Abstract
In mouse embryoid bodies, mutation of the tight junction protein cingulin results in changes in gene expression. Here, we studied the function of cingulin using a gene silencing approach in Madin-Darby canine kidney (MDCK) cells. Cingulin-depleted cells show higher protein and mRNA levels of claudin-2 and ZO-3, increased RhoA activity, activation of G1/S phase transition, and increased cell density. The effects of cingulin depletion on claudin-2 expression, cell proliferation, and density are reversed by coexpression of either a dominant-negative form of RhoA (RhoAN19) or the Rho-inhibiting enzyme C3 transferase. However, the increase in ZO-3 protein and mRNA levels is not reversed by inhibition of either RhoA, p38, extracellular signal-regulated kinase (ERK), or c-Jun NH2-terminal kinase (JNK), suggesting that cingulin modulates ZO-3 expression by a different mechanism. JNK is implicated in the regulation of claudin-2 levels independently of cingulin depletion and RhoA activity, indicating distinct roles of RhoA- and JNK-dependent pathways in the control of claudin-2 expression. Finally, cingulin depletion does not significantly alter the barrier function of monolayers and the overall molecular organization of tight junctions. These results provide novel insights about the mechanisms of cingulin function and the signaling pathways controlling claudin-2 expression in MDCK cells.
INTRODUCTION
Tight junctions (TJs) are of fundamental importance in the physiology of epithelial tissues. They form a semipermeable gasket, which seals the luminal from the internal compartment, and they contribute to the maintenance of the polarized organization of epithelial cells. TJs consist of a multiprotein complex made up of integral membrane and cytoplasmic proteins linked to the actin cytoskeleton (Gonzalez-Mariscal et al., 2003; Schneeberger and Lynch, 2004). TJs have been implicated in the regulation of gene expression, cell proliferation, and differentiation, based on the interaction of some TJ proteins with transcription factors and signaling molecules (Matter et al., 2005). However, little is known on the role of specific TJ proteins in the control of gene expression and cell proliferation and on the mechanisms involved in this control.
Cingulin is a cytoplasmic TJ protein (Citi et al., 1988, 1989) for which no invertebrate homologue has been identified so far, suggesting that it is a vertebrate-specific TJ component. The cingulin molecule is a dimer, with globular (head) and coiled-coil (rod) domains (Cordenonsi et al., 1999), and it is recruited to cell–cell junctions through the interaction of the head domain with ZO-1 (D’Atri et al., 2002; Umeda et al., 2004). In addition to ZO-1, cingulin forms a complex with several other TJ proteins (Cordenonsi et al., 1999; Bazzoni et al., 2000) and interacts through its rod domain with the RhoA activator GDP/GTP-exchange factor (GEF)-H1/Lfc (Aijaz et al., 2005). Cingulin binding to GEF-H1/Lfc inhibits RhoA activation, providing a mechanism whereby formation of TJs and accumulation of cingulin at TJs are linked to inhibition of RhoA signaling (Aijaz et al., 2005).
To explore cingulin function, we developed mouse embryonic stem (ES) cells with a targeted mutation of cingulin, resulting in the deletion of the head domain (Guillemot et al., 2004). Differentiation of cingulin-mutant ES cells into embryoid bodies (EBs) led to the formation of epithelial cells with junctions lacking cingulin. The structure and function of TJ in mutant EBs were similar to those of control EBs. However, immunoblotting, microarray analysis, and quantitative real-time PCR showed that cingulin mutation resulted in changes in the expression of many proteins and genes, including the TJ proteins claudin-2, claudin-6, claudin-7, and occludin. Because cingulin mutation also affected the expression of transcription factors controlling endodermal differentiation, we concluded that the accumulation of cingulin at the TJ contributes to regulating cell differentiation and gene expression. However, it was not clear whether the phenotype was due to changes in endogenous cingulin levels or to the expression of a truncated form of cingulin, lacking the head domain. In addition, the molecular mechanism through which cingulin controls gene expression was not investigated. Finally, it remained to be seen whether cingulin controls gene expression only in EBs or also in other experimental model systems.
Here, we addressed all these questions by developing stable clones of cultured kidney epithelial cells (Madin-Darby canine kidney; MDCK) in which endogenous cingulin was depleted by expression of short hairpin RNAs (shRNAs). The results provide novel information about the mechanisms whereby cingulin controls TJ protein expression, cell proliferation and density, and the signaling pathways controlling claudin-2 expression in MDCKII cells.
MATERIALS AND METHODS
Materials
Antibodies against cingulin (36–4401), claudin-2 (32–5600), claudin-3 (34–1700), and claudin-7 (34–9100) were from Zymed Laboratories (South San Francisco, CA). Anti-c-Jun NH2-terminal kinase (JNK) (9252) and anti-p-JNK (9255) antibodies were from Cell Signaling Technology (Beverly, MA). Other antibodies were as described in Guillemot et al. (2004). The anti-ZO-3 antibody was from K. Matter and M. Balda (University College, London, United Kingdom). Anti-RhoA (sc-179) was from Santa Cruz Biotechnology (Santa Cruz, CA). Zeocin and blasticidin S were from Invitrogen (Carlsbad, CA). The signaling inhibitors Y-27632, SB202190, and SP600125 were from Tocris Cookson Inc. (Bristol, United Kingdom). U0126 was from Promega (Madison, WI).
Cell Culture and Generation of MDCK Clones Expressing shRNAs
MDCKII cells were cultured in DMEM (Sigma Chemical, Poole, Dorset, United Kingdom) containing 10% fetal bovine serum (FBS), 1× minimal essential medium (MEM) nonessential amino acids, 100 U/ml penicillin, and 100 μg/ml streptomycin. Six shRNAs were designed from the DNA coding sequence of canine cingulin (Paschoud and Citi, unpublished data) using the Whitehead small interfering RNA (siRNA) selection program (Yuan et al., 2004), and one shRNA was chosen randomly. The sequences were submitted to a BLAST search to avoid targeting of other canine genes. Complementary oligonucleotides showing the sense and the antisense cingulin-targeting sequences separated by a nine-base pair loop region, the RNA polymerase III termination sequence and the BglII and HindIII half-sites used for ligation were synthesized. Annealed oligonucleotides were cloned downstream of the human H1 promoter in the pSUPER vector (Brummelkamp et al., 2002) and transiently transfected in MDCK cells (using Lipofectamine 2000; Invitrogen) together with a pGL3 reporter gene in which the canine cingulin sequence was cloned downstream of a luciferase gene. Luciferase assays showed that only two shRNA (#2 and #5) decreased the reporter gene activity, whereas the randomly designed shRNA#7 had no effect (our unpublished data). The sequences targeted by shRNA were as follows: 5′-AAACCTCTGTGAGGAGGAA-3′ (shRNA#1), 5′-AGAGCATGTTCCAGAAGAA-3′ (shRNA#2), 5′-AGATTCGCTTCATCACAGA-3′ (shRNA#3), 5′-CCATTGGAATGGAAGGCTA-3′ (shRNA#4), 5′-ACATGAAGCTCCTTGTGGA-3′ (shRNA#5), 5′-GGCCACTATCTATAGCATC-3′ (shRNA#6), and 5′-TCTACGACCCTTCTTCCAT-3′ (shRNA#7). To create stable cell lines, selected shRNAs (#2, #5, and #7) were subcloned downstream of the human H1 promoter/Tet operator in the pTER vector (van de Wetering et al., 2003) and transfected into MDCK cells. Clones (n = 48 for constructs #2 and #5; n = 6 for construct #7) were isolated after selection in medium containing 0.6 mg/ml zeocin and analyzed by immunoblotting using anti-cingulin antibodies.
Expression of TetR, C3 Transferase, and RhoAN19
MDCK cell clones were transfected with either pcDNA6/TR (a gift from K. Matter, University College London, London, United Kingdom), pRK5-C3 transferase, pcDNA3-RhoAN19 (a gift from E. Olson, University of Texas Southwestern Medical Center, Dallas, TX), pRK5, or pcDNA3. After 24 h, cells transfected with pRK5-C3 transferase and pRK5 were lysed for immunoblotting and quantitative real-time (qRT)-PCR. Cells transfected with other plasmids were selected in medium containing either 0.75 mg/ml neomycin or 3 μg/ml blasticidin. Stably transfected cells were lysed 2–3 wk later and analyzed by immunoblotting and qRT-PCR.
Treatment with Signaling Inhibitors and Immunoblotting
The signaling inhibitors Y-27632 (1 and 10 μM), U0126 (15 μM), SB202190 (20 μM), and SP600125 (30 μM) were added to the culture medium for 16 h before cell lysis. Inhibitor concentrations were chosen based on manufacturer’s directions, current literature (Altan and Fenteany, 2004), and preliminary titrations of three different concentrations of each inhibitor, which showed dose-dependent effects on cell morphology, indicating a biological activity. Lysates (with or without inhibitors) were prepared and analyzed by immunoblotting as described previously (Bordin et al., 2004; Guillemot et al., 2004). Protein concentrations were determined using the DC protein assay (Bio-Rad, Hercules, CA), and gel loadings were normalized by immunoblotting with antibodies against α-tubulin.
Quantitative qRT-PCR
Total RNA was prepared, reverse-transcribed, and analyzed by SYBR Green-based real-time PCR, as described previously (Guillemot et al., 2004). Forward and reverse primers are shown in Table 1 (see Supplemental Materials). Ct values were determined for each sample, and ΔCt values were obtained by subtracting the Ct value of the internal control gene HPRT from the Ct value of each target gene. ΔΔCt values were calculated as the differences in ΔCt between wild-type (WT) cells and the transfected MDCK clones. The mRNA -fold change was calculated by 2−(ΔΔCt). Student’s t test was performed using the GraphPad Prism 4 software (GraphPad Software, San Diego, CA).
RhoA and JNK Activity Assays
To measure RhoA activity, MDCK cells in 100-mm dishes were washed twice with ice-cold phosphate-buffered saline (PBS) and lysed in 1 ml Mg2+ lysis/wash buffer (25 mM HEPES, pH 7.5, 150 mM NaCl, 1% NP-40, 10 mM MgCl2, 1 mM EDTA, 10% glycerol, 25 mM NaF, 1 mM Na3VO4, 0.1 mM phenylmethylsulfonyl fluoride [PMSF], and 10 μg/ml antipain-leupeptin-pepstatin cocktail), and lysates were clarified by centrifugation at 14,000 × g. Lysates containing equivalent amounts of total RhoA protein were incubated with glutathione-Sepharose beads containing 20 μg of glutathione S-transferase (GST)-rhotekin/Rho-binding domain (RBD) (a gift from K. Burridge, University of North Carolina, Chapel Hill, NC) fusion protein. The beads were washed, resuspended in SDS sample buffer, boiled, and active RhoA was detected by immunoblotting. To determine JNK activity, MDCK cells in 100-mm dishes were washed twice with ice-cold PBS and suspended in 0.25 ml of lysis buffer (25 mM HEPES, pH 7.5, 100 mM NaCl, 1.5 mM MgCl2, 0.5 mM EGTA, 0.25 mM EDTA, 0.1% NP-40, and 10 mM NaF) containing freshly added protease and phosphatase inhibitors (40 mM β-glycerophosphate, 2 mM Na3VO4, 1 mM PMSF, and 10 μg/ml antipain-leupeptin-pepstatin cocktail). Samples were analyzed by immunoblotting, as described above. Active JNK were revealed on polyvinylidene difluoride membrane (Millipore, Billerica, MA) using anti-p-JNK antibodies.
Measurement of Transepithelial Electrical Resistance (TER) and Paracellular Flux Assay
To measure TER of MDCK monolayers, cells were plated in duplicate in 24-mm Transwell filters (Corning Life Sciences, Acton, MA) in Ca2+-free medium (S-MEM) containing 10 mM HEPES, 5% dialyzed FBS, 2 mM l-glutamine, 2 μM EGTA, and 1× MEM nonessential amino acids. Sixteen hours later, TER was measured (t = 0 h), and the low calcium medium was replaced by normal medium (calcium-switch). TER was measured 0.5, 1, 2, 3, 4, 6, 8, 24, 28, 32, and 48 h after the switch. Data from three separate experiments were averaged and are expressed as ohm · cm2.
The paracellular flux assay was as described in Marzesco et al. (2002). Briefly, MDCK cells were cultured in duplicate for 5 d to reach the steady state in 24-mm Transwells. Fluorescein isothiocyanate (FITC)-dextran (4 or 300 kDa) was added to the apical compartment at a final concentration of 2 mg/ml. The basal compartment medium was collected after 4-h incubation at 37°C, and FITC-dextran was measured with a SFM25 spectrofluorometer (excitation, 492 nm; emission, 520 nm; Kontron Instruments, Watford, Herts, United Kingdom). A minimum of two independent measurements was performed for each sample, in three separate experiments.
Wound Healing Assay
Confluent MDCK monolayers were scratched with a 10-μl pipette tip, with or without a 1-h preincubation with the DNA synthesis inhibitor mitomycin C (3 μg/ml), to block cell proliferation. Wound areas were imaged at 0 and 7 h with an Axiovert S100 TV inverted microscope (Carl Zeiss, Jena, Germany). Experiments were done in duplicate wells, and three wound areas were measured for each well by using Adobe Photoshop CS2 software (Adobe Systems, Mountain View, CA).
Immunofluorescence
MDCK cells were labeled for immunofluorescence as described previously (Bordin et al., 2004). Coverslips were mounted in ProLong antifade medium (Invitrogen) and cells were imaged using an Axiovert S100 fluorescence microscope.
Proliferation and Cell Density Analysis
To measure cell proliferation, MDCK cells were seeded at a low density (5600 cells/cm2), cultured on coverslips for 2 d, and growth-arrested in DMEM supplemented with 0.1% FBS for another 2 d. Cell cycle reentry was induced by adding medium containing 10% FBS for 16 h, in the presence of 100 μM 5-bromo-2′-deoxyuridine (BrdU) (with or without 30 μM SP600125). Cells were fixed and incubated with mouse anti-BrdU antibodies with nuclease (GE Healthcare, Little Chalfont, Buckinghamshire, United Kingdom). Cells were washed with PBS, stained with an FITC-labeled anti-mouse antibody, and mounted in medium containing 1.5 μg/ml 4,6-diamidino-2-phenylindole (DAPI). For each sample, between 2000 and 3000 cells were counted.
For cell density analysis, MDCK cells were seeded at confluence and cultured for 7 d in 12-well plates and on coverslips in 24-well plates (with or without 30 μM SP600125). Cells in 12-well plates were then trypsinized and counted in a hemacytometer or imaged by phase contrast microscopy. Cells in 24-well plates were incubated with 100 μM BrdU for 16 h and analyzed as described above.
RESULTS
Cingulin Knockdown in MDCK Cells Results in Up-Regulation of the Expression of Claudin-2, ZO-3, and RhoA, and Increased RhoA Activity
In mouse EBs, the targeted mutation of cingulin alters the expression of genes coding for TJ proteins, with claudin-2 up-regulation being quantitatively the most striking effect (21-fold; Guillemot et al., 2004). To determine whether cingulin-dependent control of gene expression is restricted to EBs or has a more general significance, stable MDCK clones in which cingulin expression was knocked down by siRNA were obtained. Among different shRNA sequences targeted against canine cingulin, two sequences (shRNA#2 and shRNA#5) were identified that decreased the activity of a reporter gene having the cingulin sequence cloned downstream of a luciferase gene, and one sequence (shRNA#7) that was inactive (our unpublished data). MDCK cells were transfected with these three shRNAs cloned in a pSUPER-derived pTER vector, and stable clones were isolated. Immunoblotting analysis showed significant down-regulation of cingulin in several clones transfected with either the shRNA#2 or the shRNA#5 constructs. Because these clones behaved identically in all of our assays, only data from three cingulin knockdown (KD) clones (A, B, and C) expressing shRNA#2 are shown here.
Immunoblotting analysis with anti-cingulin antibodies showed that stable clones A, B, and C contained significantly less cingulin (between 87 and 98%, depending on cell lysates) than WT MDCK cells (Figure 1A). On the contrary, clones expressing the control shRNA#7 construct (clones 1, 2, and 3) contained levels of cingulin protein that were indistinguishable from WT cells (Figure 1A). We next tested whether cingulin depletion affects the expression levels of other TJ proteins. Immunoblotting analysis showed that both control clones and cingulin KD clones contained amounts of ZO-1, ZO-2, occludin, JAM-1, PAR-3, ZONAB, GEF-H1/Lfc, claudin-3, claudin-6, and claudin-7, which were very similar to WT cells (Figure 1A). However, cingulin KD clones contained higher levels of claudin-2 (∼3.1-fold) and ZO-3 (∼1.9-fold), compared with WT cells or control clones (Figure 1A). Interestingly, claudin-2 mRNA levels, but not ZO-3 protein and mRNA levels, were increased in cingulin-mutant EBs (Guillemot et al., 2004). In addition, although levels of RhoA protein seemed similar in all cells, levels of active RhoA were increased in cingulin KD clones (Figure 1A; Aijaz et al., 2005).
Figure 1.
Knockdown of cingulin increases the expression of ZO-3, claudin-2, and RhoA, and RhoA activity. (A) Immunoblotting analysis of TJ proteins in WT cells, control clones (1, 2, and 3), and cingulin KD clones (A, B, and C). Note the decrease in cingulin levels and the increase in ZO-3, claudin-2, and active RhoA in cingulin KD clones. One representative example among three independent cell lysate preparations is shown. (B) qRT-PCR analysis of mRNAs coding for TJ proteins in WT cells (black bars), control (gray bars), and cingulin KD (white bars) clones. For each gene, relative mRNA levels were calculated as the ratio of the mRNA level of the control or the cingulin KD clones, normalized to that of WT cells (100%). Values represent the means ± SD of three independent RNA preparations. *p < 0.05, compared with mRNA levels of WT cells. (C and D) Specificity of the effect of cingulin knockdown on protein (C) and mRNA (D) levels. Immunoblotting (C) and qRT-PCR (D) analysis of MDCK clones expressing (+) or not expressing (−) TetR, which blocks expression of shRNA. Note that expression of TetR in cingulin KD clones results in levels of cingulin, claudin-2, and ZO-3 protein and mRNAs, which are similar to those of WT cells. In D, *p < 0.05, compared with mRNA levels of nontransfected WT cells.
To determine whether cingulin depletion affects the mRNA expression levels of TJ proteins, we used qRT-PCR. In cingulin KD clones, cingulin mRNA levels were reduced on average to ∼17% of WT cells, whereas claudin-2 and ZO-3 mRNA levels were increased ∼3.0- and ∼1.7-fold, respectively, compared with WT cells (Figure 1B). Although the protein expression level of RhoA did not seem to be affected by cingulin knockdown (Figure 1A), RhoA mRNA levels were increased ∼1.5-fold in cingulin KD clones (Figure 1B). mRNA levels of occludin and GEF-H1/Lfc were also slightly decreased in cingulin KD clones (Figure 1B), although these changes did not correlate with decreased protein levels (Figure 1A).
To demonstrate that changes in claudin-2 and ZO-3 expression were specifically due to cingulin depletion, WT cells and control and cingulin KD clones were transfected with the Tet repressor (TetR), which blocks shRNA expression by the pTER vector. Immunoblotting analysis showed that when cingulin KD clones expressed TetR, cingulin, ZO-3 and claudin-2 levels returned to the same level as control clones and WT cells (Figure 1C). In contrast, expression of TetR did not modify the amounts of cingulin, ZO-3, and claudin-2 in WT cells and control clones (Figure 1C). The rescue of the cingulin KD phenotype by coexpression of TetR was validated by qRT-PCR analysis. Indeed, the increase of mRNA levels of ZO-3, claudin-2, and RhoA in the cingulin KD clones was reversed by the expression of TetR (Figure 1D). Together, these results show that in MDCK cells, cingulin specifically controls the expression levels of claudin-2 (protein and mRNA), ZO-3 (protein and mRNA) and RhoA (mRNA).
Up-Regulation of Claudin-2, but Not ZO-3 Expression, in Cingulin-depleted Cells Is Dependent upon RhoA Activity
Cingulin inhibits RhoA activation through its interaction with GEF-H1/Lfc (Aijaz et al., 2005), and cingulin depletion results both in increased RhoA activation and changes in gene expression (Figure 1). We therefore hypothesized that cingulin depletion increases claudin-2 and ZO-3 expression through its effect on RhoA. To test this model, we transfected WT cells and control and cingulin KD clones with either the dominant-negative RhoAN19 construct (Figure 2, A and C) or the Rho-inhibiting enzyme C3 transferase (Figure 2, B and D). In both cases, the amount of active RhoA was dramatically reduced, as demonstrated by immunoblotting analysis of GST pull-down assays of active RhoA (Figure 2E). Strikingly, RhoAN19 and C3 transferase not only prevented the up-regulation of claudin-2 expression in the cingulin KD clones but also reduced it to levels below that seen in untreated WT cells (Figure 2, A and B). Control clones and WT cells also showed a reduction in claudin-2 expression upon transfection with either RhoAN19 or C3 transferase (Figure 2, A and B), indicating that a constitutive activity of RhoA contributes to maintaining claudin-2 expression. In contrast, ZO-3 protein levels were not affected by the inhibition of the RhoA activity (Figure 2, A and B). qRT-PCR analysis confirmed that in WT cells, control and cingulin KD clones claudin-2 mRNA levels were decreased, to ∼50% of WT levels, by inhibition of RhoA activity by either RhoAN19 (Figure 2C) or C3 transferase (Figure 2D). Together, these results indicate that regulation of claudin-2, but not ZO-3 levels, by cingulin is dependent upon RhoA activity.
Figure 2.
Expression of claudin-2, but not ZO-3, is dependent upon RhoA activity, but not upon ROCK kinases. (A and B) Immunoblotting analysis of claudin-2 and ZO-3 in lysates of MDCK cells transfected (+), or not transfected (−), with either dominant-negative RhoAN19 (A) or C3 transferase (B). Note the decrease in claudin-2 (but not ZO-3) protein levels upon inhibition of endogenous RhoA activity by either RhoAN19 or C3 transferase. Images are representative of two independent experiments. (C and D) Histograms showing relative claudin-2 mRNA levels, based on qRT-PCR analysis of nontransfected clones (NT, black bars in C and D), clones transfected with RhoAN19 (white bars in C), C3 transferase (white bars in D), and empty vector (gray bars in C and D). The relative claudin-2 mRNA levels were calculated as a ratio, normalized to WT cells (100%). Values represent two independent experiments. *p < 0.05, compared with the claudin-2 mRNA levels of nontransfected WT cells. (E) Immunoblotting analysis of active versus total RhoA in cingulin KD clone A. Note the decrease in RhoA activity in cells transfected with RhoAN19 and C3 transferase. Images are representative of two independent experiments. This analysis was not performed on WT and control clones, because the levels of active RhoA in these cells were too low to see a significant difference under these conditions. (F) Immunoblotting analysis of cell lysates prepared from cingulin KD clone A untreated (0) or treated with the ROCK kinase inhibitor Y-27632 (1 and 10 μM). Note that both claudin-2 and ZO-3 protein levels are not affected by inhibition of ROCK kinases. Images are representative of two independent experiments, and the same results were obtained with cingulin KD clone B (our unpublished data).
To determine whether ROCK kinases act as downstream effectors of RhoA in the up-regulation of claudin-2 induced by cingulin depletion, we treated cells with the ROCK kinase inhibitor Y-27632. As shown in Figure 2F, neither claudin-2 nor ZO-3 protein levels were affected by treatment with this inhibitor, indicating that ROCK kinases are not implicated in the control of claudin-2 expression.
JNK Regulate Claudin-2 Expression Independently of Cingulin Depletion and RhoA Activity
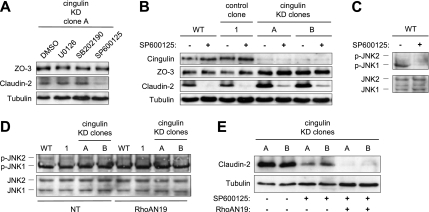
The MAPK/extracellular signal-regulated kinase (ERK) kinase pathway has been implicated in the control of claudin-2 expression (Kinugasa et al., 2000; Lan et al., 2004; Singh and Harris, 2004; Yamamoto et al., 2004; Lipschutz et al., 2005; Murata et al., 2005). To investigate the role of this and other signaling pathways in the up-regulation of claudin-2 and ZO-3 in cingulin KD clones, cingulin-depleted cells were treated with either the MAPK/ERK inhibitor U0126, the p38 kinase inhibitor SB202190, or the JNK inhibitor SP600125 (Figure 3A). None of these inhibitors affected the protein expression levels of ZO-3 (Figure 3A). However, the JNK inhibitor SP600125 significantly decreased the amount of claudin-2 in lysates of the cingulin KD clone A (Figure 3A). To study in further detail the role of the JNK pathway, WT cells, control and cingulin KD clones were treated with the JNK inhibitor SP600125, and lysates were analyzed by immunoblotting with antibodies to cingulin, ZO-3, and claudin-2 (Figure 3B). The JNK inhibitor did not affect the protein expression levels of cingulin and ZO-3 in WT cells and control and cingulin KD clones. However, in WT cells and control clones treated with the JNK inhibitor, claudin-2 protein was decreased to almost undetectable levels (Figure 3B). In treated cingulin KD clones, claudin-2 was still detectable but in much lower amounts than in untreated cells (Figure 3B). The JNK inhibitor SP600125 effectively decreased phospho-JNK (p-JNK) levels, as shown by immunoblotting analysis with anti-phospho-JNK antibodies (Figure 3C). This demonstrates a role of the JNK signaling pathway in the regulation of claudin-2 expression.
Figure 3.
The JNK signaling pathway is involved in the control of claudin-2, but not ZO-3 expression, independently of cingulin depletion and RhoA activity. (A) Immunoblotting analysis of cell lysates prepared from cingulin KD clone A treated either with solvent (dimethyl sulfoxide) or the MAPK/ERK inhibitor U0126 (15 μM), or the p38 kinase inhibitor SB202190 (20 μM), or the JNK inhibitor SP600125 (30 μM). Note the decrease in claudin-2 protein levels only in lysates from cells treated with the JNK inhibitor SP600125. Images are representative of two independent experiments, and the same results were obtained with cingulin KD clone B (our unpublished data). (B) Immunoblotting analysis of the effect of the JNK inhibitor SP600125 (30 μM) on the expression of cingulin, ZO-3, and claudin-2 in WT cells and control and cingulin KD clones. Note that SP600125 does not affect the protein expression levels of cingulin and ZO-3, but it reduces claudin-2 levels in WT cells and control and cingulin KD clones. Images are representative of two independent experiments. (C) Immunoblotting analysis of active JNK (phospho-JNK) (top) and total JNK (bottom), either in the absence (−) or in the presence (+) of the JNK inhibitor SP600125 (30 μM) in WT MDCK cells. Note that the JNK inhibitor decreases phospho-JNK levels by >75%. Similar results were obtained for control and cingulin KD clones (our unpublished data). Images are representative of two independent experiments. (D) Immunoblotting analysis of JNK activity in WT cells and control and cingulin KD clones with or without (NT) coexpression of the dominant-negative RhoA mutant RhoAN19. Note that phospho-JNK levels are not affected either by cingulin depletion or by inhibition of RhoA. Images are representative of two independent experiments. (E) Immunoblotting analysis of claudin-2 levels in cingulin KD clones A and B, either untreated, or treated with the JNK inhibitor SP600125 (30 μM), with or without coexpression of RhoAN19. Note that claudin-2 levels in cells where JNK is inhibited are further decreased when RhoA is also inhibited.
To test whether the up-regulation of claudin-2 expression in cingulin KD clones is due to a modification of the JNK activity, we examined the amount of active JNK in WT cells and control and cingulin KD clones by immunoblotting with anti-p-JNK antibodies (Figure 3D, NT). The results showed that cingulin depletion does not significantly affect the activity of JNK. Furthermore, we tested whether JNK are downstream of RhoA, by examining JNK activity in cells transfected with RhoAN19 (Figure 3D, RhoAN19). The levels of p-JNK were similar in WT cells and control and cingulin KD clones, and they were not affected by RhoAN19, showing that RhoA activity does not influence JNK activity. If RhoA and JNK independently control claudin-2 expression, the effect of their inhibition on claudin-2 levels should be cumulative. To test this hypothesis, we carried out immunoblotting analysis of claudin-2 in cingulin KD clones where both JNK and RhoA or only JNK were inhibited. The results showed that claudin-2 levels are further decreased when both JNK and RhoA are inhibited (Figure 3E). In summary, although JNK control claudin-2 expression in MDCKII cells, JNK activity is not influenced by cingulin depletion and is not involved in the RhoA-dependent up-regulation of claudin-2 expression.
Cingulin Knockdown Does Not Significantly Affect TJ Barrier Function, the Localization of TJ Proteins, and Cell Motility
We next tested whether the up-regulation of claudin-2 and ZO-3 could affect the barrier function of TJ. In MDCK cells, the barrier function of TJ can be evaluated by measurement of TER and permeability to FITC-dextran, which allow one to quantitate the flux of electrolytes and nonelectrolytes through the paracellular pathway. TJ formation in cultured monolayers was induced by the calcium-switch protocol, and the TER was measured up to 2 d after the switch (Figure 4, A and B). The time required to reach the peak of TER (6 h) was the same in WT cells, control clones (Figure 4A), and cingulin KD clones (Figure 4B). Although the TER values for the three cingulin KD clones at the peak of TER and 2 h before and after the peak were slightly (∼10–20%) lower than for WT cells (Figure 4B), these differences were not found to be statistically significant. In addition, TER values at the other time points, including in the steady state, were similar to control clones and WT cells (Figure 4, A and B). Finally, no significant difference was observed when comparing the steady-state permeability to 300-kDa FITC-dextran and 4-kDa FITC-dextran of confluent monolayers of control and cingulin KD clones, with respect to WT cells (Supplemental Figure 1A). Together, these results indicate that the barrier function of TJs is not significantly altered by cingulin depletion.
Figure 4.
Effects of cingulin knockdown on TJ barrier function and junctional localization of ZO-3 and claudin-2. (A and B) Analysis of TER of MDCK monolayers during the calcium-switch assay (see Materials and Methods). (A) WT cells and control clones. (B) Cingulin KD clones. Values represent the means ± SD of three independent experiments. (C) Immunofluorescence localization of cingulin, claudin-2, and ZO-3 in WT cells, control, and cingulin KD clones. Cells were fixed and double-stained with antibodies against cingulin and claudin-2 or ZO-3, as indicated. Data are representative of two independent experiments. Bar, 10 μm.
To characterize the effect of cingulin depletion on TJ organization, cells were immunofluorescently labeled with antibodies against cingulin, claudin-2, ZO-3 (Figure 4C), ZO-1, and occludin (our unpublished data). Cingulin KD clones showed reduced cingulin junctional immunostaining (Figure 4C), consistent with the decrease in the cingulin protein level observed by immunoblotting. The intensity of labeling for claudin-2 and ZO-3 was similar in WT cells, control and KD clones (Figure 4C), suggesting that the immunofluorescence signal was saturated and did not reflect the increase in the protein levels. Notably, the distribution of ZO-3 and claudin-2 labeling (and also ZO-1 and occludin; our unpublished data; also see Aijaz et al., 2005) were very similar in WT cells and control and cingulin KD clones (Figure 4C), indicating that cingulin depletion does not alter the organization of TJ.
Because active RhoA is increased in cingulin KD clones compared with WT cells (Figure 1A), and Rho GTPases are involved in dynamic cellular processes such as cell migration (Jaffe and Hall, 2005), we tested the effect of cingulin depletion on cell migration, using a wound healing assay. As shown in Supplemental Figure 1B, no significant difference was observed in the migration of the cingulin KD clones compared with the control clones and WT cells, both in the presence of mitomycin C, which blocks cell proliferation, or in its absence. This result indicates that cingulin depletion does not significantly affect the migration of MDCK cells.
Cingulin Controls Activation of G1/S Phase Transition and Cell Density through Modulation of RhoA Activity
Because down-regulation of GEF-H1/Lfc results in an inhibition of the G1/S phase transition, and cingulin acts as an inhibitor of GEF-H1/Lfc (Aijaz et al., 2005), we tested whether cingulin depletion stimulates G1/S phase transition. Serum-starved cells grown at low density were stimulated to enter the cell cycle by addition of serum-containing medium, and transition through the S phase was measured by determining BrdU incorporation. In WT cells and control clones, ∼55% of cells had gone through S phase 16 h after serum addition (Figure 5A). In cingulin KD clones, >75% of cells had gone through S phase, indicating that cingulin depletion activates G1/S phase transition. Moreover, expression of TetR (Figure 5A) reversed this effect, indicating that it is specifically due to cingulin depletion.
Figure 5.
Cingulin depletion activates G1/S phase transition and increases cell density through modulation of RhoA activity. (A and E) Cell proliferation assays (by measurement of BrdU incorporation; see Materials and Methods) of WT cells and control and cingulin KD clones nontransfected (NT; black bars), or after transfection with either Tet repressor (+TetR; white bars in A), empty vector (+pcDNA3; gray bars in D), or RhoAN19 (+RhoAN19; white bars in D). Data are expressed as percentage of BrdU-positive cells relative to total cell number. Values represent the means ± SD of four independent experiments. *p < 0.05, compared with nontransfected WT cells. **p < 0.05, compared with the nontransfected clones A or B. (B and F) Densities of confluent MDCK monolayers (see above for labeling of bars). Cell numbers are expressed as percentage of change, normalized to WT cells (100%). Values represent the means ± SD of three independent experiments. *p < 0.05, compared with nontransfected WT cells. (C and G) Immunofluorescence analysis of cell density and proliferation in confluent monolayers, without transfection (NT; top left) or after transfection with either TetR (+TetR; bottom left), empty vector (+pcDNA3; top right), or dominant-negative RhoAN19 (+RhoAN19; bottom right). Proliferation of cells (stained with DAPI to visualize nuclei) was assessed by measuring the percentage of BrdU-positive cells (indicated by numbers below each image). Values represent the means ± SD of three fields of one representative experiment (the images shown represent a subset of one field). Bar, 20 μm. (D) Phase contrast microscopy analysis of monolayers of WT MDCK cells and control and cingulin KD clones after 7-d culture at confluence. Note higher density and smaller cell size in cingulin KD clones. Bar, 20 μm.
We next tested whether cingulin levels regulate the density of confluent monolayers. In cingulin KD clones, cell numbers at high confluence were ∼40–55% higher than those of control clones or WT cells (Figure 5B). This effect was specific, because it was blocked by coexpression of TetR (Figure 5B). To determine whether cingulin depletion affects cell cycle arrest in high-density monolayers, cells were labeled with DAPI and BrdU at high confluence (Figure 5C). Immunofluorescent analysis with DAPI confirmed that cell density was increased in cingulin KD clones and that this effect was reversed by the expression of TetR (Figure 5C). Indeed, compared with WT cells or control clones, cingulin KD clones cultured for 7 d at confluence showed a larger number of cells per unit surface area, resulting in cells of smaller size, forming a tightly compact monolayer (Figure 5D). However, cingulin depletion did not change the proliferation rate of cells in high-density monolayers, because only ∼2–3% of cells had gone through S phase (BrdU-positive cells) in WT cells, control clones, and cingulin KD clones (Figure 5C). In summary, cingulin depletion stimulates G1/S phase transition and increases cell density in MDCK monolayers, without affecting cell cycle arrest at high confluence.
We next investigated the role of RhoA in the cingulin-dependent regulation of G1/S phase transition and cell density. Transfection with the dominant-negative RhoAN19, but not with empty vector, reversed the stimulation of G1/S phase transition induced by cingulin depletion and reduced cell proliferation both in WT cells and control clones (Figure 5E). Moreover, RhoAN19 inhibited the increase in cell density induced by cingulin depletion, without affecting either the density of WT cells and control clones (Figure 5, F and G), or cell proliferation in high-density monolayers (Figure 5G). These results indicate that in MDCK cells, the regulation of G1/S-phase transition and density by cingulin is dependent upon RhoA activity.
Although JNK activity controls claudin-2 expression independently of RhoA activity and cingulin levels, we also examined the effect of JNK inhibition on cell proliferation (Supplemental Figure 2). Short-term treatment of cells with SP600125 resulted in ∼50% decrease in cell proliferation in WT cells, control and cingulin KD clones (Supplemental Figure 2A). Long-term treatment of cells with SP600125 resulted in an ∼90% decrease in cell density and severely altered cell morphology and size (Supplemental Figure 2, B–D).
DISCUSSION
In this article, we demonstrate that in MDCKII cells cingulin regulates claudin-2 gene expression, cell proliferation, and cell density via a RhoA-dependent signaling pathway. Second, we show that cingulin depletion up-regulates ZO-3 expression, but RhoA is not involved in this regulation, indicating the presence of RhoA-dependent and -independent pathways in the control of gene expression by cingulin. Third, we show that inhibition of JNK signaling pathway reduces claudin-2 expression in WT and cingulin-depleted cells, independently of RhoA activity and cingulin levels, revealing a novel role of this pathway in the control of claudin-2 expression.
In EBs, cingulin mutation up-regulates claudin-2, claudin-6, claudin-7, occludin, ZO-2, and GEF-H1/Lfc, and down-regulates ZO-1 (Guillemot et al., 2004). In MDCK cells, cingulin depletion only seems to up-regulate the expression of claudin-2 and ZO-3. These differences could be explained by taking into account the different characteristics of the two experimental systems. EBs are heterogeneous structures, derived from undifferentiated embryonic cells, whereas MDCK cells are a differentiated kidney epithelial cell line. Their responses to experimental treatments may be qualitatively and quantitatively different. The observation that heterozygous mutant EBs had the same phenotype as homozygous mutant EBs (Guillemot et al., 2004) suggested that changes in gene expression could be due either to the reduction in junctional cingulin levels or to the expression of a mutant form of cingulin (lacking the head domain). The present study, showing that depletion of cingulin from MDCK cells results in changes of gene expression, argues in favor of the hypothesis that the phenotype of heterozygous and homozygous mutant EBs was due to a reduction in junctional cingulin, compared with WT EBs.
Binding of the RhoA activator GEF-H1/Lfc to cingulin inhibits RhoA signaling (Aijaz et al., 2005). Here, we demonstrate that RhoA, but not ROCK kinases, is involved in the up-regulation of claudin-2 expression and the increased cell proliferation and density induced by cingulin depletion. Although it is likely that RhoA activation induced by cingulin depletion in our system is due to the functional activation of GEF-H1/Lfc, additional mechanisms cannot be excluded. For example, analysis of dominant-negative mutants of ZO-3 suggests that ZO-3 acts by sequestering the armadillo family protein p120 catenin (Wittchen et al., 2003). Because p120 catenin acts as a negative regulator of RhoA (Anastasiadis et al., 2000), cingulin depletion might activate RhoA also through the increase in ZO-3 levels. However, this mechanism would not apply to EBs, where ZO-3 levels are not affected by cingulin mutation (Guillemot et al., 2004). Rho GTPases are involved in control of cell migration (Jaffe and Hall, 2005). Yet, we noted that no significant changes in cellular motility were detected in cingulin-depleted MDCK cells, which had increased active RhoA. This may due to the fact that the increase in the amount of active RhoA did not reach a threshold level, necessary to elicit changes in cytoskeletal organization in this context. Alternatively, negative feedback mechanisms may be activated in these cells, which counterbalance the effects of RhoA activation on the cytoskeleton.
Our data show that constitutive RhoA and JNK signaling pathways are required to maintain physiological levels of claudin-2 and proliferation of MDCKII cells. Claudin-2 levels were dramatically reduced by treatment with the JNK inhibitor in WT cells and control and cingulin KD clones. However, because cingulin depletion does not modify JNK activity, the effect of cingulin depletion on claudin-2 levels is unlikely to depend on JNK. More importantly, because inhibition of RhoA does not influence JNK activity, JNK are not involved as a downstream effector of RhoA in the up-regulation of claudin-2 induced by cingulin depletion. Thus, claudin-2 expression in MDCKII cells is regulated through at least two independent pathways, involving RhoA and JNK, respectively. Previous studies have indicated an involvement of the ERK pathway in the regulation of claudin-2 expression (Kinugasa et al., 2000; Lan et al., 2004; Singh and Harris, 2004; Yamamoto et al., 2004; Murata et al., 2005). However, treatment of only MDCKI, but not MDCKII cells, with the ERK inhibitor U0126 up-regulates claudin-2, suggesting that the ERK pathway is not implicated in the up-regulation of claudin-2 expression in MDCKII cells (Lipschutz et al., 2005). In contrast to what observed for claudin-2, the up-regulation of ZO-3 induced by cingulin depletion was not affected by either inhibition of RhoA, or other signaling pathways (ERK, p38, and JNK). Thus, further studies will be necessary to determine the mechanisms through which cingulin depletion up-regulates ZO-3.
The present results confirm the notion that cingulin is not an essential structural component of TJ, because the junctional localizations of claudin-2, ZO-3, ZO-1, and occludin were not significantly altered by cingulin depletion (also see Guillemot et al., 2004). With regard to the barrier function of TJs, it should be noted that exogenous claudin-2 expression decreases the TER in MDCKI cells, which lack claudin-2 (Furuse et al., 2001). However, our cingulin-depleted clones were obtained from MDCKII cells, which already express claudin-2, and have a relatively low TER. Cingulin-depleted monolayers showed a slight decrease in TER values at 4–8 h after start of junction assembly by the calcium switch, consistent with the notion that increased claudin-2 levels correlate with lower TER, due to increased permeability to cations (Amasheh et al., 2002). However, this decrease in TER was not statistically significant, and at steady-state the barrier function of cingulin-depleted clones was similar to WT cells. This suggests that increased claudin-2 expression in cingulin-depleted cells does not affect the permeability properties of the monolayer, possibly due to yet undetermined counteracting mechanisms.
Previously, it was shown that inhibition of GEF-H1/Lfc by RNA interference or by overexpression of fragments of cingulin that bind to GEF-H1/Lfc results in an inhibition of cell proliferation (Aijaz et al., 2005). Because cingulin binds to and inactivates GEF-H1/Lfc, we postulated that cingulin depletion would result in increased cell proliferation. The results presented here confirm this hypothesis, and further show that cingulin regulates cell density in a Rho-A dependent manner, resulting in tightly packed monolayers of smaller cells. These results highlight the possible role of cingulin as a junctional protein that contributes to the regulation of cell growth and shape, through its effect on RhoA. The increase in cellular cingulin content in differentiating cells (Bordin et al., 2004; Guillemot et al., 2004) may both fine-tune the expression of claudin-2 (and possibly other TJ proteins), and contribute to the regulation of cell proliferation. This could be one of a number of regulatory mechanisms that control epithelial tissue biogenesis. Indeed, several TJ-associated proteins have been implicated in signaling pathways that regulate gene expression and cell proliferation (reviewed in (Matter et al., 2005). However, very little is known about the targets of regulation by these proteins. Because of the complexity of signaling pathways controlling cell growth and differentiation, it is essential to clarify the role of each junctional component in different pathways and experimental contexts. Our work provides new insights into this direction.
Supplementary Material
ACKNOWLEDGMENTS
We thank Serge Paschoud for communicating the dog cingulin cDNA sequence and for discussions. We are grateful to the colleagues cited in the text and to the Schibler group (University of Geneva, Geneva, Switzerland) for gifts of plasmids and antibodies. This study was supported by grants from the Swiss National Fund, the Swiss Cancer League, the State of Geneva, and the Ministero dell’Istruzione, dell’Università e della Ricerca. After submission of this article, McNeill et al. (Mol. Biol. Cell [2006] 17, 1922) reported that cingulin depletion does not affect TJ barrier function in MDCK cells, in agreement with our results.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E06-02-0122) on May 24, 2006.
 The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
REFERENCES
- Aijaz S., D’Atri F., Citi S., Balda M. S., Matter K. Binding of GEF-H1 to the tight junction-associated adaptor cingulin results in inhibition of Rho signaling and G1/S phase transition. Dev. Cell. 2005;8:777–786. doi: 10.1016/j.devcel.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Altan Z. M., Fenteany G. c-Jun N-terminal kinase regulates lamellipodial protrusion and cell sheet migration during epithelial wound closure by a gene expression-independent mechanism. Biochem. Biophys. Res. Commun. 2004;322:56–67. doi: 10.1016/j.bbrc.2004.07.079. [DOI] [PubMed] [Google Scholar]
- Amasheh S., Meiri N., Gitter A. H., Schoneberg T., Mankertz J., Schulzke J. D., Fromm M. Claudin-2 expression induces cation-selective channels in tight junctions of epithelial cells. J. Cell Sci. 2002;115:4969–4976. doi: 10.1242/jcs.00165. [DOI] [PubMed] [Google Scholar]
- Anastasiadis P. Z., Moon S. Y., Thoreson M. A., Mariner D. J., Crawford H. C., Zheng Y., Reynolds A. B. Inhibition of RhoA by p120 catenin. Nat. Cell Biol. 2000;2:637–644. doi: 10.1038/35023588. [DOI] [PubMed] [Google Scholar]
- Bazzoni G., Martinez-Estrada O. M., Orsenigo F., Cordenonsi M., Citi S., Dejana E. Interaction of junctional adhesion molecule with the tight junction components ZO-1, cingulin, and occludin. J. Biol. Chem. 2000;275:20520–20526. doi: 10.1074/jbc.M905251199. [DOI] [PubMed] [Google Scholar]
- Bordin M., D’Atri F., Guillemot L., Citi S. Histone deacetylase inhibitors up-regulate the expression of tight junction proteins. Mol. Cancer Res. 2004;2:692–701. [PubMed] [Google Scholar]
- Brummelkamp T. R., Bernards R., Agami R. A system for stable expression of short interfering RNAs in mammalian cells. Science. 2002;296:550–553. doi: 10.1126/science.1068999. [DOI] [PubMed] [Google Scholar]
- Citi S., Sabanay H., Jakes R., Geiger B., Kendrick-Jones J. Cingulin, a new peripheral component of tight junctions. Nature. 1988;333:272–276. doi: 10.1038/333272a0. [DOI] [PubMed] [Google Scholar]
- Citi S., Sabanay H., Kendrick-Jones J., Geiger B. Cingulin: characterization and localization. J. Cell Sci. 1989;93:107–122. doi: 10.1242/jcs.93.1.107. [DOI] [PubMed] [Google Scholar]
- Cordenonsi M., D’Atri F., Hammar E., Parry D. A., Kendrick-Jones J., Shore D., Citi S. Cingulin contains globular and coiled-coil domains and interacts with ZO-1, ZO-2, ZO-3, and myosin. J. Cell Biol. 1999;147:1569–1582. doi: 10.1083/jcb.147.7.1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Atri F., Nadalutti F., Citi S. Evidence for a functional interaction between cingulin and ZO-1 in cultured cells. J. Biol. Chem. 2002;277:27757–27764. doi: 10.1074/jbc.M203717200. [DOI] [PubMed] [Google Scholar]
- Furuse M., Furuse K., Sasaki H., Tsukita S. Conversion of zonulae occludens from tight to leaky strand type by introducing claudin-2 into Madin-Darby canine kidney I cells. J. Cell Biol. 2001;153:263–272. doi: 10.1083/jcb.153.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Mariscal L., Betanzos A., Nava P., Jaramillo B. E. Tight junction proteins. Prog. Biophys. Mol. Biol. 2003;81:1–44. doi: 10.1016/s0079-6107(02)00037-8. [DOI] [PubMed] [Google Scholar]
- Guillemot L., Hammar E., Kaister C., Ritz J., Caille D., Jond L., Bauer C., Meda P., Citi S. Disruption of the cingulin gene does not prevent tight junction formation but alters gene expression. J. Cell Sci. 2004;117:5245–5256. doi: 10.1242/jcs.01399. [DOI] [PubMed] [Google Scholar]
- Jaffe A. B., Hall A. Rho GTPases: biochemistry and biology. Annu. Rev. Cell Dev. Biol. 2005;21:247–269. doi: 10.1146/annurev.cellbio.21.020604.150721. [DOI] [PubMed] [Google Scholar]
- Kinugasa T., Sakaguchi T., Gu X., Reinecker H. C. Claudins regulate the intestinal barrier in response to immune mediators. Gastroenterology. 2000;118:1001–1011. doi: 10.1016/s0016-5085(00)70351-9. [DOI] [PubMed] [Google Scholar]
- Lan M., Kojima T., Osanai M., Chiba H., Sawada N. Oncogenic Raf-1 regulates epithelial to mesenchymal transition via distinct signal transduction pathways in an immortalized mouse hepatic cell line. Carcinogenesis. 2004;25:2385–2395. doi: 10.1093/carcin/bgh248. [DOI] [PubMed] [Google Scholar]
- Lipschutz J. H., Li S., Arisco A., Balkovetz D. F. Extracellular signal-regulated kinases 1/2 control claudin-2 expression in Madin-Darby canine kidney strain I and II cells. J. Biol. Chem. 2005;280:3780–3788. doi: 10.1074/jbc.M408122200. [DOI] [PubMed] [Google Scholar]
- Marzesco A. M., Dunia I., Pandjaitan R., Recouvreur M., Dauzonne D., Benedetti E. L., Louvard D., Zahraoui A. The small GTPase Rab13 regulates assembly of functional tight junctions in epithelial cells. Mol. Biol. Cell. 2002;13:1819–1831. doi: 10.1091/mbc.02-02-0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matter K., Aijaz S., Tsapara A., Balda M. S. Mammalian tight junctions in the regulation of epithelial differentiation and proliferation. Curr. Opin. Cell Biol. 2005;17:453–458. doi: 10.1016/j.ceb.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Murata M., Kojima T., Yamamoto T., Go M., Takano K., Osanai M., Chiba H., Sawada N. Down-regulation of survival signaling through MAPK and Akt in occludin-deficient mouse hepatocytes in vitro. Exp. Cell Res. 2005;310:140–151. doi: 10.1016/j.yexcr.2005.07.017. [DOI] [PubMed] [Google Scholar]
- Schneeberger E. E., Lynch R. D. The tight junction: a multifunctional complex. Am. J. Physiol. 2004;286:C1213–C1228. doi: 10.1152/ajpcell.00558.2003. [DOI] [PubMed] [Google Scholar]
- Singh A. B., Harris R. C. Epidermal growth factor receptor activation differentially regulates claudin expression and enhances transepithelial resistance in Madin-Darby canine kidney cells. J. Biol. Chem. 2004;279:3543–3552. doi: 10.1074/jbc.M308682200. [DOI] [PubMed] [Google Scholar]
- Umeda K., Matsui T., Nakayama M., Furuse K., Sasaki H., Furuse M., Tsukita S. Establishment and characterization of cultured epithelial cells lacking expression of ZO-1. J. Biol. Chem. 2004;279:44785–44794. doi: 10.1074/jbc.M406563200. [DOI] [PubMed] [Google Scholar]
- van de Wetering M., Oving I., Muncan V., Pon Fong M. T., Brantjes H., van Leenen D., Holstege F. C., Brummelkamp T. R., Agami R., Clevers H. Specific inhibition of gene expression using a stably integrated, inducible small-interfering-RNA vector. EMBO Rep. 2003;4:609–615. doi: 10.1038/sj.embor.embor865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittchen E. S., Haskins J., Stevenson B. R. NZO-3 expression causes global changes to actin cytoskeleton in Madin-Darby canine kidney cells: linking a tight junction protein to Rho GTPases. Mol. Biol. Cell. 2003;14:1757–1768. doi: 10.1091/mbc.E02-08-0486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T., Kojima T., Murata M., Takano K., Go M., Chiba H., Sawada N. IL-1beta regulates expression of Cx32, occludin, and claudin-2 of rat hepatocytes via distinct signal transduction pathways. Exp. Cell Res. 2004;299:427–441. doi: 10.1016/j.yexcr.2004.06.011. [DOI] [PubMed] [Google Scholar]
- Yuan B., Latek R., Hossbach M., Tuschl T., Lewitter F. siRNA Selection Server: an automated siRNA oligonucleotide prediction server. Nucleic Acids Res. 2004;32:W130–W134. doi: 10.1093/nar/gkh366. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.