Abstract
Five Spain9V-3 Streptococcus pneumoniae strains were isolated from a patient with bronchiectasis who had received long-term ciprofloxacin therapy. One ciprofloxacin-susceptible strain was isolated before treatment, and four ciprofloxacin-resistant strains were isolated during treatment. The resistant strains were derived from the susceptible strain either by a parC mutation (low-level resistance) or by parC and gyrA mutations (high-level resistance). This study shows that ciprofloxacin therapy in a patient colonized by susceptible S. pneumoniae may select fluoroquinolone-resistant mutants.
Streptococcus pneumoniae remains a major etiological agent of community-acquired pneumonia, meningitis, and acute otitis media. The emergence of resistance to antibiotics commonly used for the treatment of pneumococcal infections (13, 23) has highlighted the importance of the new fluoroquinolones that have been recommended for the treatment of respiratory tract infections (5). Although the prevalence of ciprofloxacin (CIP) resistance in S. pneumoniae is still low in Spain (3 to 7%) (1, 16, 27) and Canada (2%) (7), prior fluoroquinolone administration is a risk factor for resistant strain selection, as observed for infections caused by CIP-resistant (Cipr) (28) and levofloxacin-resistant (8, 34) S. pneumoniae. Likewise, resistance has been reported in blood isolates of viridans group streptococci from neutropenic cancer patients who received fluoroquinolone prophylaxis (11, 35).
The targets of the fluoroquinolones are the DNA gyrase (gyrase; GyrA2GyrB2) and DNA topoisomerase IV (topo IV; ParC2ParE2) enzymes (9). The pneumococcal parC and parE genes are homologous to gyrA and gyrB, respectively (3, 19, 26). Biochemical studies have established that CIP preferentially inhibits pneumococcal topo IV rather than gyrase enzymes (10, 18, 24). Genetic studies have identified fluoroquinolone resistance mutations in a discrete region of ParC, ParE, and GyrA termed the quinolone resistance-determining region (QRDR). Low-level (LL) Cipr strains had mutations altering the QRDRs of one of the two subunits of topo IV: S79 or D83 of ParC (12, 15, 19, 26, 32) or D435 of ParE (29). High-level (HL) Cipr strains had changes affecting the QRDRs of both ParC and GyrA (S81 and E85) (12, 15, 19, 26, 32) or ParE and GyrA (29). Direct biological evidence showing that those mutations are involved in resistance has been obtained by transformation experiments. Single parC mutations confer LL CIP resistance (14, 19, 32), and once the cells have acquired this LL Cipr phenotype, it is possible to transform them to a higher level of resistance by using DNA containing the gyrA QRDR from the HL Cipr strains (14, 19).
We describe herein the in vivo emergence of fluoroquinolone resistance in S. pneumoniae strains isolated from a patient who received multiple courses of CIP therapy for the treatment of bronchiectasis persistently infected by Pseudomonas aeruginosa infection.
Patient history.
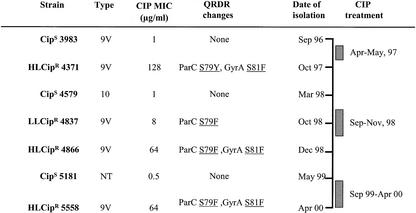
A 64-year-old man with a long-standing history of chronic cough productive of purulent sputum was first seen in September 1996. In his first clinical evaluation, a high-resolution thoracic scanner demonstrated the presence of bilateral bronchiectasis and a CIP-sensitive (Cips) S. pneumoniae strain (3983) was isolated from his sputum. In April 1997, he was first admitted to the hospital with severe hypercapnic respiratory failure and a Cips P. aeruginosa strain was isolated. Subsequently, the patient received CIP (500 mg/12 h for 10 days) for the exacerbations. In October 1997, an HL Cipr S. pneumoniae strain (4371) was isolated. The patient remained under control and free of exacerbations until March 1998, when a Cips S. pneumoniae strain (4579) was isolated. In September 1998, because of the isolation of Cips Haemophilus influenzae and Moraxella catarrhalis strains, CIP therapy was reintroduced and 1 month later, an LL Cipr S. pneumoniae strain (4837) was isolated. In December 1998, the patient had hypercapnic respiratory failure and an HL Cipr S. pneumoniae strain (4866) and a Cips P. aeruginosa strain were isolated. In May 1999, he was readmitted to the hospital with a new infectious episode, and a Cips S. pneumoniae strain (5181) and a Cips P. aeruginosa strain were isolated from his sputum. From September 1999 to April 2000, the patient was treated regularly with CIP. On a visit to the respiratory outpatient clinic in April 2000, his sputum yielded a Cipr P. aeruginosa strain and an HL Cipr S. pneumoniae strain (5558). The patient died 2 weeks later because of irreversible hypercapnic respiratory failure.
Characterization of S. pneumoniae isolates.
The antibiotic resistance patterns of the strains, their serotypes, the MICs of selected fluoroquinolones (determined as previously described [20]), and QRDR mutations are shown in Table 1. PCR products containing the gyrA, gyrB, parC, and parE QRDRs were obtained as previously described (11), separated in agarose gels (30), purified, and sequenced on both strands. The HL Cipr (MIC, ≥64 μg/ml) strains showed cross-resistance to other fluoroquinolones. Given the fluoroquinolone MICs for LL Cipr strain 4837, this strain could be considered susceptible on the basis of the NCCLS breakpoint criteria (21). However, this strain has mutations that would favor the appearance of HL Cipr strains and perhaps those breakpoints should be revised correspondingly. LL Cipr strain 4837 (MIC of 8 μg/ml) had a parC mutation, and the HL Cipr strains had parC and gyrA mutations.
TABLE 1.
Susceptibilities to fluoroquinolones and mutations in the topoisomerase QRDRs of S. pneumoniae clinical isolates
| Strain | Type | PFGEa | Resistance patternb | MIC (μg/ml)c
|
Mutation(s) in the QRDRd of:
|
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| CIP | LVX | GAT | MXF | ParC | GyrA | ParE | ||||
| R6 | NTe | S | 0.5 | 0.5 | 0.25 | 0.12 | None | None | None | |
| ATCC 49619 | 19F | PEN | 1 | 1 | 0.25 | 0.12 | None | Y74 (TAT) | None | |
| ATCC 700671 | 9V | Spain9V-3 | PENSXT | 1 | 0.5 | 0.25 | 0.12 | K137N | Y74 (TAT) | 1460V |
| 3983 | 9V | Spain9V-3 | PENSXT | 1 | 0.5 | 0.25 | 0.12 | K137N | Y74 (TAT) | 1460V |
| 4371 | 9V | Spain9V-3 | PENSXT | 128 | 32 | 8 | 4 | S79Y, K137N | S81F, Y74 (TAT) | 1460V |
| 4579 | 10 | B | S | 1 | 0.5 | 0.25 | 0.12 | K137N | — | — |
| 4837 | 9V | Spain9V-3 | PENSXT | 8 | 2 | 0.5 | 0.25 | S79F, K137N | Y74 (TAT) | 1460V |
| 4866 | 9V | Spain9V-3* | PENSXT | 64 | 16 | 4 | 4 | S79F, K137N | S81F, Y74 (TAT) | 1460V |
| 5181 | NT | D | S | 0.5 | 0.5 | 0.25 | 0.12 | K137N | — | — |
| 5558 | 9V | Spain9V-3* | PENSXT | 64 | 16 | 4 | 4 | S79F, K137N | S81F, Y74 (TAT) | 1460V |
An asterisk indicates that the strain is a Spain9V-3 clone subtype.
S, susceptible to all antibiotics tested; PEN, resistant to penicillin (MICs of 2 to 4 μg/ml, except for ATCC 49619; for which the MIC was 0.25 μg/ml); SXT: resistant to trimethropim-sulfamethoxazole (MICs of 4 and 76 μg/ml, respectively).
LVX, levofloxacin; GAT, gatifloxacin; MXF, moxifloxacin.
Residue changes involved in fluoroquinolone resistance are underlined. Mutations (by reference to the R6 DNA sequence) were as follows: ParC, S79F (TCT→TTT) and K137N (AAG→AAT); GyrA, S81F (TCC→TTC); ParE, 1460V (ATC→GTC). The strains indicated harbored a silent mutation (TAC→TAT) at codon 74 of the gyrA sequence. No changes were found in the QRDR of GyrB. —, not determined.
NT, not typeable.
Strains 3983, 4371, and 4837 have the same serotype (9V) and pulsed-field gel electrophoresis (PFGE) pattern (determined as described previously [31]) as the Spain9V-3 clone (17). The last two 9V serotype HL Cipr isolates (4866 and 5558) have the same PFGE pattern, which differs from that of the Cips 3983 and ATCC 700671 strains by three bands, and are considered Spain9V-3 subtypes (33). Despite these PFGE pattern differences, which could be a consequence of genome rearrangements that are common among S. pneumoniae (6), all Spain9V-3 strains showed identical polymorphisms in their QRDRs with respect to the sequence of the R6 strain: a K137N change in ParC, an I460V change in ParE, and a change in the Y74 codon of GyrA (TAT instead TAC) (Table 1). A genealogy of the strains was derived. HL Cipr strain 4371 could have been derived from Cips strain 3983 by the acquisition of two changes: ParC S79Y and GyrA S81F. Although the LL Cipr strain from which strain 4371 (October 1997) has been derived has not been identified in this work because sputum cultures were not performed between April and September 1997, that strain could have been present in the respiratory tract of the patient during that period. Likewise, LL Cipr strain 4837 could also have been derived from Cips strain 3983 by the acquisition of an S79F ParC change. The last two HL Cipr isolates (strain 4866 and, 16 months later, strain 5558) showed S79F ParC and S91F GyrA changes.
Analysis of the S. pneumoniae strains sequentially isolated from this patient shows that resistance develops during treatment because of mutations in the primary (topo IV) and secondary (gyrase) targets. The patient was initially infected and colonized by a Cips Spain9V-3 strain (3983) that underwent serial mutagenesis while he received CIP therapy, yielding different degrees of CIP resistance (Fig. 1). This in vivo acquisition of resistance is consistent with genetic transformation experiments (15, 19) and with generation of Cipr mutants (25) under laboratory conditions. The emergence of Cipr S. pneumoniae occurred during CIP treatment and could have been favored by the low achievable concentration of this compound in serum (1.5 to 3 μg/ml), which is close to the MIC (0.5 to 1 μg/ml) for Cips strains. On the other hand, two Cips strains (4579 and 5181) with different serotypes, PFGE types, and gene polymorphisms appeared after periods without treatment, showing that without antibiotic pressure, there was no selection of resistant mutants.
FIG. 1.
Time course of CIP treatments and emergence of S. pneumoniae strains. Only mutations involved in fluoroquinolone resistance are indicated. NT, not typeable.
In our patient, previous chronic use of fluoroquinolones for a persistent bronchial infection was a risk factor for the development of antibiotic resistance, not only in the microorganisms considered causative of infectious exacerbations, such as P. aeruginosa, but also for those microorganisms colonizing or coinfecting bronchiectasis. Results obtained by our group demonstrated that prior fluoroquinolone use, purulent bronchitis, and prior hospitalization are risk factors for the development of respiratory tract infections caused by Cipr pneumococci (J. Liñares, F. Tubau, R. Pallarés, M. J. Ferrándiz, M. A. Domínguez, F. Manresa, A. G. de la Campa, and R. Martín, Abstr. 40th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 2106, 2000).
Since infectious episodes are frequent and recurrent in chronic obstructive pulmonary disease and bronchiectasis, antibiotics are generally prescribed in an empirical way, without bacteriological studies. Should the clinician reuse a fluoroquinolone in a patient with bronchiectasis once it has already been used? According to our own experience and previously published data (8), a high risk of fluoroquinolone resistance development by S. pneumoniae may exist in patients with recent fluoroquinolone therapy, and this must be considered before the empirical introduction of an antibiotic. In our experience, previous use of fluoroquinolones may develop cross-resistance to levofloxacin and other newer fluoroquinolones. Thus, the empirical and systematic use of levofloxacin for the treatment of exacerbations of chronic obstructive pulmonary disease or bronchiectasis must be questioned and modification of the American Thoracic Society (2) and Infectious Disease Society of America (4) guidelines may be necessary.
Restricted use of fluoroquinolones and performance of susceptibility studies to monitor the prevalence of fluoroquinolone-resistant pneumococci are recommended. It is important to keep in mind that most patients infected with invasive multiresistant pneumococci may still be treated with an appropriate β-lactam such as amoxicillin or ceftriaxone (22, 23).
Acknowledgments
We thank A. Fenoll, Spanish Pneumococcus Reference Laboratory (Centro Nacional de Microbiología, Instituto de Salud Carlos III, Majadahonda, Madrid), for checking serotypes. We thank M. A. Dominguez for performing the PFGE analysis and critically reading the manuscript.
This work was supported by grants 00/0258 and 01/1267 from the Fondo de Investigación Sanitaria and by grant BIO2002-01398 from the Ministerio de Ciencia y Tecnología.
REFERENCES
- 1.Alou, L., M. Ramirez, C. García-Rey, J. Prieto, and H. de Lencastre. 2001. Streptococcus pneumoniae isolates with reduced susceptibility to ciprofloxacin in Spain: clonal diversity and appearance of ciprofloxacin-resistant epidemic clones. Antimicrob. Agents Chemother. 45:2955-2957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.American Thoracic Society. 2001. Guidelines for the management of adults with community-acquired pneumonia: diagnosis, assessment of severity, antimicrobial therapy, and prevention. Am. J. Respir. Crit. Care Med. 163:1730-1754. [DOI] [PubMed] [Google Scholar]
- 3.Balas, D., E. Fernández-Moreira, and A. G. de la Campa. 1998. Molecular characterization of the gene encoding the DNA gyrase A subunit of Streptococcus pneumoniae. J. Bacteriol. 180:2854-2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bartlett, J. G., S. F. Dowell, L. A. Mandell, T. L. File, Jr, D. L. Musher, and M. J. Fine. 2000. Guidelines from IDSA: practice guidelines for the management of adults with community-acquired pneumonia in adults. Clin. Infect. Dis. 31:347-382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bartlett, J. G., R. F. Breiman, L. Mandell, and T. M. File. 1998. Community-acquired pneumonia in adults: guidelines for management. Clin. Infect. Dis. 26:811-838. [DOI] [PubMed] [Google Scholar]
- 6.Claverys, J.-P., M. Prudhomme, I. Martier-Barrière, and B. Martin. 2000. Adaptation to the environment: Streptococcus pneumoniae, a paradigm for recombination-mediated genetic plasticity? Mol. Microbiol. 35:251-259. [DOI] [PubMed] [Google Scholar]
- 7.Chen, D. K., A. McGeer, J. C. de Azavedo, and D. E. Low. 1999. Decreased susceptibility of Streptococcus pneumoniae to fluoroquinolones in Canada. N. Engl. J. Med. 341:233-239. [DOI] [PubMed] [Google Scholar]
- 8.Davidson, R., R. Cavalcanti, J. L. Brunton, D. J. Bast, J. C. S. de Azevedo, P. Kibsey, C. Fleming, and D. E. Low. 2002. Resistance to levofloxacin and failure of treatment of pneumococcal pneumonia. N. Engl. J. Med. 346:747-750. [DOI] [PubMed] [Google Scholar]
- 9.Drlica, K., and X. Zhao. 1997. DNA gyrase, topoisomerase IV, and the 4-quinolones. Microbiol. Mol. Biol. Rev. 61:377-392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fernández-Moreira, E., D. Balas, I. González, and A. G. de la Campa. 2000. Fluoroquinolones inhibit preferentially Streptococcus pneumoniae DNA topoisomerase than DNA gyrase native proteins. Microb. Drug Res. 6:259-267. [DOI] [PubMed] [Google Scholar]
- 11.González, I., M. Georgiou, F. Alcaide, D. Balas, J. Liñares, and A. G. de la Campa. 1998. Fluoroquinolone resistance mutations in the parC, parE, and gyrA genes of clinical isolates of viridans group streptococci. Antimicrob. Agents Chemother. 42:2792-2798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gootz, T. D., R. Zaniewski, S. Haskell, B. Schmieder, J. Tankovic, D. Girard, P. Courvalin, and R. J. Polzer. 1996. Activity of the new fluoroquinolone trovafloxacin (cp-99,219) against DNA gyrase and topoisomerase IV mutants of Streptococcus pneumoniae selected in vitro. Antimicrob. Agents Chemother. 40:2691-2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoffman, J., M. S. Cetron, M. M. Farley, W. S. Baughman, R. R. Facklam, J. A. Elliot, K. A. Deaver, and R. F. Breiman. 1995. The prevalence of drug-resistant Streptococcus pneumoniae in Atlanta. N. Engl. J. Med. 333:481-486. [DOI] [PubMed] [Google Scholar]
- 14.Janoir, C., I. Podglajen, M. D. Kitzis, C. Poyart, and L. Gutmann. 1999. In vitro exchange of fluoroquinolone resistance determinants between Streptococcus pneumoniae and viridans streptococci and genomic organization of the parE-parC region in S. mitis. J. Infect. Dis. 180:555-558. [DOI] [PubMed] [Google Scholar]
- 15.Janoir, C., V. Zeller, M.-D. Kitzis, N. J. Moreau, and L. Gutmann. 1996. High-level fluoroquinolone resistance in Streptococcus pneumoniae requires mutations in parC and gyrA. Antimicrob. Agents Chemother. 40:2760-2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liñares, J., A. G. de la Campa, and R. Pallarés. 1999. Fluoroquinolone resistance in Streptococcus pneumoniae. N. Engl. J. Med. 341:1546-1548. [DOI] [PubMed] [Google Scholar]
- 17.McGee, L., L. McDougal, J. Zhou, B. G. Spratt, F. C. Tenover, R. George, R. Hakenbeck, W. Hryniewicz, J. C. Lefévre, A. Tomasz, and K. P. Klugman. 2001. Nomenclature of major antimicrobial-resistant clones of Streptococcus pneumoniae defined by the Pneumococcal Molecular Epidemiology Network. J. Clin. Microbiol. 39:2565-2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morrissey, I., and J. George. 1999. Activities of fluoroquinolones against Streptococcus pneumoniae type II topoisomerases purified as recombinant proteins. Antimicrob. Agents Chemother. 43:2579-2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Muñoz, R., and A. G. de la Campa. 1996. ParC subunit of DNA topoisomerase IV of Streptococcus pneumoniae is a primary target of fluoroquinolones and cooperates with DNA gyrase A subunit in forming resistance phenotype. Antimicrob. Agents Chemother. 40:2252-2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.NCCLS. 2000. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 5th ed. Publication M7-A5. NCCLS, Wayne, Pa.
- 21.NCCLS. 2002. Performance standards for antimicrobial susceptibility testing; twelfth informational supplement. Publication M100-S12. NCCLS, Wayne, Pa.
- 22.Pallarés, R., O. Capdevila, J. Liñares, I. Grau, H. Onaga, F. Tubau, M. H. Schulze, P. Hohl, and F. Gudiol. 2002. The effect of cephalosporin resistance on mortality in adult patients with nonmeningeal systemic pneumococcal infections. Am. J. Med. 113:120-126. [DOI] [PubMed] [Google Scholar]
- 23.Pallarés, R., J. Liñares, M. Vadillo, C. Cabellos, F. Manresa, P. F. Viladrich, R. Martín, and F. Gudiol. 1995. Resistance to penicillin and cephalosporins and mortality from severe pneumococcal pneumonia in Barcelona, Spain. N. Engl. J. Med. 333:474-480. [DOI] [PubMed] [Google Scholar]
- 24.Pan, X. S., and L. M. Fisher. 1999. Streptococcus pneumoniae DNA gyrase and topoisomerase IV: overexpression, purification, and differential inhibition by fluoroquinolones. Antimicrob. Agents Chemother. 43:1129-1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pan, X.-S., J. Ambler, S. Mehtar, and L. M. Fisher. 1996. Involvement of topoisomerase IV and DNA gyrase as ciprofloxacin targets in Streptococcus pneumoniae. Antimicrob. Agents Chemother. 40:2321-2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pan, X.-S., and L. M. Fisher. 1996. Cloning and characterization of the parC and parE genes of Streptococcus pneumoniae encoding DNA topoisomerase IV: role in fluoroquinolone resistance. J. Bacteriol. 178:4060-4069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pérez-Trallero, E., C. Fernández-Mazarrasa, C. García-Rey, E. Bouza, L. Aguilar, J. García-de-Lomas, F. Baquero, and The Spanish Group for Respiratory Pathogens. 2001. Antimicrobial susceptibilities of 1,684 Streptococcus pneumoniae and 2,039 Streptococcus pyogenes isolates and their ecological relationships: results of a 1-year (1998-1999) multicenter study in Spain. Antimicrob. Agents Chemother. 45:3334-3340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pérez-Trallero, E., J. M. García-Arenzana, J. A. Jiménez, and A. Peris. 1990. Therapeutic failure and selection of resistance to quinolones in a case of pneumococcal pneumonia treated with ciprofloxacin. Eur. J. Clin. Microbiol. Infect. Dis. 9:905-906. [DOI] [PubMed] [Google Scholar]
- 29.Perichon, B., J. Tankovic, and P. Courvalin. 1997. Characterization of a mutation in the parE gene that confers fluoroquinolone resistance in Streptococcus pneumoniae. Antimicrob. Agents Chemother. 41:166-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 31.Soares, S., K. G. Kristisson, J. M. Musser, and A. Tomasz. 1993. Evidence for the introduction of a multiresistant clone of serotype 6B Streptococcus pneumoniae from Spain to Iceland in the late 1980s. J. Infect. Dis. 168:158-163. [DOI] [PubMed] [Google Scholar]
- 32.Tankovic, J., B. Perichon, J. Duval, and P. Courvalin. 1996. Contribution of mutations in gyrA and parC genes to fluoroquinolone resistance of mutants of Streptococcus pneumoniae obtained in vivo and in vitro. Antimicrob. Agents Chemother. 40:2505-2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tenover, F. C., R. Arbeit, R. V. Goering, P. A. Mickelsen, B. E. Murray, D. H. Persing, and B. Swaminathan. 1995. Interpreting chromosomal DNA restriction patterns produced by pulse-field gel electrophoresis: criteria for bacterial strain typing. J. Clin. Microbiol. 33:2233-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Urban, C., N. Rahman, X. Zhao, N. Mariano, S. A. Segal-Maurer, K. Drlica, and J. J. Rahal. 2001. Fluoroquinolone-resistant Streptococcus pneumoniae associated with levofloxacin therapy. J. Infect. Dis. 184:794-798. [DOI] [PubMed] [Google Scholar]
- 35.Venditti, M., P. Baiocchi, C. Barandimarte, P. Serra, G. Gentile, C. Girmenia, and P. Martino. 1989. Antimicrobial susceptibilities of Streptococcus species that cause septicemia in neutropenic patients. Antimicrob. Agents Chemother. 33:580-582. [DOI] [PMC free article] [PubMed] [Google Scholar]