration buffer (199 medium, 10 mM HEPES, pH 7.4, 1 mM MgCl2, and 0.5% bovine serum albumin). Cells were added on the upper part of an 8.0-μm pore size gelatin-coated polycarbonate membrane separating the upper and lower chambers of a 6.5-mm transwell. Cells were left to adhere for 1 h. Then, 10 ng/ml VEGF was added in the lower chamber. Two hours later, the cells on the upper part of the membrane were scraped, and those that migrated to the lower part were stained with Meyer’s hematoxylin and were counted under an inverted microscope. Assays were done in duplicates or triplicates. HUVECs were electroporated with an siRNA that specifically targets GAPDH mRNA or with increasing concentrations of siRNA that specifically target human FAK mRNA (Dharmacon). Thereafter, cells were transfected for 48 h with 1 μg of nonhuman forms (chicken/mouse) of FAK that share >90% identity between each other but that are insensitive to human FAK sRNA. Then, HUVECs were treated with 5 ng/ml VEGF and processed for a migration assay in modified Boyden chambers. Coelectroporation of an EGFP-expressing plasmids allows evaluating migration of electroporated cells only.
RESULTS
FAK Is Phosphorylated on Ser732 in Response to VEGF, and the Phosphorylation Is Impaired by Inhibiting HSP90 or ROCK
FAK is phosphorylated on several tyrosyl residues in response to VEGF (Abu-Ghazaleh et al., 2001; Eliceiri et al., 2002; Le Bœuf et al., 2004). This kinase also possesses four potentially phosphorylatable serine residues located in its C terminus: Ser722, Ser732, Ser843, and Ser910. We previously reported that FAK is phosphorylated on Tyr407 downstream from VEGFR2-mediated RhoA–ROCK activation (Le Bœuf et al., 2004). In the present study, we investigated whether the FAK serine residues are phosphorylated by VEGF treatment, in an attempt to characterize their function, and whether they influence phosphorylation of FAK on Tyr407.
Primary cultures of HUVECs were treated with 5 ng/ml VEGF for increasing periods. Thereafter, cell extracts were prepared, and FAK phosphorylation on Ser residues was determined by Western blot using phospho-specific antibodies. Results show that VEGF did not induce the phosphorylation of Ser722 and 910 (Figure 1A). In contrast, the phosphorylation of Ser732 (arrow) and Ser843 was increased in response to VEGF, although the kinetics of phosphorylation was different (Figure 1A). Phosphorylation of Ser843 started only after 5 min to reach a peak at 15 min, whereas Ser732 was phosphorylated more rapidly reaching a peak between 5 and 10 min (Figure 1A). Interestingly, the kinetics of phosphorylation of Ser732 and Tyr407 (arrows) were comparable particularly for the first 10 min of treatment (Figure 1B). Moreover, the phosphorylation of Ser732 but not of Ser843 was inhibited after the inhibition of HSP90 and ROCK with geldanamycin and Y27632, respectively (Figure 2). Given that phosphorylation of Tyr407 necessitates the association of HSP90 with the C-terminal end of VEGFR2 and then the sequential activation of RhoA and ROCK, our findings highlight the possibility that the phosphorylation of Ser732 and Tyr407 shares a common cascade of activation and that they are mutually interdependent.
A FAK Nonphosphorylatable Mutant of Ser732 Abrogates the Phosphorylation of Tyr407 and the Recruitment of Vinculin at the Ventral Focal Adhesions
We next investigated the causal relationship between phosphorylation of Ser732 and Tyr407. HUVECs were electroporated with plasmids expressing wild-type HA-FAK, HA-FAK Tyr407Phe, or HA-FAK Ser732Ala and were treated or not with VEGF. Then, the HA-tag constructs were immunoprecipitated, and phosphorylated Ser732 was immunodetected in cell extracts expressing FAK Tyr407Phe (Figure 3A). Reciprocally, phosphorylated Tyr407 was immunodetected in cell extracts expressing FAK Ser732Ala (Figure 3B). We found that the expression of the mutant FAK Tyr407Phe had no effect on the phosphorylation of Ser732 induced by VEGF (Figure 3A). In contrast, the expression of the mutant Ser732Ala impaired the phosphorylation of FAK Tyr407 in response to VEGF (Figure 3B). These results suggest that phosphorylation of Ser732 within FAK is a prerequisite for the phosphorylation of Tyr407. Given that Tyr397 is the autophosphorylation site of FAK and thereby is importantly involved in FAK-mediated events, we next verified whether the mutants Ser732 or Tyr407 compromised the phosphorylation of Tyr397. HUVECs were electroporated, as described above, with plasmids expressing wild-type HA-FAK, HA-FAK Tyr407Phe, or HA-FAK Ser732Ala and were treated or not with VEGF. Then, the HA-TAG constructs were immunoprecipitated, and phosphorylated Tyr397 was immunodetected in cell extracts expressing the various forms of HA-FAK. The results show that the phosphorylation of Tyr397 was not affected either by the mutant Ser732Ala or Tyr407Phe (Figure 3C).
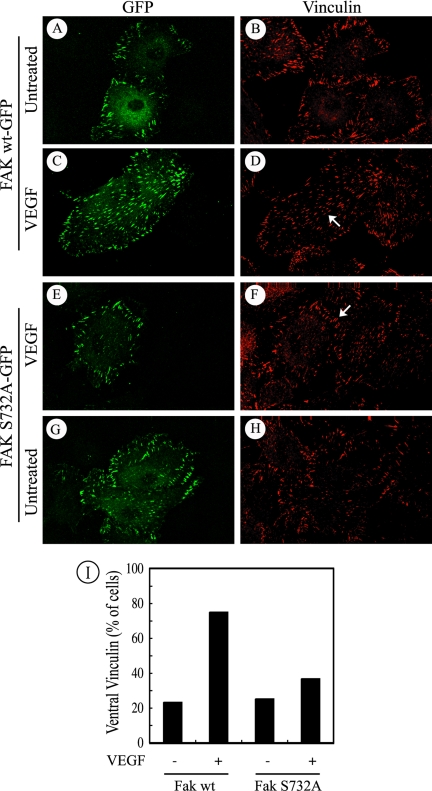
We previously reported that phosphorylation of Tyr407 is required for the recruitment of vinculin to the ventral focal adhesions (Le Bœuf et al., 2004). In this context, one may expect that phosphorylation of Ser732 is required for the recruitment of vinculin to focal contacts. We next verified this possibility. HUVECs were electroporated with plasmids expressing GFP-tagged versions of wild-type FAK or Ser732Ala mutated form of FAK and were treated or not with VEGF. Then, cells were examined in fluorescence microscopy to detect vinculin in GFP-FAK–expressing cells. The results showed that, in cells expressing both wild-type and Ser732Ala forms of FAK, vinculin remained at the periphery of the cells (Figure 4, A and B, G and H). In the presence of VEGF, vinculin was recruited to the ventral adhesion plaques in ∼80% of the cells expressing wild-type (wt) FAK (Figure 4, C and D, arrow, and I). Conversely, even in the presence of VEGF, vinculin still remained at cell periphery in most of the cells expressing the Ser732Ala-mutated form of FAK (Figure 4, E–F, arrow, and I). Of note, after VEGF treatment, the GFP-FAK Ser732Ala mutant remained itself at the periphery of the cells, whereas the wt GFP-FAK localized to ventral focal adhesions. Overall, these results suggest that phosphorylation of FAK on Ser732 is required to trigger the formation of the ventral focal adhesions.
Figure 4.
Phosphorylation FAK on Ser732 is necessary for recruitment of vinculin in response to VEGF. Quiescent HUVECs transiently expressing wild-type FAK fused to GFP-tag or mutant FAK-Ser732Ala fused to GFP-tag were plated on gelatin-coated Lab-Tek chambers and left untreated (A and B, G and H) or were exposed to 5 ng/ml VEGF for 5 min (C–F). After treatments, cells were fixed, permeabilized, and costained for GFP detection (A, C, E, and G) using an anti-GFP antibody and then an anti-rabbit IgG-fluorescein isothiocyanate-conjugated antibody for detection in confocal fluorescence microscopy. Vinculin (B, D, F, and H) was detected using specific antibody coupled with a biotin-labeled anti-mouse IgG and revealed with Texas Red-conjugated streptavidin. Representative fields from two separate experiments are shown. The arrow in D denotes the recruitment of vinculin to the ventral focal adhesions in cells transfected with wild-type FAK and treated with 5 ng/ml VEGF. The arrow in F shows that, even in the presence of VEGF, vinculin remains at the periphery of the cells expressing the Ser732Ala mutant. (I) Quantification of cells showing recruitment of vinculin to the ventral focal adhesions. Quantification has been done on 400–500 cells for each condition.
ROCK Directly Phosphorylates Ser732 within FAK in Response to VEGF
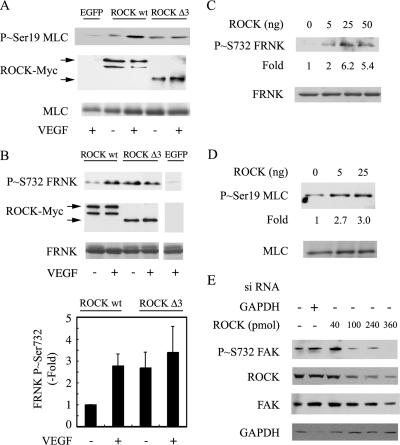
Because the phosphorylation of Ser732 was impaired by inhibiting ROCK with Y27632, we developed an immunocomplex kinase assays to assess whether ROCK may directly phosphorylate Ser732 within FAK. BAECs were transfected with plasmids expressing Myc-tagged versions of wild-type ROCK (wt-ROCK) or constitutively active ROCK I (ROCK-Δ3) (Ishizaki et al., 1997). Thereafter, the cells were treated with VEGF, and the different forms of transfected Myc-tagged ROCK were immunopreciptated with an anti-Myc antibody. The immunoprecipitates were used to assay the kinase activity of ROCK using purified GST-FRNK or MLC as substrates. FRNK is a 45-kDa noncatalytic peptide of FAK that contains Ser732 (Parsons, 2003). We used FRNK as substrate instead of full-length FAK to eliminate any catalytic activity of FAK. MLC was used as a positive control substrate to set up the assay, because it is a well-known target of ROCK (Totsukawa et al., 2000; Matsumura, 2005). As expected, we found that VEGF increased the phosphorylation of MLC by immunoprecipitated wt-ROCK and that ROCK-Δ3 increased by itself the phosphorylation of MLC. VEGF did not affect the phosphorylation level of MLC by constitutively active ROCK (Figure 5A). These results indicated that the ROCK assay was functional, and they confirmed that ROCK was activated by VEGF. Similar results were obtained when FRNK was used as a substrate to ascertain the ability of ROCK to phosphorylate Ser732. We found that VEGF increased by 2.7-fold the phosphorylation of Ser732 by immunoprecipitated wt-ROCK. Moreover, ROCK-Δ3 increased by itself by 2.5-fold the phosphorylation of Ser732, and VEGF did not significantly modify the level of phosphorylation of Ser732 by ROCK-Δ3 (Figure 5B). As expected, in cells transfected only with EGFP, no myc-ROCK and no phosphorylation of Ser732 was detected after immunoprecipitation with the anti-Myc-antibody. These results suggested that ROCK directly phosphorylated FAK on Ser732 in response to VEGF. To eliminate the possible influence of another kinase that might have coimmunoprecipitated with myc-ROCK in the immunocomplex assay, we proceed to a direct in vitro kinase assay. In this assay, purified activated ROCK I was incubated with purified FRNK or MLC and thereafter, the phosphorylation of Ser732 within FRNK and Ser19 within MLC were evaluated in Western blot. We found that activated ROCK induced the phosphorylation of MLC on Ser19 and FRNK on Ser732 (Figure 5, C and D). Together, these results indicate that ROCK directly phosphorylates FAK on Ser732 in response to VEGF. The in vivo relevance of ROCK I in phosphorylating Ser732 in whole cells treated by VEGF was obtained by showing that the knockdown of ROCK I with siRNAs (smart pools from Dharmacon that target ROCK I mRNA) was associated with a proportional decrease in the phosphorylation of FAK on Ser732 (Figure 5E).
Figure 5.
ROCK directly phosphorylates FAK on Ser732 in response to VEGF. (A) BAECs transiently expressing EGFP, wild-type myc-tagged-ROCK I or constitutively active Myc-tagged ROCK I-Δ3 were treated or not with 10 ng/ml VEGF for 5 min and extracted. The Myc-tagged ROCK protein was immunoprecipitated using anti-Myc mouse antibody. Myc-tagged ROCK protein was processed for an immunocomplex in vitro kinase assay using MLC as substrate in the presence of 0.5 mM ATP during 20 min. Proteins were separated by SDS-PAGE and transferred to a nitrocellulose membrane. The membrane was processed for immunodetection of phospho-Ser19 within MLC (top) and Myc-tagged ROCKs (middle). Ponceau Red staining of total MLC is shown as internal control for the amount of added substrate. (B) BAECs were processed as in described A, except that FRNK was used as substrate. The membrane was processed for immunodetection of phospho-Ser732 within FAK (top). Myc-tagged ROCKs are shown in the middle panel, and Ponceau Red staining to monitor FRNK in the bottom panel. Representative blots are shown, and quantification of the blots from duplicate samples from five separate experiments (means ± SD) is shown. (C and D) Direct in vitro kinase assay was performed using increasing amounts of constitutively active form of ROCK I that was incubated with FRNK (C) or MLC (D) as substrates. Proteins were separated by SDS-PAGE and transferred to a nitrocellulose membrane. The membrane was processed for immunodetection of phospho-Ser732 within FRNK or phospho-Ser19 within MLC. Immunodetection of total FRNK and total MLC are shown (bottom). (E) Quiescent HUVECs were electroporated with 40 pmol of siRNA that specifically targets GAPDH mRNA or with increasing concentrations of siRNAs that specifically target ROCK I mRNA (smart pools from Dharmacon). Forty-eight hours later, cells were treated for 10 min with 5 ng/ml VEGF, and proteins were extracted separated by SDS-PAGE and transferred to a nitrocellulose membrane. The membrane was processed for immunodetection of phospho Ser732 FAK, ROCK I, total FAK, or GAPDH.
VEGF Induces the Phosphorylation of Pyk-2, Which Leads to Phosphorylation of Tyr407 within FAK
The FAK-related kinase Pyk2 is recruited to Tyr747 within integrin β3 in adhering leukocytes (Butler and Blystone, 2005). Moreover, Pyk2 has been proposed to phosphorylate FAK on Tyr407 during epithelial mesenchymal transdifferentiation (Li et al., 1999; Nakamura et al., 2001). Considering that integrin αvβ3 is implicated in transmitting the VEGF signal to FAK in endothelial cells maintained on matrices, such as vitronectin and gelatin, that bind αvβ3 (Petitclerc et al., 1999; Masson-Gadais et al., 2003), we investigated whether Pyk2 was involved in VEGF signaling and especially in phosphorylating FAK on Tyr407.
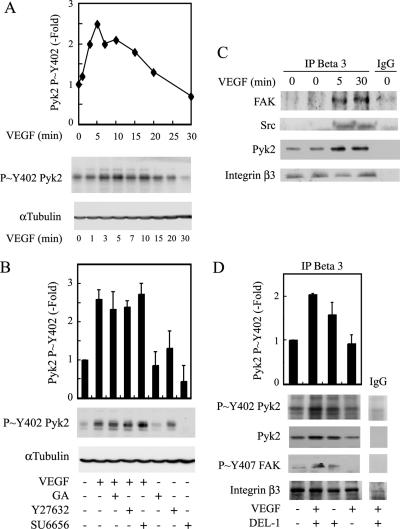
We first verified whether VEGF triggers the activation of Pyk2. HUVECs cultivated on gelatin were treated for various periods in the presence of VEGF. Then, cell extracts were prepared, and the activation of Pyk2 was evaluated by quantifying the phosphorylation level of its autophosphorylation site Tyr402 in Western blot. Results showed that the activation of Pyk2 was quickly increased by VEGF to reach a peak at 5 min, after which it progressively decreased to return to basal level after 20 min (Figure 6A). Interestingly, the VEGF-induced activation of Pyk2 was independent of both HSP90 and ROCK, being not inhibited by geldanamycin or Y27632 (Figure 6B). These results suggest that Pyk2 is not in the same linear pathway as that linking ROCK to VEGFR2. Of note, the activation of Pyk2 was also independent of Src kinases, being not inhibited by SU6656 (Figure 6B).
Figure 6.
Pyk2 is phosphorylated in an HSP90- and ROCK-independent manner in response to VEGF, and it associates with integrin β3 subunit. (A) Quiescent HUVECs were treated or not with 5 ng/ml VEGF for increasing periods. Cells were then extracted, and proteins were separated by SDS-PAGE and transferred to a nitrocellulose membrane. The membrane was processed for immunodetection of phospho-Pyk2 Tyr402 (top), and total α-tubulin (bottom) to monitor loading. Representative autoradiograms are shown, and data points represent means of two separate experiments. (B) Quiescent HUVECs were pretreated for 60 min with 1 μg/ml geldanamycin (GA) or vehicle (0.25% DMSO), or for 120 min with 25 μM Y27632 or 5 μM SU6656. Cells were treated or not with 5 ng/ml VEGF for 5 min and were extracted and processed as described in A. Representative blots are shown, and data points represent means ± SD of three experiments. (C) Quiescent HUVECs were treated or not with 5 ng/ml VEGF for 5 and 30 min. Cells were extracted, and integrin β3 was immunoprecipitated. Control immunoprecipitation was done similarly using preimmune mouse IgG. Proteins were separated by SDS-PAGE and transferred to a nitrocellulose membrane. The membrane was processed for Western blot using anti-FAK mouse antibody, anti-Src mouse antibody, and anti-Pyk2 mouse antibody. The membrane was reprobed for total integrin β3 using anti-β3 mouse antibody to ensure equal protein loading (fourth panel). (D) HUVECs were trypsinized and left in suspension for 20 min in serum-free medium. Then, peptide 75 nM DEL-1 was added or not for 30 min. Suspended cells were treated or not with 5 ng/ml VEGF for the last 5 min. Cells were then extracted, and proteins were separated on SDS-PAGE and transferred on nitrocellulose membrane. The membrane was processed for immunodetection of phospho-Pyk2 Tyr402, total Pyk2, and phospho-FAK Tyr407. The membrane was reprobed for total integrin β3 to ensure equal protein loading (fourth panel). Representative blots are shown, and data points represent means ± SD of duplicate sample of two experiments.
We investigated next whether the activation of Pyk2 by VEGF may derive from the activation of integrin subunit β3, to which it is recruited along with Src and FAK in a VEGF-sensitive manner (Figure 6C). To demonstrate this point, we used HUVECs in suspension, because integrins are not engaged in cells maintained in suspension. We then treated the cells with VEGF and/or DEL-1, a peptide that specifically induces the clustering of integrin αvβ3 even in cells in suspension (Penta et al., 1999). Results show that DEL-1 increased the association of activated Pyk2 (p∼Y402 Pyk2) with integrin β3. The effect was more pronounced in the presence of VEGF (Figure 6D, top). These findings suggest that the activation of Pyk2 derives from the activation of integrin β3 associated with VEGFR2. Interestingly, the phosphorylation of Tyr407 within FAK was phosphorylated in suspended cells exposed to both VEGF and DEL-1 but not DEL-1 alone (Figure 6D, third panel). From this result, we hypothesize that Pyk2 may contribute to phosphorylate Tyr407 and that this might be associated with a VEGF-induced conformational change that renders FAK Tyr407 accessible to Pyk2 activated in response to activation of αvβ3. However, further studies, including crystallographic studies, should be done to ascertain this possibility.
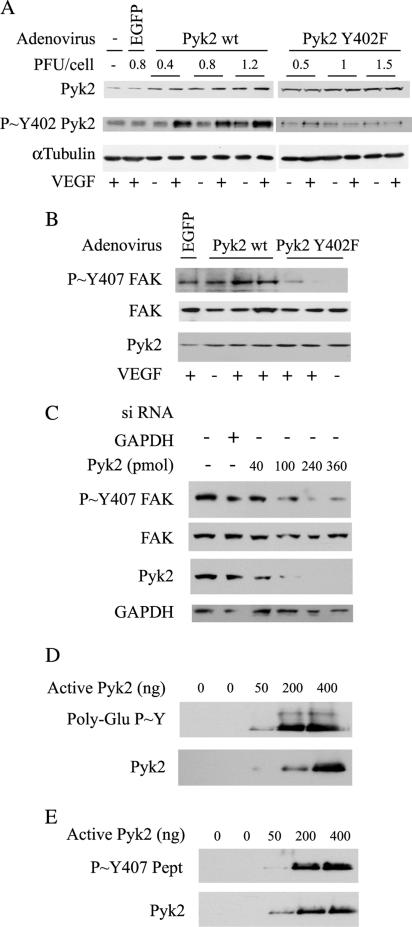
To more firmly ascertain that Pyk2 was involved in phosphorylating Tyr407, we realized experiments in which we measured the VEGF-mediated phosphorylation of Tyr407 in HUVECs expressing a wt form or a Tyr402 phosphorylation-deficient mutant form of Pyk2 (Tyr402Phe) after adenoviral gene transfer (Melendez et al., 2004). We first determined the amount of adenovirus required for optimal expression of both forms of Pyk2 by infecting the cells with increasing quantities of viruses. We found that 15 ml (6 × 105 plaque-forming units [PFU]) was the volume of virus that yields the best expression and the best VEGF-induced activation of Pyk2 without conferring any observable toxic effect (Figure 7A). Indeed, this volume of virus corresponds to a multiplicity of infection of 1.2 PFU/cells, which is compatible with the infection rate of >90% that we found in fluorescence microscopy (Supplemental Data 1). As expected, VEGF did not induce the phosphorylation of Pyk2 at any concentration of viruses expressing the mutant Tyr402Phe. We next investigated the involvement of Pyk2 in contributing to phosphorylate FAK on Tyr407. HUVECs were infected with viruses carrying the wt form of Pyk2 or the mutant Pyk2 (Tyr402Phe). Then, the phosphorylation of Tyr407 was evaluated. Results showed that the VEGF-induced phosphorylation of Tyr407 was increased in cells expressing wt-Pyk2 and inhibited in cells expressing the nonphosphorylatable Tyr402Phe mutant (Figure 7B). Similarly, we found that the knockdown of Pyk2 with siRNAs (smart pools from Dharmacon that target human Pyk2 mRNA) was associated with a marked decrease in the phosphorylation of FAK on Tyr407 (Figure 7C). To determine whether the Pyk2-mediated phosphorylation of Tyr407 within FAK was direct, we proceed to a direct in vitro kinase assay. In this assay, purified activated Pyk2 was incubated with a synthetic Tyr407 peptide EIIDEEDTY407TMPSTRD or using the [GG(EEEEY)10EE] biotin conjugate peptide as a positive control. Thereafter, the phosphorylation of Tyr407 within the Tyr407FAK peptide and the poly Glu-Tyr peptide were evaluated in Western blot. We found that activated Pyk2 induced the tyrosine phosphorylation of both peptides (Figure 7, D and E). Overall, these findings are strong indications that Pyk2 contributes to directly phosphorylate FAK on Tyr407 in response to VEGF.
Figure 7.
Pyk2 mediates the phosphorylation of FAK on Tyr407 in response to VEGF. (A) Exponentially growing HUVECs were infected or not with increasing quantity of adenoviral vectors carrying EGFP, wild-type Pyk2, or mutant Pyk2 Tyr402Phe. Twenty-four hour later, the medium was changed for serum-free medium and grown for 16 h. Quiescent cells were then treated or not with 5 ng/ml VEGF for 10 min. Proteins were extracted, run into SDS-PAGE, and transferred on nitrocellulose membrane. Immunodetections with mouse anti-Pyk2, rabbit anti-P∼Y402-Pyk2, and mouse anti-α-tubulin are shown. (B) Exponentially growing HUVECs were infected with adenoviral vector carrying EGFP (0.8 PFU/cell), wild-type Pyk2 (1.2 PFU/cell), or mutant Pyk2 Tyr402Phe (1.2 PFU/cell) and were treated or not for 10 min with 5 ng/ml VEGF. Then, proteins were processed as described in A. Immunodectections with rabbit anti-P∼Y407-FAK (top), mouse anti-FAK (middle), and mouse anti-Pyk2 (bottom) are shown. Representative autoradiograms from two separated experiments are shown. (C) Quiescent HUVECs were electroporated with siRNA that specifically targets GAPDH mRNA or with increasing concentrations of siRNAs that specifically target Pyk2 mRNA (smart pools from Dharmacon). Forty-eight hours later, cells were treated for 10 min with 5 ng/ml VEGF, and proteins were extracted. Proteins were separated by SDS-PAGE and transferred to a nitrocellulose membrane. The membrane was processed for immunodetection of phospho-Tyr FAK407, total FAK, Pyk2, or GAPDH. (D and E) Direct in vitro kinase assay was performed using increasing amounts of constitutively active form of Pyk2 that was incubated with a poly-Glu-Tyr peptide (D) or synthetic FAK-Tyr407 peptide (E) as substrates. Proteins were separated by Tris-Tricine SDS-PAGE and transferred to a nitrocellulose membrane. The membrane was processed for immunodetection of phospho-Tyr407 within the FAK-Tyr407 peptide or phospho-Tyr (E) within the poly-Glu-Tyr peptide (D).
Phosphorylation of Ser732 and Tyr407 Is Required for Cell Migration in Response to VEGF
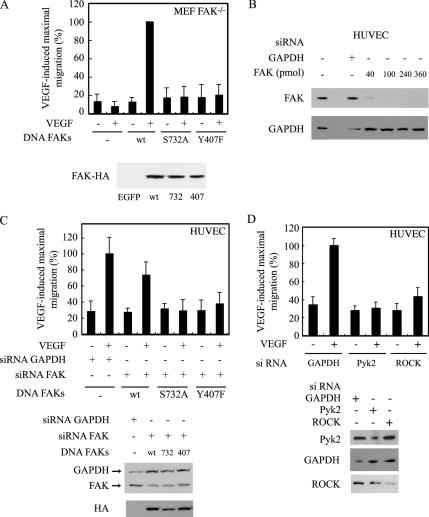
We previously reported that the VEGFR2-HSP90 ROCK-axis is essential to trigger endothelial cell migration (Le Bœuf et al., 2004). This raised the possibility that the phosphorylation of Ser732 and Tyr407 was required to promote cell migration in response to VEGF. To prove this point, we first used a fibroblastic cell line that is null for FAK (FAK−/−) (Ilic et al., 1995) and in which we transfected vectors expressing VEGFR2 along with vectors expressing FAK wt or the FAK mutants (FAK Ser732Ala and FAK Tyr407Phe). The cellular concentration of FAK is critical for cell migration, because cells that adhere too strongly or too weakly do not migrate or migrate slowly (Palecek et al., 1997; Richardson et al., 1997). Hence, we first determined the concentration of FAK plasmids that induced maximal migration in response to VEGF using the Boyden chamber assay. We found that cell migration in response to VEGF was maximally increased with FAK plasmids added in concentration between 1 and 2 μg (Supplemental Data 2). Thereafter, we transfected FAK−/− cells with 1 μg of vectors expressing wt-FAK or the same amount of the mutants FAK Ser732Ala or FAKTyr407Phe together with 4 μg of vector expressing VEGFR2. As expected, we found that cell migration was completely impaired in cells that expressed the mutants being in marked contrast with the fivefold increase induced by VEGF in cells expressing wt form of FAK. Interestingly, at this concentration of plasmid, FAK does not promote cell migration by itself indicating that VEGF is required (Figure 8A). Given that MEF−/− cells may differ from endothelial cells in their response to VEGF, we next electroporated HUVECs with siRNA to specifically target human FAK mRNA and knockdown endogenous FAK. In comparison, we used siRNA against GAPDH as controls. Thereafter, we reintroduced siRNA-insensitive nonhuman wt form of FAK or the mutant forms Tyr407Phe or Ser 732Ala at the concentration of 1 μg of vectors. After 48 h, we proceeded to a cell migration assay in Boyden chamber using or not VEGF as chemoattractant in the lower chamber. Results showed that VEGF increased by 2.5-fold the migration of HUVECs in which we have reintroduced wt-FAK. However, the migration remains to basal level in cells in which the mutant Ser732Ala or Tyr407Phe were added (Figure 8B). Interestingly, the siRNA against GAPDH did not affect cell migration. It did not affect either the expression of FAK, whereas the FAK siRNA almost completely impaired the expression of endogenous FAK (Figure 8C).
Figure 8.
Phosphorylation of Ser732 and Tyr407 is required for cell migration in response to VEGF. (A) MEF FAK−/− cells were transfected with 1 μg of EGFP, wild-type FAK (wt), FAK Ser732Ala (S732A), or FAK Tyr407Phe (Y407F) mutants, along with a constant concentration of VEGFR2 (4 μg /ml). Then, the cells were processed as described in A for a migration assay in a modified Boyden chamber. Data points represent the mean ± SD of two separate experiments in triplicate and duplicates. To monitor the expression of transfected FAK, MEF cells used for migration were processed for immunodetection of HA-FAK proteins (bottom). (B) Quiescent HUVECs were electroporated with siRNA that specifically targets GAPDH mRNA or with increasing concentrations of siRNA that specifically target human FAK mRNA (from Dharmacon). Forty-eight hours later, proteins were extracted, separated by SDS-PAGE, and transferred to a nitrocellulose membrane. The membrane was processed for immunodetection of total FAK or GAPDH. (C) Quiescent HUVECs were electroporated as described above with siRNAs to knockdown human GAPDH or FAK (40 pmol of siRNA). Thereafter, cells were transfected with 1 μg of nonhuman forms of FAK and were processed as described in B for a migration assay in response to 5 ng/ml VEGF using a modified Boyden chamber. Data points represent the mean ± SD of triplicate samples. The efficacy of siRNA knockdowns was determined in Western blotting as described in B (middle). To monitor the expression of transfected FAK, HUVECs used for migration were processed for immunodetection of HA-FAK proteins (bottom). (D) Quiescent HUVECs were electroporated as described above with GAPDH (40 pmol) or Pyk2 or ROCKI (360 pmol each) siRNAs to knockdown the expression of human GAPDH, Pyk2, or ROCK. Thereafter, the cells were processed as described in A for a migration assay in a modified Boyden chamber. Data points represent the mean ± SD of triplicate samples. The efficacy of siRNA knockdowns was determined in Western blotting as described in C (bottom). The results of cell migration are expressed as the percentage of maximal increase in cell migration induced by VEGF (mean number of transfected cells counted in controls, 10 cells). Similar results were obtained in two separate experiments.
Given that ROCK I and Pyk2 were, respectively, required to directly phosphorylate Ser732 and Tyr407, we then decided to ascertain whether these kinases regulate endothelial cell migration. To prove this point, we still relied on the use of siRNA to knockdown ROCK1 or Pyk2 and then looked at the effect of VEGF on cell migration evaluated in Boyden chamber. As expected, we found that the knockdown of both kinases was associated with an inhibition of VEGF-induced cell migration (Figure 8D).
Overall, these findings highlight for the first time that the ROCK-mediated phosphorylation of Ser732- and Pyk2-mediated phosphorylation of Tyr407 is sequentially required to initiate cell migration in response to VEGF.
DISCUSSION
Endothelial cell migration induced by VEGF requires its binding to VEGFR2 and the association of ligand-bound VEGFR2 with integrin αvβ3 (Bernatchez et al., 1999; Soldi et al., 1999; Borges et al., 2000; Rousseau et al., 2000b; Masson-Gadais et al., 2003). The VEGF-VEGFR2/αvβ3 complex is then involved in initiating signals that activate various pathways, including p38 and FAK. FAK is a nonreceptor kinase whose activation is required to drive the cytoskeleton reorganization essential for cell migration (Rousseau et al., 2000b; Bohnsack and Hirschi, 2003; Parsons, 2003). The mechanisms by which FAK is activated to trigger cell migration by VEGF involve the phosphorylation of Tyr407 and Tyr576 (Holmqvist et al., 2004; Le Bœuf et al., 2004). However, the mechanisms that govern the phosphorylation of these residues are ill defined. In the present study, we show the first series of evidence indicating that the phosphorylation of FAK on Tyr407 relies on the activation of Pyk2 and is dependent on ROCK-mediated phosphorylation of FAK on Ser732.
We previously reported that phosphorylation of Tyr407 within FAK requires the activation of ROCK (Le Bœuf et al., 2004). However, that ROCK (both ROCK I and ROCK II) has serine-threonine kinase activity indicates that it cannot directly phosphorylates Tyr407 within FAK. A major contribution of the present study is to show for the first time that ROCK directly phosphorylates FAK on Ser732 in response to VEGF. This is supported by the results of the immunocomplex kinase assays in which we found that VEGF induced the phosphorylation of Ser732 within FRNK by immunoprecipitated wt-ROCK and that a constitutively active form of ROCK could phosphorylate Ser732. Moreover, similar results were obtained when using MLC as substrate. Given that MLC is a known target of ROCK, this finding confirms the suitability of the assay and that VEGF activates ROCK. Overall, these results converge on the concept that ROCK can directly phosphorylate Ser732 within FAK. This conclusion is further emphasized by our finding that ROCK can directly phosphorylate Ser732 within FRNK in a direct in vitro assay. Moreover, the in vivo relevance of ROCK in mediating phosphorylation of Ser732 is supported by the observations that the knockdown of ROCK I with siRNA is associated with an inhibition of the phosphorylation of FAK on Ser732 and of cell migration. Hence, based on our findings we believe that ROCK is the major kinase involved in phosphorylating directly Ser732 within FAK in response to VEGF. However, one cannot exclude the possibility that another kinase particularly cdk5 might also contribute to the direct phosphorylation of this site, as it does in neurons during corticogenesis (Xie et al., 2003). Of note, the various ROCK constructs that we used in our study express the ROCK I isoform, which supports the involvement of ROCK I in phosphorylating Ser732. However, the possible participation of ROCK II cannot be excluded.
Another important contribution of our study was to show that Ser732 within FAK should be phosphorylated to allow phosphorylation of FAK on Tyr407. In particular, the kinetics of activation of Ser732 and Tyr407 are comparable and are both inhibited by impairing HSP90-mediated events with geldanamycin and ROCK with Y27632. More directly, that Ser732 should be phosphorylated before phosphorylation of Tyr407 is supported by the finding that expression of a nonphosphorylatable form of Ser732 (Ser732Ala) within FAK inhibits the phosphorylation of Tyr407 induced by VEGF, whereas expression of Tyr407Phe does not inhibit the phosphorylation of Ser732. We previously reported that phosphorylation of Tyr407 is required for the assembly of focal adhesions. In accordance with the fact that Ser732 should be phosphorylated to allow phosphorylation of Tyr407, we found that the Ser732Ala mutant impairs the recruitment of vinculin to the ventral focal adhesions and that FAK Ser732Ala does not relocalize to the ventral plaques. Overall, these results indicate that ROCK-dependent phosphorylation of Ser732 is essential to elicit phosphorylation of Tyr407 and P∼Tyr407–mediated events, possibly by changing the conformation of FAK in a way that renders Tyr407 accessible to a tyrosine kinase. Interestingly, VEGF should induce a conformation change in FAK configuration to allow its association with integrin αvβ5 (Eliceiri et al., 2002; Schaller, 2004). This finding constitutes a major paradigm in favor that VEGF regulates FAK function in a spatial manner.
Pyk2, a member of the FAK family of nonreceptor protein kinase, has been proposed as a potential candidate to directly phosphorylate FAK on Tyr407 (Li et al., 1999; Nakamura et al., 2001). Indeed, using an in vitro kinase assay, we found that a constitutively active form of Pyk2 can directly phosphorylate Tyr407 within a synthetic FAK peptide. Moreover, the siRNA knockdown of Pyk2 is associated with an inhibition of FAK phosphorylation on Tyr407 in vivo. In line with these findings indicating that Pyk2 is a kinase directly upstream of Tyr407, we found that VEGF induces the phosphorylation of Pyk2 on its autophosphorylation site Tyr402 and that this occurs within the same time frame as phosphorylation of Tyr407 within FAK. However, the VEGF-induced phosphorylation of Tyr402 within Pyk2 is not sensitive to geldanamycin, Y27632, or SU6656, indicating that it is independent of HSP90, ROCK, or Src kinases. The discovery that activation of Pyk2 is independent of HSP90 and ROCK is a strong indication that the activation of Pyk2 does not directly emanate from the same VEGFR2 signaling pathway that phosphorylates Ser732. VEGFR2 signaling requires its association with integrin αvβ3 (Soldi et al., 1999; Borges et al., 2000; Byzova et al., 2000; Masson-Gadais et al., 2003). Moreover, αvβ3 ligation induces phosphorylation of Pyk2 on Tyr402 and favors its association with the cytoplasmic tail of β3 in K562 leukocytes adhering to vitronectin (Butler and Blystone, 2005). Hence, activation of Pyk2 may derive from integrin αvβ3. Accordingly, Pyk2 is recruited to integrin β3 and FAK after exposure of HUVECs to VEGF. Most importantly, we obtained direct evidence that activation of Pyk2 by VEGF derives from integrin αvβ3. Indeed, P∼Tyr402 on Pyk2 is found at low levels in endothelial cells maintained in suspension, a condition that precludes clustering of integrins (Germer et al., 1998; Stupack et al., 1999). In contrast, the specific clustering and activation of integrin αvβ3 by treating HUVECs in suspension with the peptide DEL-1 (Penta et al., 1999) is followed by the recruitment of Pyk2 to integrin β3 and is associated with an increase in the level of tyrosine phosphorylation of its Tyr402. Moreover, both effects are further increased by coexposure of cells to VEGF and DEL-1, whereas they remain at basal level in the presence of VEGF alone. Interestingly, the DEL-1 peptide-mediated clustering of integrin αvβ3 and recruitment and activation of Pyk2 is not sufficient by itself to increase the phosphorylation of Tyr407 within FAK. However, phosphorylation of Tyr407 is increased when the cells are treated by both DEL-1 and VEGF, which is consistent with the aforementioned possibility that the VEGFR2-dependent activation of Ser732 is required to induce a conformational change that renders Tyr407 accessible to activated Pyk2.
Phosphorylation of Ser732 and Tyr407 within FAK has both been associated with increased cell migration in neuron and during epithelial mesenchymal transdifferentiation (Nakamura et al., 2001; Xie et al., 2003). However, our study is the first to bring direct evidence that the phosphorylation of both Ser732 and Tyr407 are essential for endothelial cell migration induced by VEGF. This is supported first by the observation that the expression of Ser732Ala and Tyr407Phe mutants of FAK in FAK MEF−/− cells or in HUVECs depleted of FAK by means of siRNA does not promote cell migration in response to VEGF, in contrast to the expression of the wt form of FAK. Second, this is also supported by our findings that the knockdowns of ROCK I or Pyk2, the kinases respectively responsible for the phosphorylation of Ser732 and Tyr407, are also associated with an inhibition of VEGF-induced cell migration. Interestingly, neither the Ser732Ala nor the Tyr407Phe mutants modified the autophosphorylation of FAK on Tyr397, which excludes the possibility that the effect of these mutants on cell migration results from an in inhibition of the phosphorylation of FAK on Tyr397. In this context, it was previously reported that the kinase activity of FAK and its phosphorylation on Tyr397 are both independent of phosphorylation of Ser732 (Xie et al., 2003). Given that the phosphorylation of Ser732 depends on signaling events initiated by the activation of VEGFR2–HSP90–ROCK pathway, these results are important, because they explain why this pathway is essential for cell migration (Le Bœuf et al., 2004). In addition, the observation suggesting that the phosphorylation of Ser732 induces a conformational change that is necessary to trigger the phosphorylation of Tyr407 through activated Pyk2 explains why phosphorylation of Tyr407 is also essential for cell migration. Incidentally, that phosphorylation of Tyr407 requires the activation of Pyk2 might explain why the integrin and chemokine-stimulated motility is impaired in macrophages that are defective in Pyk2, and that the inhibition is not compensated by FAK expression (Okigaki et al., 2003; Mitra et al., 2005). The result is also consistent with previous results showing that Pyk2 is a regulator of VEGF-induced cell migration (Avraham et al., 2003). However, a recent report shows that Pyk2 is not involved in mediating LPA-induced increased cell migration of intestinal epithelial cells IEC-6 and IEC-18 grown on fibronectin (Jiang et al., 2006). A possible explanation is that the lysophosphatidic acid receptor does not associate with αvβ3 integrin to concourse to activation of Pyk2 in response to VEGF.
Overall, the results of our study indicate that the VEGF-induced endothelial cell migration requires integrin αvβ3-dependent Pyk2-mediated phosphorylation of FAK on Tyr407, an event that requires appropriate conformational change induced in FAK by VEGFR2-mediated phosphorylation of Ser732 by ROCK.
Supplementary Material
ACKNOWLEDGMENTS
We thank Drs. Josée N. Lavoie (Université Laval) for help in setting up the ROCK in vitro kinase assay, S. Narumiya for the ROCK constructs, and Dusko Ilic (University of California, San Francisco, San Francisco, CA) for the MEF FAK−/− cells. We also thank Drs. Jun-Lin Guan for providing the human wt-HA-FAK cDNA, Zhigang Xie and Li-Huei Tsai (Harvard University, Cambridge, MA) for FAK S732A and FAK-GST constructs, and Mark Sussman (San Diego State University Heart Institute, San Diego, CA) for adenoviral Pyk2 constructs. We thank Drs. Darren Richard (Université Laval) and Martin Sirois (Institut de Cardiologie de Montréal, Montréal, Canada) for providing BAECs. Dr. Darren Richard also provided the siRNA to target GAPDH mRNA and the porcine anti-GAPDH antibody. Finally, we thank Dr. Jean-Philippe Gratton (Institut de Cardiologie de Montréal) for critically reading the manuscript. This work was supported by The Canadian Institutes of Health Research Grant MOP 15402. F. L. holds a studentship from Le Fonds de la Recherche en Santé du Québec.
Abbreviations used:
- BAEC
bovine aortic endothelial cell
- GAPDH
glyceraldehyde-3-phosphate-dehydrogenase
- FAT
focal adhesion targeting
- FERM
protein four.1, ezrin, radixin, moesin
- FRNK
FAK related nonkinase domain
- HSP90
heat-shock protein of 90 kDa
- HUVEC
human umbilical vein endothelial cell
- MLC
myosin light chain
- Pyk2
proline-rich tyrosine kinase-2
- ROCK
Rho-dependent kinase
- VEGF
vascular endothelial growth factor.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E05-12-1158) on June 7, 2006.
 The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
REFERENCES
- Abedi H., Zachary I. Vascular endothelial growth factor stimulates tyrosine phosphorylation and recruitment to new focal adhesions of focal adhesion kinase and paxillin in endothelial cells. J. Biol. Chem. 1997;272:15442–15451. doi: 10.1074/jbc.272.24.15442. [DOI] [PubMed] [Google Scholar]
- Abu-Ghazaleh R., Kabir J., Jia H., Lobo M., Zachary I. Src mediates stimulation by vascular endothelial growth factor of the phosphorylation of focal adhesion kinase at tyrosine 861, and migration and anti-apoptosis in endothelial cells. Biochem. J. 2001;360:255–264. doi: 10.1042/0264-6021:3600255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avraham H. K., Lee T. H., Koh Y., Kim T. A., Jiang S., Sussman M., Samarel A. M., Avraham S. Vascular endothelial growth factor regulates focal adhesion assembly in human brain microvascular endothelial cells through activation of the focal adhesion kinase and related adhesion focal tyrosine kinase. J. Biol. Chem. 2003;278:36661–36668. doi: 10.1074/jbc.M301253200. [DOI] [PubMed] [Google Scholar]
- Bernatchez P. N., Soker S., Sirois M. G. Vascular endothelial growth factor effect on endothelial cell proliferation, migration, and platelet-activating factor synthesis is Flk-1-dependent. J. Biol. Chem. 1999;274:31047–31054. doi: 10.1074/jbc.274.43.31047. [DOI] [PubMed] [Google Scholar]
- Bohnsack B. L., Hirschi K. K. The FAKs about blood vessel assembly. Circ. Res. 2003;92:255–257. doi: 10.1161/01.res.0000059260.91342.6e. [DOI] [PubMed] [Google Scholar]
- Borges E., Jan Y., Ruoslahti E. Platelet-derived growth factor-receptor-(β) and EGF-receptor-2 bind to the (β)3 integrin through its extracellular domain. J. Biol. Chem. 2000;275:39867–39873. doi: 10.1074/jbc.M007040200. [DOI] [PubMed] [Google Scholar]
- Butler B., Blystone S. D. Tyrosine phosphorylation of beta3 integrin provides a binding site for Pyk2. J. Biol. Chem. 2005;280:14556–14562. doi: 10.1074/jbc.M411765200. [DOI] [PubMed] [Google Scholar]
- Byzova T. V., Kim W., Midura R. J., Plow E. F. Activation of integrin alpha(V)beta(3) regulates cell adhesion and migration to bone sialoprotein. Exp. Cell Res. 2000;254:299–308. doi: 10.1006/excr.1999.4765. [DOI] [PubMed] [Google Scholar]
- Calalb M. B., Polte T. R., Hanks S. K. Tyrosine phosphorylation of focal adhesion kinase at sites in the catalytic domain regulates kinase activity: a role for Src family kinases. Mol. Cell. Biol. 1995;15:954–963. doi: 10.1128/mcb.15.2.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornillon J., Campos L., Guyotat D. [Focal adhesion kinase (FAK), a multifunctional protein] Med. Sci. 2003;19:743–752. doi: 10.1051/medsci/20031967743. [DOI] [PubMed] [Google Scholar]
- Dikic I., Dikic I., Schlessinger J. Identification of a new Pyk2 isoform implicated in chemokine and antigen receptor signaling. J. Biol. Chem. 1998;273:14301–14308. doi: 10.1074/jbc.273.23.14301. [DOI] [PubMed] [Google Scholar]
- Eliceiri B. P., Puente X. S., Hood J. D., Stupack D. G., Schlaepfer D. D., Huang X. Z., Sheppard D., Cheresh D. A. Src-mediated coupling of focal adhesion kinase to integrin alpha(v)beta5 in vascular endothelial growth factor signaling. J. Cell Biol. 2002;157:149–160. doi: 10.1083/jcb.200109079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germer M., Kanse S. M., Kirkegaard T., Kjoller L., Felding-Habermann B., Goodman S., Preissner K. T. Kinetic analysis of integrin-dependent cell adhesion on vitronectin–the inhibitory potential of plasminogen activator inhibitor-1 and RGD peptides. Eur. J. Biochem. 1998;253:669–674. doi: 10.1046/j.1432-1327.1998.2530669.x. [DOI] [PubMed] [Google Scholar]
- Guan J. L., Trevithick J. E., Hynes R. O. Fibronectin/integrin interaction induces tyrosine phosphorylation of a 120-kDa protein. Cell Regul. 1991;2:951–964. doi: 10.1091/mbc.2.11.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmqvist K., Cross M. J., Rolny C., Hagerkvist R., Rahimi N., Matsumoto T., Claesson-Welsh L., Welsh M. The adaptor protein Shb binds to tyrosine 1175 in the VEGFR-2 and regulates VEGF-dependent cellular migration. J. Biol. Chem. 2004;279:22267–22275. doi: 10.1074/jbc.M312729200. [DOI] [PubMed] [Google Scholar]
- Huot J., Houle F., Marceau F., Landry J. Oxidative stress-induced actin reorganization mediated by the p38 mitogen-activated protein kinase/heat shock protein 27 pathway in vascular endothelial cells. Circ. Res. 1997;80:383–392. doi: 10.1161/01.res.80.3.383. [DOI] [PubMed] [Google Scholar]
- Huot J., Houle F., Rousseau S., Deschesnes R. G., Shah G. M., Landry J. SAPK2/p38-dependent F-actin reorganization regulates early membrane blebbing during stress-induced apoptosis. J. Cell Biol. 1998;143:1361–1373. doi: 10.1083/jcb.143.5.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilic D., Furuta Y., Kanazawa S., Takeda N., Sobue K., Nakatsuji N., Nomura S., Fujimoto J., Okada M., Yamamoto T. Reduced cell motility and enhanced focal adhesion contact formation in cells from FAK-deficient mice. Nature. 1995;377:539–544. doi: 10.1038/377539a0. [DOI] [PubMed] [Google Scholar]
- Ishizaki T., Naito M., Fujisawa K., Maekawa M., Watanabe N., Saito Y., Narumiya S. p160ROCK, a Rho-associated coiled-coil forming protein kinase, works downstream of Rho and induces focal adhesions. FEBS Lett. 1997;404:118–124. doi: 10.1016/s0014-5793(97)00107-5. [DOI] [PubMed] [Google Scholar]
- Jiang X., Jacamo R., Zhukova E., Sinnett-Smith J., Rozengurt E. RNA interference reveals a differential role of FAK and Pyk2 in cell migration, leading edge formation and increase in focal adhesions induced by LPA in intestinal epithelial cells. J. Cell. Physiol. 2006;207:816–828. doi: 10.1002/jcp.20629. [DOI] [PubMed] [Google Scholar]
- Kornberg L., Earp H. S., Parsons J. T., Schaller M., Juliano R. L. Cell adhesion or integrin clustering increases phosphorylation of a focal adhesion-associated tyrosine kinase. J. Biol. Chem. 1992;267:23439–23442. [PubMed] [Google Scholar]
- Lamalice L., Houle F., Jourdan G., Huot J. Phosphorylation of tyrosine 1214 on VEGFR2 is required for VEGF-induced activation of Cdc42 upstream of SAPK2/p38. Oncogene. 2004;23:434–445. doi: 10.1038/sj.onc.1207034. [DOI] [PubMed] [Google Scholar]
- Le Bœuf F., Houle F., Huot J. Regulation of vascular endothelial growth factor receptor 2-mediated phosphorylation of focal adhesion kinase by heat shock protein 90 and Src kinase activities. J. Biol. Chem. 2004;279:39175–39185. doi: 10.1074/jbc.M405493200. [DOI] [PubMed] [Google Scholar]
- Li X., Dy R. C., Cance W. G., Graves L. M., Earp H. S. Interactions between two cytoskeleton-associated tyrosine kinases: calcium-dependent tyrosine kinase and focal adhesion tyrosine kinase. J. Biol. Chem. 1999;274:8917–8924. doi: 10.1074/jbc.274.13.8917. [DOI] [PubMed] [Google Scholar]
- Masson-Gadais B., Houle F., Laferriere J., Huot J. Integrin alphavbeta3, requirement for VEGFR2-mediated activation of SAPK2/p38 and for Hsp90-dependent phosphorylation of focal adhesion kinase in endothelial cells activated by VEGF. Cell Stress Chaperones. 2003;8:37–52. doi: 10.1379/1466-1268(2003)8<37:ivrfva>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto T., Claesson-Welsh L. VEGF receptor signal transduction. Sci. STKE. 2001;2001:RE21. doi: 10.1126/stke.2001.112.re21. [DOI] [PubMed] [Google Scholar]
- Matsumura F. Regulation of myosin II during cytokinesis in higher eukaryotes. Trends Cell Biol. 2005;15:371–377. doi: 10.1016/j.tcb.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Melendez J., Turner C., Avraham H., Steinberg S. F., Schaefer E., Sussman M. A. Cardiomyocyte apoptosis triggered by RAFTK/pyk2 via Src kinase is antagonized by paxillin. J. Biol. Chem. 2004;279:53516–53523. doi: 10.1074/jbc.M408475200. [DOI] [PubMed] [Google Scholar]
- Mitra S. K., Hanson D. A., Schlaepfer D. D. Focal adhesion kinase: in command and control of cell motility. Nat. Rev. Mol. Cell Biol. 2005;6:56–68. doi: 10.1038/nrm1549. [DOI] [PubMed] [Google Scholar]
- Nakamura K., Yano H., Schaefer E., Sabe H. Different modes and qualities of tyrosine phosphorylation of Fak and Pyk2 during epithelial-mesenchymal transdifferentiation and cell migration: analysis of specific phosphorylation events using site-directed antibodies. Oncogene. 2001;20:2626–2635. doi: 10.1038/sj.onc.1204359. [DOI] [PubMed] [Google Scholar]
- Neagoe P. E., Lemieux C., Sirois M. G. Vascular endothelial growth factor (VEGF)-A165-induced prostacyclin synthesis requires the activation of VEGF receptor-1 and -2 heterodimer. J. Biol. Chem. 2005;280:9904–9912. doi: 10.1074/jbc.M412017200. [DOI] [PubMed] [Google Scholar]
- Okigaki M., Davis C., Falasca M., Harroch S., Felsenfeld D. P., Sheetz M. P., Schlessinger J. Pyk2 regulates multiple signaling events crucial for macrophage morphology and migration. Proc. Natl. Acad. Sci. USA. 2003;100:10740–10745. doi: 10.1073/pnas.1834348100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen J. D., Ruest P. J., Fry D. W., Hanks S. K. Induced focal adhesion kinase (FAK) expression in FAK-null cells enhances cell spreading and migration requiring both auto- and activation loop phosphorylation sites and inhibits adhesion-dependent tyrosine phosphorylation of Pyk2. Mol. Cell. Biol. 1999;19:4806–4818. doi: 10.1128/mcb.19.7.4806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palecek S. P., Loftus J. C., Ginsberg M. H., Lauffenburger D. A., Horwitz A. F. Integrin-ligand binding properties govern cell migration speed through cell-substratum adhesiveness. Nature. 1997;385:537–540. doi: 10.1038/385537a0. [DOI] [PubMed] [Google Scholar]
- Parsons J. T. Focal adhesion kinase: the first ten years. J. Cell Sci. 2003;116:1409–1416. doi: 10.1242/jcs.00373. [DOI] [PubMed] [Google Scholar]
- Penta K., Varner J. A., Liaw L., Hidai C., Schatzman R., Quertermous T. Del1 induces integrin signaling and angiogenesis by ligation of alphaVbeta3. J. Biol. Chem. 1999;274:11101–11109. doi: 10.1074/jbc.274.16.11101. [DOI] [PubMed] [Google Scholar]
- Petitclerc E., Stromblad S., von Schalscha T. L., Mitjans F., Piulats J., Montgomery A. M., Cheresh D. A., Brooks P. C. Integrin alpha. (v) beta3 promotes M21 melanoma growth in human skin by regulating tumor cell survival. Cancer Res. 1999;59:2724–2730. [PubMed] [Google Scholar]
- Richardson A., Malik R. K., Hildebrand J. D., Parsons J. T. Inhibition of cell spreading by expression of the C-terminal domain of focal adhesion kinase (FAK) is rescued by coexpression of Src or catalytically inactive FAK: a role for paxillin tyrosine phosphorylation. Mol. Cell. Biol. 1997;17:6906–6914. doi: 10.1128/mcb.17.12.6906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousseau S., Houle F., Huot J. Integrating the VEGF signals leading to actin-based motility in vascular endothelial cells. Trends Cardiovasc. Med. 2000a;10:321–327. doi: 10.1016/s1050-1738(01)00072-x. [DOI] [PubMed] [Google Scholar]
- Rousseau S., Houle F., Kotanides H., Witte L., Waltenberger J., Landry J., Huot J. Vascular endothelial growth factor (VEGF)-driven actin-based motility is mediated by VEGFR2 and requires concerted activation of stress-activated protein kinase 2 (SAPK2/p38) and geldanamycin-sensitive phosphorylation of focal adhesion kinase. J. Biol. Chem. 2000b;275:10661–10672. doi: 10.1074/jbc.275.14.10661. [DOI] [PubMed] [Google Scholar]
- Rousseau S., Houle F., Landry J., Huot J. p38 MAP kinase activation by vascular endothelial growth factor mediates actin reorganization and cell migration in human endothelial cells. Oncogene. 1997;15:2169–2177. doi: 10.1038/sj.onc.1201380. [DOI] [PubMed] [Google Scholar]
- Schaller M. D. FAK and paxillin: regulators of N-cadherin adhesion and inhibitors of cell migration? J. Cell Biol. 2004;166:157–159. doi: 10.1083/jcb.200406151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaepfer D. D., Hauck C. R., Sieg D. J. Signaling through focal adhesion kinase. Prog. Biophys. Mol. Biol. 1999;71:435–478. doi: 10.1016/s0079-6107(98)00052-2. [DOI] [PubMed] [Google Scholar]
- Soldi R., Mitola S., Strasly M., Defilippi P., Tarone G., Bussolino F. Role of alphavbeta3 integrin in the activation of vascular endothelial growth factor receptor-2. EMBO J. 1999;18:882–892. doi: 10.1093/emboj/18.4.882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stupack D. G., Li E., Silletti S. A., Kehler J. A., Geahlen R. L., Hahn K., Nemerow G. R., Cheresh D. A. Matrix valency regulates integrin-mediated lymphoid adhesion via Syk kinase. J. Cell Biol. 1999;144:777–788. doi: 10.1083/jcb.144.4.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Totsukawa G., Yamakita Y., Yamashiro S., Hartshorne D. J., Sasaki Y., Matsumura F. Distinct roles of ROCK (Rho-kinase) and MLCK in spatial regulation of MLC phosphorylation for assembly of stress fibers and focal adhesions in 3T3 fibroblasts. J. Cell Biol. 2000;150:797–806. doi: 10.1083/jcb.150.4.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q., Xie Y., Du Q. S., Wu X. J., Feng X., Mei L., McDonald J. M., Xiong W. C. Regulation of the formation of osteoclastic actin rings by proline-rich tyrosine kinase 2 interacting with gelsolin. J. Cell Biol. 2003;160:565–575. doi: 10.1083/jcb.200207036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Z., Sanada K., Samuels B. A., Shih H., Tsai L. H. Serine 732 phosphorylation of FAK by Cdk5 is important for microtubule organization, nuclear movement, and neuronal migration. Cell. 2003;114:469–482. doi: 10.1016/s0092-8674(03)00605-6. [DOI] [PubMed] [Google Scholar]
- Xie Z., Tsai L. H. Cdk5 phosphorylation of FAK regulates centrosome-associated miocrotubules and neuronal migration. Cell Cycle. 2004;3:108–110. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.