Abstract
Chemokine receptors (CKRs) are important physiological mediators of immune defense, inflammatory responses, and angiogenesis, and they have also been implicated in a number of viral disease processes. Here, we report that the Nef protein of human immunodeficiency virus (HIV) reduces cell surface levels of eight different members of the CC- and CXC-family of CKRs by up to 92%. This broad-range activity required specific elements in HIVSF2 Nef, including the proline-rich motif P73P76P79P82 as well as the acidic cluster motif E66E67E68E69, and Nef expression induced a marked perinuclear accumulation of CKRs. Surprisingly, receptor mutagenesis demonstrated that the cytoplasmic tail of CCR5 and CXCR4, which is critical for basal and ligand-mediated endocytosis, was completely dispensable for this Nef activity. In contrast, triple-mutation of the highly conserved DRY motif in the second intracellular CKR loop abolished the Nef-mediated down-regulation of CXCR4 independently of this motif’s role in CKR binding to heterotrimeric G proteins and signaling via the Gαi subunit. Thus, we identify the lentiviral pathogenicity factor Nef as a unique and broad-range modulator of CKR cell surface levels. Nef uses a mechanism that is distinct from well-established pathways orchestrating CKR metabolism and offers an interesting tool to study the multifaceted biology of CKRs.
INTRODUCTION
Chemokine receptors (CKRs) are a specialized subset of seven-transmembrane (7-TM) G protein-coupled receptors (GPCRs) that is broadly grouped into CC, CXC, CX3C, and C classes based on the structure of their cognate agonists (Murphy et al., 2000). All CKRs are composed of an extracellular amino-terminal domain, seven hydrophobic transmembrane domains, and a cytoplasmic carboxy-terminal tail that harbors important motifs for basal and ligand-induced signaling, desensitization, and endocytosis. CKRs transduce signals via multiple mediators, i.e., heterotrimeric G proteins, β-arrestin, and GPCR kinases. Signal transduction after ligand binding is initiated by stabilizing the CKR in an active conformation that enables the binding and activation of heterotrimeric G proteins (Scheer et al., 1997; Rasmussen et al., 1999; Scheer et al., 2000; Seifert and Wenzel-Seifert, 2003). A highly conserved sequence starting with Asp-Arg-Tyr (DRY) in the second intracellular loop of all CKRs plays a critical role in mediating the binding and signaling via heterotrimeric G proteins. After agonist engagement, desensitization of CKRs rapidly occurs by the interaction of β-arrestin with phosphorylated Ser/Thr residues in the cytoplasmic tail of CKRs. Phosphorylation abolishes the signaling via heterotrimeric G proteins, and β-arrestin binding lowers the receptor cell surface expression by targeting the molecule for endocytosis (Krupnick and Benovic, 1998). In addition, some CKRs contain a dileucine-based element in their cytoplasmic tail that provides a second independent motif for receptor endocytosis. Receptor internalization, recycling, and/or degradation are key mechanisms governing CKR signaling activity and are therefore regulated by diverse pathways, including clathrin-mediated endocytosis and internalization via caveolae. The basal and ligand-induced receptor internalization rate and the fate of the receptors after endocytosis depend on multiple factors that have only partially been elucidated for some CKR family members. Both processes are influenced by determinants such as the type and extracellular concentration of ligand, the presence of endocytosis motifs in the individual CKR cytoplasmic tail, the phosphorylation status of the CKR, and the cell type-specific expression pattern of adaptor molecules for endocytosis (Neel et al., 2005).
Physiologically, CKRs regulate a variety of processes involving leukocytes, including inflammatory responses, chemotaxis as well as angiogenesis (Rossi and Zlotnik, 2000; Johnson et al., 2004; Esche et al., 2005). However, CKRs and GPCRs have also been implicated in viral disease processes: herpesviruses and poxviruses encode for proteins that are homologous to cellular GPCR ligands or specifically modulate the expression of host cell CKRs (Sodhi et al., 2004). Some DNA viruses even support their own replication by encoding for proteins that are homologous to cellular CKRs but mediate altered signal transduction in response to host chemokines. Moreover, primate lentiviruses typically engage a specific CKR, in conjunction with CD4, for virus entry. For human immunodeficiency virus type 1 (HIV-1), most viral isolates use the human CKRs CCR5 and/or CXCR4 as coreceptor. However, depending on their ability to interact with the viral envelope glycoprotein (Env), several other CKRs can facilitate HIV-1 entry. The cellular expression pattern of these receptors critically determines the tropism of HIV-1 in vivo and the concentration of CCR5 or CXCR4 on the cell surface can be rate limiting for infection (Platt et al., 1998; Peters et al., 2004).
HIV-1, HIV-2, and simian immunodeficiency virus (SIV) encode the accessory protein Nef, which is a key factor for lentiviral pathogenesis and disease progression after infection (Geyer et al., 2001; Greene and Peterlin, 2002). Nef has no enzymatic activity; however, by acting as a multivalent adaptor protein, it causes numerous host cell modulations, including the alteration of T-cell receptor signaling to prime infected T-cells for activation as well as the interference with intracellular vesicle trafficking (Arora et al., 2002; Fackler and Baur, 2002). As one of its cardinal activities, HIV-1 Nef specifically modulates the surface expression of a rapidly growing number of cell surface receptors such as CD3, CD4, and CD8; major histocompatibility complex (MHC) class I (MHC-I); CD1a; CD1d; the invariant chain of immature MHC-II (CD74); mature MHC-II; DC-SIGN; mannose receptor; tumor necrosis factor; LIGHT; CD80; CD86; transferrin receptor; and hemochromatosis protein HFE (Garcia and Miller, 1991; Schwartz et al., 1996; Bell et al., 1998; Lama and Ware, 2000; Stumptner-Cuvelette et al., 2001; Sol-Foulon et al., 2002; Shinya et al., 2004; Chaudhry et al., 2005; Cho et al., 2005; Drakesmith et al., 2005; Madrid et al., 2005; Stove et al., 2005; Vigerust et al., 2005). Nef’s ability to serve as a sorting adapter is believed to be important for many of these receptor-modulating activities (Tolstrup et al., 2004), although the molecular details are poorly understood. Despite the large number of receptors that are affected, Nef’s receptor cell surface modulation is not the consequence of generally altered plasma membrane turnover induced by this amino-terminally myristoylated protein. Abundant cell surface receptors such as the epidermal growth factor receptor are not affected by the expression of Nef (Madrid et al., 2005). Furthermore, at least two distinct subsets of cell surface receptors exist that are targeted by Nef via two independent, genetically separable mechanisms (Mangasarian et al., 1999; Geyer et al., 2001). Thus, discriminative mutations in Nef can completely abrogate down-regulation of one receptor subset, whereas levels of other receptors are still drastically reduced. What governs this specificity and how these individual receptors are targeted by Nef is currently unknown. Recently, we demonstrated that expression of HIV Nef induces the reduction of surface-exposed CCR5 and CXCR4 (Michel et al., 2005). The Nef-induced down-regulation of CD4 and CCR5 independently and synergistically protected Nef-expressing target cells from HIV-1 superinfection due to an inhibited Env-mediated fusion of the viral with the target cell membrane (Michel et al., 2005). CXCR4 down-regulation by Nef also impedes the agonist-induced chemotaxis of Jurkat T-cells (Hrecka et al., 2005).
Here, we asked whether Nef proteins from human and simian immunodeficiency virus target multiple members of the family of CKRs or whether the Nef activity is specific for CKRs that can function as coreceptors for HIV entry. Because the modulation of CKR cell surface expression is a key regulatory mechanism for the signaling activity, we were particularly interested in mapping viral and cellular determinants to shed light on the strategy used by Nef to affect the cell surface expression of this receptor family.
MATERIALS AND METHODS
Plasmids
Plasmids encoding the wild-type HIV-1SF2 Nef or the mutants NefG2A, NefEDAA, NefLLAA, NefAxxA, and NefE4A, either bicistronically with green fluorescent protein (GFP) (from the pIRES2-EGFP vector; Clontech, Mountain View, CA) or as a GFP-fusion protein (from the pEGFP-N1 vector; Clontech) have been reported previously (Krautkramer et al., 2004; Keppler et al., 2005; Michel et al., 2005). To create the expression construct for Nef.RFP, the gene encoding for monomeric red fluorescent protein (mRFP)1 was amplified using pRSETB mRFP1 as template (kindly provided by Dr. Roger Tsien, Department of Neuroscience, University of California, San Diego, CA). The amplified mrfp1 gene was used to replace the egfp gene in the pEGFP-N1 vector by using two newly introduced 5′ BamHI and 3′ NotI sites. The resulting pmRFP-N1 expression vector has intermediate sequences between the multicloning site and the fluorescent gene open reading frame identical to those of the pEGFP-N1 vector as has been confirmed by sequence analysis. The HIV-1SF2 nef gene was subsequently introduced via BglII and EcoRI, resulting in the vector pmRFP-Nef. Correct expression of RFP and Nef.RFP was confirmed using confocal microscopy. Coexpression of Nef.GFP and Nef.RFP in Chinese hamster ovary (CHO) cells resulted in a pronounced colocalization of both proteins (Haller et al., 2006).
Nef alleles from HIV-1SF2, HIV-1NL4–3, HIV-1NA-7, HIV-2NEP, and SIVmac239 were expressed using the bicistronic GFP expression vector pCG (Greenberg et al., 1997; Lock et al., 1999; Michel et al., 2005). The human CCR5 wild type and the CCR5 mutants encoding the first 308 amino acids (CCR5Δ308) or the first 327 amino acids (CCR5Δ327) of CCR5 were amplified by PCR using the plasmid pCCR5hygro (Keppler et al., 2001) as template and appropriate primers that introduce a 5′ BamHI site and a 3′ stop codon immediately followed by a HindIII site. The obtained genes were first cloned in the vector pBK CMV (Stratagene, La Jolla, CA) and then subcloned in the eukaryotic expression vectors pcDNA3.1/Hygro(−) and pcDNA3.1/Zeo(−) (Invitrogen, Carlsbad, CA). The CCR5R126N mutant was generated by amplifying an internal fragment of the CCR5 gene by PCR with primers that introduce the R126N mutation and allow the exchange of the wild-type CCR5 sequence with the mutant fragment in the CCR5-encoding pcDNA3.1/Hygro(−) vector via the internal ClaI and 3′XhoI site. The CCR5NAA mutant was generated by site-directed mutagenesis with the QuikChange XL kit (Stratagene) using the plasmid pcDNA3.1/Hygro(−) CCR5 as template and primers that introduce the mutations D125N, R126A, Y127A. The CCR5-SSSS/AAAA mutant (CCR5S4A) expression vector was kindly provided by Dr. Martin Oppermann (Department of Cellular and Molecular Immunology, Universität Göttingen, Göttingen, Germany) (Kraft et al., 2001). The expression plasmid pFX4, encoding CXCR4 wild type was a gift from Dr. Mark Goldsmith (Gladstone Institute of Virology and Immunology, University of California, San Francisco, CA). Dr. Stephen Peiper (Medical College of Georgia, Augusta, GA) kindly provided the expression plasmid for wild-type CCR3 and expression plasmids for mutated CXCR4 proteins with either a carboxy-terminal truncation after amino acid 316 together with alanine replacement mutations of T311A and S312A (CXCR4Δ316) or the mutations D133N, R134A, Y135A (CXCR4NAA) (Doranz et al., 1999). The CXCR4R134N mutant was generated by site-directed mutagenesis with the QuikChange XL kit (Stratagene) using the plasmid pFX4 as template and primers that introduce the R134N mutation. The expression plasmid for CCR2 was kindly provided by Dr. Mark Goldsmith. The expression vector for the vesicular stomatitis virus glycoprotein (VSV-G) (Emi et al., 1991) was a kind gift from Dr. Jane Burns (Department of Pediatrics, University of California, San Diego, CA). The pBR HIV-1NL4–3 internal ribosome entry site (IRES)-GFP plasmids (Δnef, HIV-1NA-7 nef or HIV-2BEN nef, encoding replication-competent viruses with a nef-IRES-gfp element (Schindler et al., 2003) were kind gifts of Dr. Frank Kirchhoff (Universitätsklinikum Ulm, Ulm, Germany). The expression plasmid for the Rab11 protein fused to GFP (Rab11.GFP) was kindly provided by Dr. Serge Benichou (Department of Infectious Diseases, Institut Cochin, Universite Paris 5, France) (Madrid et al., 2005).
Cells and Transfection
All cell lines were cultivated under standard conditions in DMEM or RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS), 1% penicillin- streptomycin, and 1% l-glutamine (all from Invitrogen). Stable transfectants for CHO hCD4/hCCR5, CHO hCXCR4, and HeLa-derived TZM cells, stably expressing CD4, CCR5, and an HIV LTR-driven β-galactosidase gene, have been reported (Keppler et al., 2001; Keppler et al., 2005). CHO cells stably expressing hemagglutinin (HA)-tagged human CCR1, CCR2, CCR3, CCR4, CCR5, CXCR1, CXCR2, or CXCR4 were kindly provided by Dr. Mark Marsh (University College London, London, United Kingdom) (Bron et al., 1997). Human TZM hCCR5 high cells have been reported previously (Michel et al., 2005). Human embryonic kidney (HEK) 293 cells were obtained from the American Type Culture Collection (Manassas, VA). Primary human CD4 T-cells were purified and activated as described previously (Keppler et al., 2002). All cells were transiently transfected using calcium phosphate precipitation, Metafectene (Biontex, München, Germany), DIMRIE-C (Invitrogen), or Lipofectamine 2000 (Invitrogen) as recommended by the manufacturers.
Flow Cytometry
Cells were stained in fluorescence-activated cell sorting (FACS) medium (3% FBS and 0.05% sodium azide in phosphate-buffered saline [PBS]) with monoclonal antibodies (mAbs), which were either nonconjugated, or conjugated to fluorescein isothiocyanate, phycoerythrin (PE), PE-cyanin dye 7, Alexa Fluor 647, allophycocyanin (APC), or biotin. The following mAbs were used in this study: anti-human CD4 mAb (clone RPA-T4; BD Biosciences PharMingen, San Diego, CA), anti-human CXCR4 (clone 12G5; BD Biosciences PharMingen), anti-human CCR5 mAb (clone 2D7; BD Biosciences PharMingen), anti-human CCR1 mAb (clone 53504; BD Biosciences PharMingen), anti-human CCR2 mAb (clone 48607; R&D Systems, Minneapolis, MN), anti-human CCR3 mAb (clone 61828; R&D Systems), anti-human CCR4 mAb (clone 1G1; BD Biosciences PharMingen), anti-human CXCR1 (clone 5A12; BD Biosciences PharMingen), anti-human CXCR2 (clone 6C6; BD Biosciences PharMingen), anti-HA-tag mAb (clone 4C12; Abcam, Cambridge, United Kingdom), and anti-flag-tag mAb (clone M2; Stratagene). For unconjugated mAbs, secondary staining was performed with APC- or R-PE–conjugated goat anti-mouse antibodies (Jackson ImmunoResearch Laboratories, West Grove, PA). For biotinylated mAbs, streptavidin Alexa Fluor 660 (Invitrogen) was used as secondary reagent. An FACSCalibur with BD CellQuest Pro 4.0.2 software (BD Biosciences PharMingen) was used for analysis.
HIV Infections
Viral stocks were generated as described previously (Schindler et al., 2003). HEK 293 cells, transiently transfected with a CCR3 expression plasmid 1 d earlier, were infected with replication-competent VSV-G pseudotyped HIV-1NL4-3 IRES-GFP viruses (∼200–1500 ng of p24/8 × 105 cells) carrying either a defective nef gene (Δnef), an HIV-1NA-7 nef, or an HIV-2BEN nef allele. Twenty-two hours after infection, cells were harvested, stained for cell surface-expressed CCR3, and subsequently fixed for 1.5 h in 2% paraformaldehyde/ PBS. Analysis was performed by flow cytometry. Primary CD4 T-cells were infected and analyzed for CXCR4 surface expression in principle as reported previously (Keppler et al., 2006).
Confocal Immunofluorescence Microscopy
Transfected cells growing on coverslips were fixed with 4% paraformaldehyde and permeabilized for 25 min with 0.2% saponin in PBS. Cells were blocked for 45 min with 1% bovine serum albumin (BSA) in PBS, stained with anti-HA-tag mAb (clone F7; Santa Cruz Biotechnology, Santa Cruz, CA), and goat anti-mouse mAb Alexa Fluor 568 (Molecular Probes). TGN46 was stained with sheep anti-human TGN46 antibody (Serotec, Oxford, United Kingdom) and donkey anti-sheep mAb Alexa Fluor 568 (Molecular Probes), and CCR5 was stained with anti-CCR5 mAb (clone 2D7; BD Biosciences PharMingen) and goat anti-mouse mAb Alexa Fluor 660. Coverslips were mounted in Histoprime (Linaris, Wertheim-Bettingen, Germany) and analyzed with a Zeiss LSM510 confocal microscope with a 100× PLAN-APO objective lens. Images were recorded with the Zeiss proprietary software LSM5 and processed with Adobe Photoshop CS2 (Adobe Systems, Mountain View, CA).
Intracellular Calcium Mobilization
Intracellular calcium release in hCXCR4- or hCCR5-expressing CHO cells and human SupT1 T-cells was measured using the Fluo-4 NW Calcium Assay kit (Molecular Probes) as recommended by the manufacturer. In brief, CHO cells were grown in 96-well plates (3 × 104/well) for 20 h and then washed with PBS and loaded with 100 μl/well Fluo-4 dye in assay buffer (Hank’s balanced salt solution and 20 mM HEPES) for 45 min at 37°C, 5% CO2. SupT1 cells were directly seeded in 96-well plates at a density of 1.25 × 105/well in assay buffer. After 60-min incubation at 37°C, 5% CO2, 50 μl of 2× Fluo-4 dye in assay buffer was added, and cells were incubated for an additional 45 min. For measurements, the cells were transferred in a 37°C preheated SAFIRE fluorometer (Tecan, Durham, NC). Intracellular calcium release was recorded in a kinetic by monitoring fluorescence emission at 516 nm (with λex = 494 nm) in the presence or absence of 125–250 nM CXCL12 or 50–100 nM CCL5. Maximum fluorescence (Fmax) and minimum fluorescence (Fmin) were measured after adding ionomycin or EDTA, respectively. The intracellular calcium concentration (Cai) was calculated with the equation Cai = 400 [(F − Fmin)]/(Fmax − Fmin)] as has been described for the related assay system based on the dye Fluo-3 (Lagane et al., 2005).
Statistics
Statistical analysis was calculated with the software Microsoft Excel 2003 using the unpaired Student’s t test. A result was considered significant when p < 0.05.
RESULTS
Cell Surface Levels of Eight Different 7-TM CKRs Are Reduced by Nef
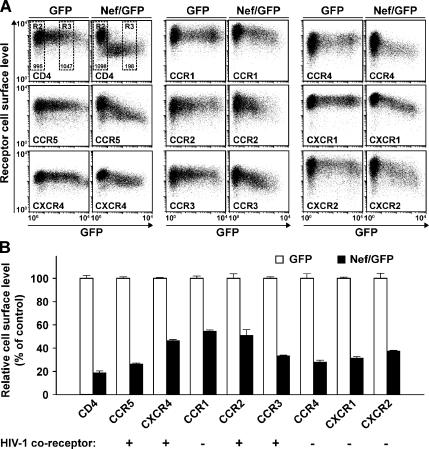
We recently observed that expression of HIV Nef reduces the cell surface level of the CKRs CCR5 and CXCR4 by up to 80% (Michel et al., 2005). Here, we first explored whether the effect of Nef was specific for those CKRs that can serve as coreceptors for HIV entry. To this end, CHO cells, which stably express human CCR1, CCR2, CCR3, CCR4, CXCR1, or CXCR2 (Bron et al., 1997), or CD4 in combination with CCR5 or CXCR4 (Michel et al., 2005), were transiently transfected with bicistronic expression vectors encoding for GFP together with HIV-1SF2 Nef (Nef/GFP) or GFP alone and analyzed 1 d later by flow cytometry (Figure 1). Among these receptors, CCR2, CCR3, CCR5, and CXCR4, but not CCR1, CCR4, CXCR1, and CXCR2 have been reported to function as HIV-1 coreceptors (Berger et al., 1999). For all CKRs analyzed, markedly reduced surface levels were observed in Nef/GFP-expressing cells. The Nef-specific down-modulation of CKRs ranged from 45% for CCR1 to 74% for CCR5 (Figure 1, A and B, details on FACS gating and statistical analysis are described in the figure legend). Interestingly, the dependence of the receptor down-regulation on the Nef concentration differed strikingly for CD4 and the CKRs. The CKR down-regulation followed a near logarithmic correlation, whereas CD4 down-regulation followed a near polynomial correlation (Supplemental Figure 1). An efficient Nef-induced down-modulation of CD4 from the cell surface occurred already at extremely low concentrations of Nef/GFP (Figure 1A and Supplemental Figure 1). In contrast, CKR down-regulation required higher Nef expression levels and the minimal concentration of Nef required for efficient down-regulation varied for different receptors. In conclusion, HIV-1 Nef reduces the cell surface exposure of eight different CC- and CXC-CKRs, six of which have previously not been reported. This identifies the first family of receptors affected by the viral protein and, notably, Nef’s activity showed no preference for those CKRs that can function as HIV coreceptors for viral entry. To our knowledge, Nef represents the first single stimulus that is able to simultaneously down-regulate eight different CKRs.
Figure 1.
The cell surface exposure of eight different CKRs is reduced by HIV-1 Nef. (A) CHO cell lines, stably expressing the indicated human receptors, were transfected with bicistronic expression constructs encoding GFP alone or GFP together with HIV-1SF2 Nef. One day after transfection, cells were stained with either receptor-specific mAbs or the anti-HA-tag mAb, and steady-state surface levels of human receptors were quantified by flow cytometry. Dot plots of GFP expression (x-axis; FL-1 channel) relative to expression of the indicated receptors by using APC-conjugated mAbs (y-axis; FL-4 channel) are shown for viable cells, expressing GFP (left) or Nef/GFP (right). Fluorescence compensation adjustments were not required, because there is no cross-talk in the detection of GFP (FL-1) and APC (FL-4). (B) MFI for cell surface-exposed receptors was quantified on cells with medium GFP expression within the R3 gate (see A, top left for gating) relative to the MFI of GFP-negative cells in the R2 gate as described previously (Michel et al., 2005). Identical R2 and R3 gates were applied for the statistical analyses of all panels shown in Figures 1, 3, and 4. For each cell line, the ratio of the MFI of cells in R3/R2 takes into account variations in staining intensity that may exist between individual samples, and this ratio most accurately reflects the Nef-mediated down-regulation of the analyzed receptor. The arithmetic mean of R3/R2 ratios obtained from triplicates for CHO cell lines expressing GFP alone were arbitrarily set to 100%. Histogram bars represent the arithmetic means of triplicates + SD from one representative experiment of at least three independent experiments.
CCR3 Is Down-regulated in HIV-infected Human Cells in a Nef-dependent Manner
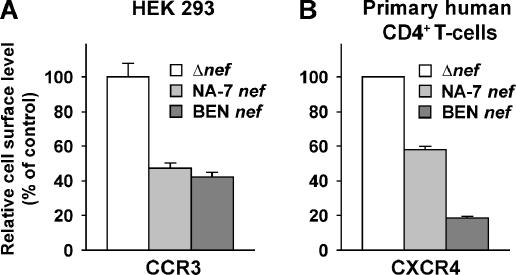
Next, we explored whether one of the CC-CKRs newly identified as a target of transiently expressed HIV-1 Nef was also down-regulated from the surface of HIV-infected human cells. Replication-competent HIV-1NL4-3–based reporter viruses, which carry either a nef IRES gfp element or a Δnef IRES gfp element, allow for a flow cytometry-based assessment of Nef-dependent receptor changes in productively infected cells (Schindler et al., 2003; Keppler et al., 2006). In this context, GFP serves as a sensitive marker for productive HIV infection and a quantitative marker for Nef expression. Human HEK 293 cells, transiently expressing CCR3, were infected with VSV-G pseudotyped HIV reporter viruses (encoding either no Nef protein (Δnef), or Nef proteins from HIV-1NA-7 or HIV-2BEN) and analyzed 1 d later. A marked down-regulation of CCR3 from the surface, ranging from 55 to 58%, was observed in cells infected with Nef-expressing viruses relative to cells infected with the isogenic Δnef virus (Figure 2A). As an informative reference, HEK 293 cells, transiently cotransfected with a CCR3 expression vector and the bicistronic HIV-1SF2 Nef/GFP or GFP expression vector, showed a comparable Nef-dependent down-regulation (data not shown). Importantly, we also observed a significant Nef-dependent down-regulation of CXCR4 in HIV-infected primary CD4 T-cells, the degree of which was more pronounced for the HIV-2BEN allele, reaching 82% (Figure 2B). Thus, besides CCR5 and CXCR4 (Hrecka et al., 2005; Michel et al., 2005; this study), the alternative HIV coreceptor CCR3 is down-regulated from the surface of HIV-infected human cells in a Nef-dependent manner.
Figure 2.
Nef-dependent reduction of CXCR4 and CCR3 cell surface levels in HIV-infected human cells. (A) HEK 293 cells were transiently transfected with an expression construct encoding for human CCR3. One day later, washed cells were infected with VSV-G pseudotyped HIV-1NL4-3 Δnef IRES gfp, HIV-1NL4-3 nefHIV-1 NA-7 IRES gfp, or HIV-1NL4-3 nefHIV-2 BEN IRES gfp viruses. Twenty-two hours after infection, cells were stained with an APC-conjugated anti-CCR3 mAb, fixed and analyzed by flow cytometry. (B) Mitogen/IL-2-blasted primary human CD4 T-cells were infected with the same panel of VSV-G pseudotyped HIV-1NL4-3 viruses and analyzed for surface expression of CXCR4 by flow cytometry two days later in principle as reported (Keppler et al., 2006). To quantify the Nef-induced alterations of CCR3 surface levels, the MFI of CCR3 staining on viable, GFP-negative, uninfected cells was compared with the MFI on GFP-positive, infected cells within the same sample. Ratios obtained for samples challenged with Δnef viruses were set to 100%. Histogram bars represent the arithmetic means of triplicates + SD from one of two comparable experiments.
Down-Modulation of 7-TM CKRs Is a Conserved Function of Nef Proteins from HIV-1, HIV-2, and SIV
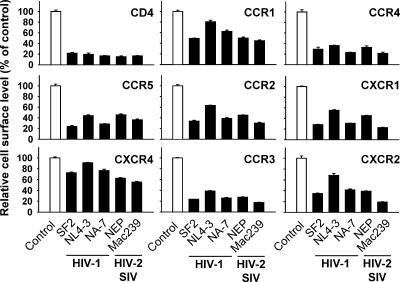
We then examined whether the ability to down-modulate multiple members of the CKR family is a characteristic of Nef proteins from different primate lentiviruses. CHO cell lines, stably expressing one of the CKRs or CD4, were transfected with bicistronic GFP expression vectors for Nef alleles from HIV-1 (SF2, NL4-3, NA-7), HIV-2 (NEP), or SIV (Mac239). One day after transfection receptor levels were quantified by flow cytometry. All Nef alleles tested down-modulated the eight CC- and CXC-CKRs (Figure 3). The potency of the different alleles for reducing the cell surface levels of CD4, and, importantly, the CKRs, was fairly comparable. The Nef protein from HIV-1NL4-3 was somewhat of an exception displaying weaker effects for CCR1, CCR2, CXCR1, CXCR2, and CXCR4. Thus, the ability to down-modulate surface levels of human CKRs is conserved among lentiviral Nef proteins, and different CKRs apparently share a common determinant that is targeted by the viral protein.
Figure 3.
Nef proteins from HIV-1, HIV-2, and SIV down-modulate human CC- and CXC-CKRs. CHO cell lines, stably expressing the indicated receptors, were transfected with bicistronic expression constructs encoding GFP alone (control) or GFP together with one of the indicated lentiviral Nef proteins from either HIV-1 (SF2, NL4-3, and NA-7), HIV-2 (NEP), or SIV (Mac239). Twenty hours after transfection, steady-state cell surface levels of CKRs and CD4 were analyzed by flow cytometry after staining with either receptor-specific mAbs or the anti-HA-tag mAb. Relative cell surface levels were calculated as described in the legend to Figure 1, and values obtained for cells expressing GFP alone were set to 100%. Histogram bars represent the arithmetic means of triplicates + SD from two similar experiments.
Reduction of Cell Surface Levels of All Eight 7-TM CKRs Requires Identical Nef Motifs
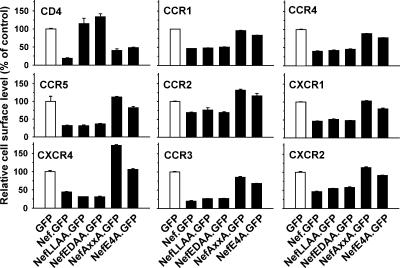
To identify the molecular determinants in Nef required for down-regulation of these CKRs, we tested several well-characterized mutants of the Nef protein from HIV-1SF2 (Geyer et al., 2001; Michel et al., 2005). CHO cells expressing the respective CKRs were transfected with vectors encoding for amino-terminal GFP fusion proteins of Nef wild type (Nef.GFP) or the indicated Nef mutants and analyzed 1 d later by flow cytometry (Figure 4). The Nef.GFP fusion protein, like the bicistronically expressed Nef/GFP, potently reduced the cell surface level of all CKRs. Alanine substitution mutation of amino acids in the Nef flexible loop region (amino acids E178D179 [NefEDAA] or amino acids L168L169 [NefLLAA]), which are required for the interaction with adaptor proteins for clathrin-mediated endocytosis and endosomal trafficking (Geyer et al., 2001), did not affect Nef’s ability to down-modulate the CKRs, whereas both of these mutations, expectedly, rendered the Nef protein incapable of reducing CD4 cell surface levels. Conversely, alanine mutation of the src homology 3 (SH3) binding motif of Nef (amino acids P76P79 [NefAxxA]) or alanine mutation of the acidic cluster motif (amino acids E66E67E68E69 [NefE4A]), required for the interaction with phosphofurin acidic cluster sorting protein (PACS)-1 and the adaptin (AP)-1/MHC-I complex (Piguet et al., 2000; Williams et al., 2005), almost completely abrogated Nef’s potential to reduce CKR cell surface levels. In contrast, the potency of these mutant Nef proteins to down-modulate CD4 was well preserved. In summary, down-modulation of CC- and CXC-CKRs by Nef is genetically separable from CD4 down-modulation, suggesting that these activities are mediated by the interaction with distinct cellular pathways.
Figure 4.
HIV-1 Nef uses its interaction surfaces with the PACS-1 sorting adaptor and SH3 domain-containing cellular proteins to down-modulate CKRs. CHO cell lines, stably expressing the indicated human receptors, were transiently transfected with expression constructs encoding GFP fusion proteins of Nef wild-type from HIV-1SF2 or the indicated Nef mutants, or GFP alone. Twenty hours after transfection, surface levels of CC-CKRs, CXC-CKRs, and CD4 were quantified as the MFI of viable cells, and relative cell surface levels were calculated as described in the legend to Figure 1. Histogram bars represent the arithmetic means of triplicates + SD from one of at least three independent experiments.
Nef accelerates the endocytosis rate of several human cell surface receptors, and this, at least partially, contributes to the Nef-mediated reduction of steady-state surface levels of CD4, MHC-I, and CCR5 (Aiken et al., 1994; Schwartz et al., 1996; Michel et al., 2005). We also observed a moderate acceleration of CCR3 endocytosis in Nef/GFP-expressing CHO CCR3 cells relative to GFP-expressing or untransfected cells in an established kinetic endocytosis assay (supplemental Materials and Methods and Supplemental Figure 2). This effect was most pronounced at early time points of the kinetic (<5 min). In particular at later time points, kinetic endocytosis assays can be affected by secondary effects of receptor recycling. Therefore, at this point we cannot exclude that Nef also targets CCR3 cell surface expression by inhibition of receptor recycling.
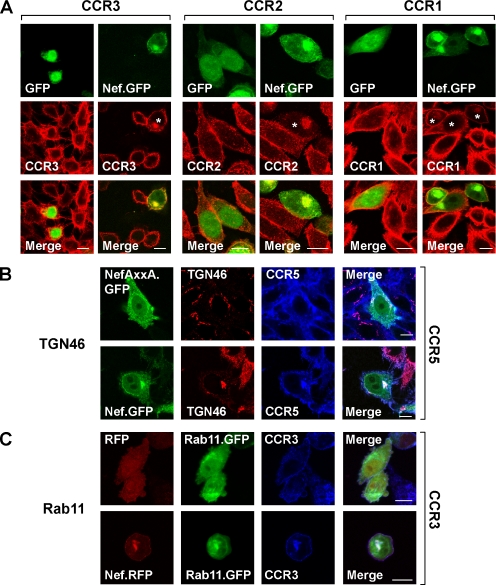
Nef Targets CCR1, CCR2, and CCR3 to a Perinuclear Compartment, Where These Receptors Accumulate and Partially Colocalize with Nef
Next, we explored the subcellular localization of three newly identified CKRs that are targeted by Nef, namely, CCR1, CCR2, and CCR3. As judged by confocal microscopy and in line with the flow cytometric analyses presented in Figure 4, cells expressing GFP-fusion proteins of HIV-1SF2 Nef typically exposed less CKRs on the cell surface than GFP-expressing control cells (Figure 5A). Importantly, cells expressing low-to-medium levels of Nef.GFP harbored prominent clusters of the CC-CKRs in the perinuclear region (Figure 5A, white asterisks mark cells with perinuclear CKR accumulation). A significant fraction of Nef.GFP was also detected in this region and partially colocalized with all three receptors, as revealed in merged images (Figure 5A). Nef’s perinuclear localization is not induced by the presence of human CKRs, because it can also be observed in cells that do not express these receptors (Supplemental Figure 3). Accentuated CKR staining in the perinuclear region was occasionally seen also in GFP-transfected or untransfected control cells, suggesting that this perinuclear compartment may be involved also in the physiological life cycle of these CKRs. We then sought to define this compartment more closely by performing colocalization studies with established markers for the trans-Golgi network (TGN46; Figure 5B) and for recycling endosomes (Rab11.GFP; Figure 5C). Both of these compartment markers showed a considerable degree of colocalization with Nef and CKRs at the maximum resolution of this imaging technique. Furthermore, in cells expressing very high levels of Nef.GFP, the CKRs were detected neither on the cell surface nor in perinuclear compartments (data not shown). This observation is consistent with an accelerated Nef-induced receptor degradation as was previously seen for CCR5 in pulse-chase analyses (Michel et al., 2005). In summary, Nef targets multiple CKRs to perinuclear compartments, where they accumulate and partially colocalize with the viral protein.
Figure 5.
Expression of Nef from HIV-1SF2 induces a perinuclear accumulation of human CCR1, CCR2, CCR3, and CCR5. (A and C) Stable CHO transfectants or (B) human TZM hCCR5 high cells were plated on coverslips and transfected with expression constructs encoding for Nef.GFP or GFP alone (A), Nef.GFP or NefAxxA.GFP (B), or Nef.RFP or RFP alone together with Rab11.GFP (C). One day after transfection, receptors were indirectly immunostained with Alexa Fluor 568 (red) (A) or with Alexa Fluor 660 (blue) (B and C) together with indirect immunostaining of TGN46 with Alexa Fluor-568 (red) (B). Images were taken with a Zeiss LSM510 confocal microscope with a 100× PLAN-APO objective. Shown are individual sections (GFP-, RFP-, Alexa Fluor 568- or Alexa Fluor 660-stained receptors, or Alexa Fluor 568-stained TGN46; and merged images) from the center of representative cells. White asterisk indicates cells with perinuclear CKR accumulation. White bars in merged images, 10 μm.
The Cytoplasmic Tail of CCR5 and CXCR4 Is Dispensable for the Down-Modulation by Nef
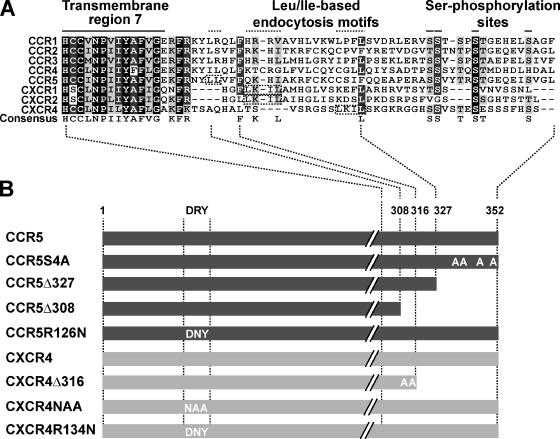
Because all CC- and CXC-CKRs tested were down-modulated by the identical motifs in the viral Nef protein, we sought to identify conserved motifs in CKRs that may serve as anchor points for this Nef activity. To this end, we analyzed different carboxy-terminal truncation and point mutants of the well-characterized CKR CCR5 that lack receptor elements critical for basal and ligand-induced internalization. Specifically, we included mutants with alanine replacement of the carboxy-terminal serine phosphorylation sites S336, S377, S342, and S349 of CCR5 (CCR5S4A); then a deletion of the 25 carboxy-terminal CCR5 amino acids (CCR5Δ327); and finally, the additional deletion of carboxy-terminal amino acids disrupting the alternative di-leucine based receptor endocytosis motifs L308L309 in CCR5 (CCR5Δ308) (Figure 6, A and B). For CXCR4, a mutant was studied that lacks all carboxy-terminal amino acids after A316 and has T311 and S312 replaced by alanine (CXCR4Δ316; Doranz et al., 1999) and therefore is devoid of all potential serine/threonine phosphorylation sites and the alternative I328L329 endocytosis motif in the carboxy terminus (Signoret et al., 1998) (Figure 6, A and B).
Figure 6.
Sequence alignment of CC- and CXC-CKRs cytoplasmic tails and analyzed CCR5 and CXCR4 mutants. (A) Sequence alignment (amino acids from transmembrane domain 7 to carboxy terminus) of the cytoplasmic tails of CC- and CXC-CKRs analyzed in this study (ClustalW method: BLOSOM scoring matrix, opening/end gap penalty = 10, extending/separating gap penalty = 0.05). Conserved carboxy-terminal motifs, including the transmembrane region 7, the Leu/Ile-based endocytosis motifs, and four serine residues are indicated. Identical amino acids are shaded in black, conserved residues are in dark gray, similar residues are in light gray, and unrelated amino acids in white. Below a consensus amino acid sequence is shown (formatted and consensus calculated with BOXSHADE). (B) Carboxy-terminal mutants and DRY box mutants in the second intracellular loop of CCR5 and CXCR4 that were used in subsequent experiments.
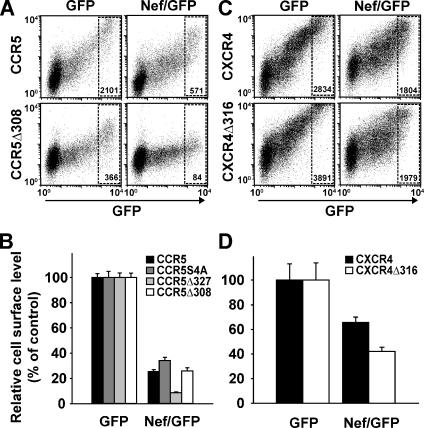
To evaluate the ability of Nef to down-modulate the carboxy-terminal truncated CKRs, we transiently coexpressed wild-type or mutant CKRs together with either Nef/GFP or GFP, and subsequently, quantified levels of surface-exposed receptors by flow cytometry. In these cotransfection experiments, the mean fluorescence intensity (MFI) for surface expression of each CKR mutant on cells with high-level GFP expression was set to 100% (Figure 7, A and C; FACS gate indicated in dot plots). Remarkably, all CCR5 mutants, including the CCR5Δ308 mutant, were down-modulated by Nef as efficiently as the wild-type CCR5 protein, with remaining surface levels ranging from 9 to 34% (Figure 7, A and Β). As an informative control, steady-state cell surface levels of the CCR5Δ308 mutant were completely unaffected by treatment with the natural ligand CCL5 (regulated upon activation, normal T-cell expressed and secreted) (data not shown), confirming a previous report (Kraft et al., 2001). In contrast, surface-exposure of CCR5 wild type, CCR5Δ327, and CCR5-4SA mutants was markedly reduced by CCL5 treatment (data not shown; Kraft et al., 2001). Of note, the CCR5Δ308 mutant was expressed ∼5–10 times less efficiently on the surface compared with CCR5 wild type. Similarly, the CXCR4Δ316 mutant was down-modulated by Nef at least as efficiently as CXCR4 wild type (Figure 7, C and D). Thus, the CCR5 and CXCR4 carboxy termini, which harbor the classical and highly conserved motifs that are critical for basal and ligand-induced receptor desensitization and endocytosis, are dispensable for the Nef-mediated reduction of CKR surface exposure.
Figure 7.
Down-regulation of CCR5 and CXCR4 by Nef does not require motifs in the CKR cytoplasmic tail. CHO cells were transiently cotransfected with expression plasmids encoding the indicated CCR5 (A and B) or CXCR4 (C and D) molecules together with bicistronic expression plasmids for GFP (left) or Nef/GFP (right). (A and B) CCR5 wild type (CCR5); CCR5ΔS4A, in which 4 conserved serine residues at position 336, 337, 342, and 349 are replaced by alanine (Kraft et al., 2001); or the carboxy-terminal truncation mutants CCR5Δ327 or CCR5Δ308 were analyzed. (C and D) CXCR4 wild type (CXCR4) or the carboxy-terminal truncation mutant CXCR4Δ316 (Doranz et al., 1999) were analyzed. One day after transfection, cell surface-exposed CKR molecules were stained with either unconjugated anti-CCR5 mAbs (clone 2D7) (A and B) or anti-CXCR4 mAbs (clone 12G5) (C and D) followed by APC-conjugated secondary antibodies. Subsequently, cells were analyzed by flow cytometry. (A and C) Representative dot plots of GFP or Nef/GFP expression (x-axis; FL-1) relative to CCR5 or CXCR4 expression (y-axis; FL-4), respectively, in cotransfected cells. The indicated gates encompass cells with high GFP expression, and numbers within the gates represent MFI values of CKR expression. MFI values within these gates obtained for cells expressing GFP alone were set to 100%. (B and D) Histogram bars depict relative CKR cell surface levels and represent the arithmetic means of triplicates + SD from one of three comparable experiments.
The DRY Motif of CXCR4 Is a Central Element for the Nef-induced Modulation of Cell Surface Expression
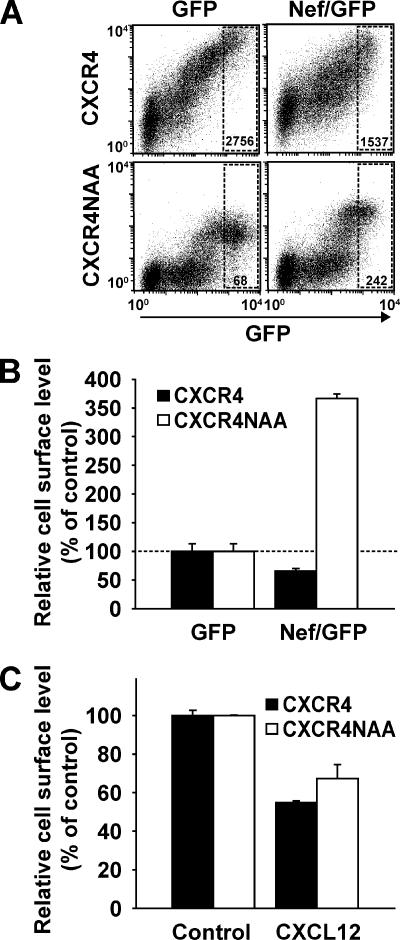
Sequence alignment of the CKRs targeted by Nef highlights that in addition to the carboxy-terminal endocytosis motifs, only the DRY motif in the second intracellular loop, which plays an important role in GPCR activation and signaling via heterotrimeric G proteins (Scheer et al., 1997; Rasmussen et al., 1999; Scheer et al., 2000; Seifert and Wenzel-Seifert, 2003), is highly conserved among all receptors analyzed herein. We therefore explored the importance of this DRY motif for the Nef-induced down-regulation by investigating a CXCR4 protein with its DRY motif triple-mutated to Asn-Ala-Ala (CXCR4NAA) (Doranz et al., 1999). CXCR4NAA is able to bind CXCL12 (stromal cell-derived factor-1α) and can serve as HIV coreceptor. However, CXCR4NAA has been reported to be markedly impaired in CXCL12-mediated signaling and calcium mobilization (Doranz et al., 1999). CXCR4NAA is generally expressed less efficiently on the cell surface compared with CXCR4 wild type (Figure 8A; Doranz et al., 1999).
Figure 8.
The DRY box mutant CXCR4NAA is up-regulated by Nef and down-regulated by CXCL12. (A and B) Effect of Nef from HIV-1SF2 on the surface-exposure of CXCR4 wild type and CXCR4NAA was evaluated in cotransfection experiments in CHO cells as described in the legend to Figure 7. (C) In parallel, CHO cells transiently expressing CXCR4 wild type or CXCR4NAA were treated with 100 nM CXCL12 overnight and analyzed for surface levels of CXCR4 by flow cytometry. Histogram bars represent the arithmetic means of triplicates + SD from one of two to five comparable experiments.
Using the above-described cotransfection assay (Figure 7), we made the astounding observation that expression of Nef/GFP induced a 3.5-fold up-regulation of surface-exposed CXCR4NAA receptors, whereas CXCR4 wild-type receptors were, expectedly, markedly down-regulated in the presence of the viral protein (Figure 8, A and B). In contrast, CXCL12 down-regulated both the wild-type receptor and the NAA mutant with comparable efficiency (Figure 8C), demonstrating a preserved capacity of the CXCR4NAA mutant for ligand-induced internalization and excluding a gross deficiency of the integrity and membrane localization of this receptor mutant. We also constructed CCR2NAA, CCR3NAA, and CCR5NAA mutants, the poor cell surface expression of which unfortunately precluded further analyses (data not shown). Thus, at least for CXCR4, the full integrity of the DRY motif, which is highly conserved among CKRs, critically determined the susceptibility of the receptor to Nef-mediated up- or down-regulation.
Nef-Induced Down-Regulation of CCR5 and CXCR4 from the Cell Surface Does Not Depend on the Gαi-mediated Signaling via Heterotrimeric G Proteins
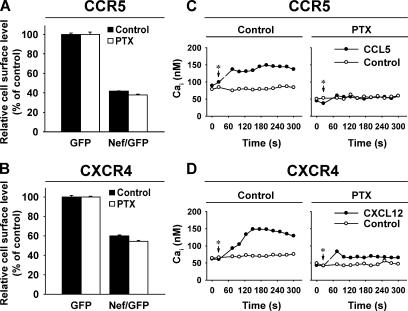
Mutation of the DRY motif severely reduces or abolishes the capacity of CKRs, including CXCR4 and CCR5, to bind to and signal through the Gαi subunit of the heterotrimeric G protein (Doranz et al., 1999; Lagane et al., 2005). To examine the role of signaling via the Gαi subunit for the Nef-induced down-regulation, CXCR4- or CCR5-expressing CHO cells were cultured in the presence or absence of the Gαi inhibitor Bordetella pertussis toxin (PTX), and, subsequently, cells were transfected with a bicistronic Nef/GFP or GFP expression vector and analyzed for receptor surface levels 1 d later.
Intriguingly, neither CCR5 nor CXCR4 down-regulation by Nef was affected by the continuous presence of PTX (Figure 9, A and B). Conversely, PTX strongly diminished the CCL5- or CXCL12-induced Gαi-mediated signaling in CHO CCR5 and CHO CXCR4 cells, respectively, as measured in a standard calcium mobilization assay performed in parallel (Figure 9, C and D), demonstrating the efficacy of the inhibitor under these experimental conditions. Similarly, a lack of PTX effect on CXCR4 down-regulation by Nef was also seen in HIV-infected human SupT1 cells, whereas PTX abolished the CXCL12-triggered calcium mobilization in these T-cells (data not shown). Thus, pharmacological interference with Gαi-mediated signaling did not affect the Nef-induced CKR down-regulation.
Figure 9.
The Nef-mediated reduction of CCR5 and CXCR4 surface levels is PTX insensitive. CHO cells, stably expressing CCR5 or CXCR4, were cultured in the presence or absence of 100 ng/ml PTX for 18 h. Subsequently, cells were either transfected with a bicistronic GFP or Nef/GFP expression vector (A and B) or left untransfected (C and D). PTX-pretreated cells were cultivated for one more day in the presence of PTX. All cells were analyzed in parallel for either receptor surface levels relative to GFP expression (A and B) or calcium mobilization (C and D) in response to 50 nM CCL5 (C) or 125 nM CXCL12 (D) as described in Materials and Methods. Asterisks indicate the time of ligand addition.
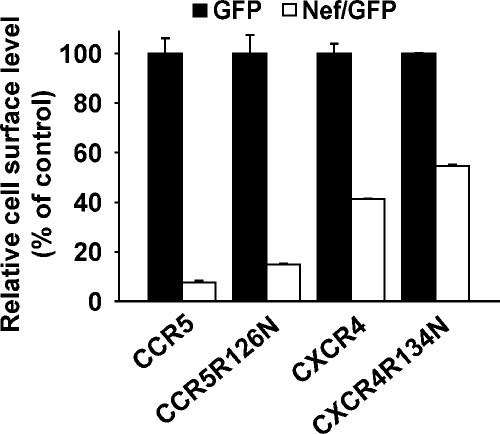
Next, we investigated the role of the DRY motif integrity for the Nef-induced CKR down-regulation with respect to its role in receptor signaling in more detail. For CCR5, a mutant protein with a single mutation of the DRY box (CCR5R126N) was generated that preserves the well-conserved arginine-surrounding aspartic acid and tyrosine residues of the DRY motif but is still deficient in constitutive and ligand-induced Gαi protein binding and signaling (Lagane et al., 2005). In addition, a corresponding DRY motif mutant of CXCR4 was constructed (CXCR4R134N). Both of these CKR mutant proteins were down-regulated by Nef with efficiencies comparable with the wild-type receptors (Figure 10). In summary, these results highlight that the DRY motif is critically involved in the Nef-mediated reduction of CXCR4 cell surface levels but that the DRY motif requirement is apparently not related to its role in facilitating conformational changes of the CKR leading to G protein coupling and subsequent signaling via Gαi subunits. The importance of the DRY motif for the Nef-mediated CXCR4 down-regulation only becomes apparent when less conservative triple mutations are present. This suggests a critical role of the DRY motif in the CKR biology beyond G protein coupling and signaling that is particularly important for the fate of the receptor in the presence of HIV Nef.
Figure 10.
DRY mutants CCR5R126N and CXCR4R134N are down-regulated by Nef with an efficiency comparable with the wild-type CKRs. CHO cells were transiently cotransfected with expression plasmids encoding CXCR4R134N, CXCR4 wild type, CCR5R126N, or CCR5 wild type together with bicistronic expression plasmids for GFP or Nef/GFP. Cell surface levels of mutant and wild-type CKRs were analyzed in principle as described in the legend to Figure 7. Histogram bars represent the arithmetic means of triplicates + SD from one of two comparable experiments.
DISCUSSION
This study demonstrates that lentiviral Nef proteins efficiently reduce the cell surface levels of multiple members of the family of G protein-coupled CC- and CXC-CKRs. Nef apparently uses a nonclassical, yet general pathway to regulate the surface exposure of CKRs. This pathway does not require carboxy-terminal CKR motifs necessary for agonist-driven receptor endocytosis, but, at least for CXCR4, depends on the DRY box motif in the second intracellular loop. Surprisingly, the DRY box dependence was unrelated to CKR G protein coupling and signaling through the Gαi subunit; two activities previously established to be controlled by CKR conformational changes that are governed by this motif.
The versatile functions of CKRs in leukocyte biology require a precise regulation of signal initiation and subsequent desensitization after ligand engagement. The modulation of the cell surface density is a key mechanism regulating the signaling activity of all CKRs (Neel et al., 2005). The CKRs analyzed in this study share only 24–39% sequence homology and possess many individual characteristics, i.e., bind specific subsets of ligands, mediate distinct intracellular signals, and have individual, cell type-dependent characteristics concerning receptor endocytosis, recycling, and degradation (Murphy et al., 2000; Johnson et al., 2004; Neel et al., 2005). The fact that all of these diverse CKRs were targeted with comparable efficacy by the identical motifs in Nef from HIV-1SF2 suggests that a universal, possibly indirect mechanism may be used by the viral protein. Regarding the motifs in Nef, the acidic cluster motif (amino acids E66E67E68E69), which is required for interaction with the PACS-1 sorting adapter, and the Nef motif for binding to SH3 domain-containing proteins (amino acids P73xxP76xxP79xxP82) were both mandatory elements for the surface reduction of CKRs. Interestingly, Nef uses the identical motifs to reduce the surface expression of structurally unrelated MHC-I molecules. For Nef’s effect on MHC-I, the acidic cluster motif, besides mediating the PACS-1 interaction, is also required for interaction with the MHC-I cytoplasmic tail (Williams et al., 2005). In contrast, motifs in the flexible loop region of Nef (amino acids E178D179 and L168L169), which are important for the recruitment of AP-1 and AP-3 adaptor proteins and are critical for the down-modulation of CD4, are dispensable for Nef’s effect both on MHC-I and CKR cell surface levels. One model of the Nef-induced receptor modulation postulates that the viral protein acts via a direct physical interaction with the respective receptor (Peterlin and Trono, 2003). However, of the currently >20 cell surface receptors reported to be affected by Nef, only low-affinity interactions were demonstrated for two receptors, namely, CD4 and MHC-I, in some studies (Williams et al., 2002; Cluet et al., 2005). In line with the scarcity of reported interactions, we did not find evidence for a physical association of Nef and CCR5 in coimmunoprecipitation studies from CHO cells (data not shown). In light of the rapidly growing list of only distantly related receptor molecules, the surface exposure of which is modulated by Nef, a more general mode of action, that may not necessarily require physical interaction, seems likely. Recently, a generalized alteration of endocytic vesicle trafficking induced by Nef was proposed as a mechanism for affecting cell surface receptors, including the transferrin receptor (Madrid et al., 2005). The requirement for distinct Nef motifs in the down-modulation of different receptors, as for example CD4 and the CKRs, may thus reflect the unique role of these motifs for Nef’s effect on specific intracellular trafficking compartments that are encountered by the respective receptors during their journey through the cell. In line with such distinct sites of Nef action, we found markedly different degrees of concentration dependence for down-regulation of CD4 and CKRs (Figure 1 and Supplemental Figure 1). Also relevant in this context, we have recently compared the expression of HIV-1 Nef after transfection of the identical Nef expression plasmids, that were used in the current study, and HIV-1 infection and found comparable expression levels of the viral protein (Keppler et al., 2006).
Our kinetic endocytosis assays and localization studies revealed that Nef only moderately accelerated the rate of CKR endocytosis. Because a robust reduction of CCR3 and CCR5 steady-state cell surface levels was observed already 4 h after transfection of CHO cells with Nef.GFP expression plasmids (data not shown), it seems likely that mechanisms other than accelerated endocytosis of surface-exposed receptors contribute to this Nef-mediated phenotype. In the case of MHC-I (Blagoveshchenskaya et al., 2002; Kasper and Collins, 2003), these include Nef-mediated disturbance of receptor recycling to the cell surface and diversion of newly synthesized molecules during anterograde transport. Conceivably, the sum of such interferences may result in the net reduction of cell surface CKR levels and the pronounced perinuclear accumulation observed at steady state in Nef-expressing cells. Irrespective of the sorting mechanism used, our previous results obtained for CCR5 (Michel et al., 2005) and the intracellular localization studies presented herein suggest CKR degradation as a possible consequence of Nef-mediated perturbance of CKR transport pathways. Of note, the Nef-mediated CKR cell surface level reduction does apparently not involve an interference with CKR de novo synthesis, because CCR5-transgene expression was not affected in Nef-expressing human TZM cells that display a profound CCR5 down-modulation (Michel et al., 2005).
It is conceivable that Nef acts at the early endosomal compartment (Madrid et al., 2005), through which CKRs traffic after internalization, irrespective of their preferred endocytosis route (Neel et al., 2005), and thereby mediates its effect on CKR cell surface expression. Nef possibly perturbs the rapid recycling of CKRs to the cell surface and reroutes the receptors to a perinuclear compartment. This compartment may be related to the slow recycling compartment that was found to be involved in normal trafficking of some CKRs (Neel et al., 2005), as supported by our colocalization of Nef and CCR3 with Rab11.GFP. However, we also found a significant colocalization of the trans-Golgi network marker TGN46 with CCR5 in Nef-expressing TZM cells, in line with a previous report on the localization of MHC-I in Nef-expressing HeLa-CD4 and A7 cells (Blagoveshchenskaya et al., 2002). In our opinion the resolution of confocal microscopy does not allow a clear distinction between these and other closely adjacent perinuclear compartments. The nature of the perinuclear accumulation of CKRs and MHC-I in Nef-expressing cells still remains to be clarified in future studies.
Our data show that lentiviral Nef proteins have evolved a general strategy to affect the cell surface levels of CKRs. Importantly, Nef-specific CKR down-regulation occurs in HIV-infected human cells, including primary human T-cells (Michel et al., 2005). The existence of a mechanism that targets such a broad class of cell surface receptors may reflect the need of HIV to down-modulate both of the divergent major entry coreceptors CCR5 and CXCR4 to efficiently protect productively infected cells from superinfection (Michel et al., 2005; data not shown). However, the newly found Nef activity of down-regulation of at least eight different CKRs is not restricted to those receptors that function as HIV coreceptors. Conceivably, and as reported for CXCR4 (Hrecka et al., 2005), down-modulation of these receptors may profoundly alter the responsiveness of Nef-expressing cells to chemokine attractants. The reduced CKR expression on these cells may disturb the coordinated action of host immune responses against virus-infected cells (Esche et al., 2005), and this may assist the immune evasion of HIV.
At present, there is only limited knowledge about the regulation of the internalization rate and intracellular trafficking of individual CKRs under physiological conditions in the absence or presence of ligand. In general, parameters known to influence receptor endocytosis include the type and concentration of ligand, composition of the CKR cytoplasmic tail, receptor phosphorylation status, and abundance of endocytosis-associated adaptor molecules in the cytoplasm (Neel et al., 2005). The mechanism by which lentiviral Nef proteins reduce CKR cell surface exposure differs by several criteria from known pathways used by individual CKRs for internalization and intracellular trafficking. First, the Nef-induced CCR5 and CXCR4 down-regulation is completely independent of agonist engagement. This contrasts the situation under physiological conditions, during which chemokine ligands are the main trigger for CKR cell surface level reduction that is primarily mediated by an accelerated clathrin-mediated receptor endocytosis. A lipid raft/caveolae-associated pathway has also been proposed as an alternative internalization route for some CKRs, including CCR5 and CXCR4, in this context (Mueller et al., 2002; Venkatesan et al., 2003; Mariani et al., 2004). However, this alternative pathway seems to be restricted to certain cell types and the evaluation of its importance in vivo has so far not been possible (Signoret et al., 2000, 2005; Venkatesan et al., 2003; Neel et al., 2005).
Second, classical endocytosis motifs in the CKR cytoplasmic tail are not required for the Nef-mediated receptor down-modulation. On the contrary, a hallmark of the major clathrin-dependent CKR endocytosis pathway under physiological conditions is its dependence on motifs within the cytoplasmic tail of CKRs that mediate interaction with the adaptor molecule AP-2. AP-2 binds CKRs either directly via the dileucine motifs or indirectly via β-arrestin, which preferably engages the CKR after ligand-induced Ser/Thr phosphorylation and desensitization. Although clathrin-mediated endocytosis may still be involved, our data clearly demonstrate an autonomous regulation of CKR trafficking by Nef that does not require regulatory elements within the cytoplasmic tail.
Third, Nef’s capability to down-regulate CKRs is independent of heterotrimeric G protein binding as well as Gαi signaling. Neither pharmacological interference nor genetic mutation of critical receptor elements for G protein binding and downstream signaling had an influence on the Nef-mediated CKR down-regulation. On the other hand the DRY motif, at least for CXCR4, was identified as a critical receptor-borne element for the Nef-induced down-modulation. One might speculate that the NAA triple-mutation of the CXCR4 DRY box channels the receptor to intracellular sorting pathways that are affected by Nef in a fundamentally different manner thereby resulting in an enhanced cell surface presentation as opposed to the down-regulation of wild-type receptor. Conceivably, other DRY motif–containing GPCRs may also be a target for Nef-mediated down-regulation from the cell surface and this would be an interesting extension of our present work. Recent studies indicate that the DRY motifs of some CKRs like CCR2, CCR5, and CXCR4, besides G protein coupling, act as docking sites for additional cellular adaptor proteins involved in signaling and endocytosis, including JAK2 kinase and β-arrestins (Mellado et al., 1998; Roland et al., 2003; Lagane et al., 2005). Future studies will need to explore their potential role in the modulation of CKR cell surface expression by Nef.
Finally, Nef is the first broad-spectrum modulator of CC- and CXC-CKRs cell surface levels, because it affects the cell surface expression of at least eight rather diverse CKRs with comparable efficiencies and diverts these receptors from their individual intracellular trafficking pathway to a probably common perinuclear compartment. This is in contrast to the individual cell surface turnover rates and the multifaceted internalization and intracellular trafficking pathways of different CKRs under physiological conditions (Neel et al., 2005).
The apparently nonclassical mechanism Nef uses to affect CKR internalization and intracellular trafficking makes this viral protein a valuable tool for future studies on the complex regulation of CKR signaling activity and/or cell surface expression. Furthermore, understanding the impact of Nef’s effect on the CKR family, that critically controls many aspects of leukocyte biology in the host immune system, may help to gain insight into Nef’s key role in HIV replication and pathogenesis.
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. Hans-Georg Kräusslich for support and stimulating discussions. We thank Drs. Serge Benichou, Jane Burns, Mark Goldsmith, Frank Kirchhoff, Mark Marsh, Martin Oppermann, Stephen Peiper, Jacek Skowronski, and Roger Tsien for kindly providing reagents. This work was supported by grants from the Deutsche Forschungsgemeinschaft to O.T.K. (Ke 742/2) and subcontract R0051-B from the J. David Gladstone Institutes to O.T.K. O.T.F. is recipient of a Chica und Heinz Schaller Stiftung fellowship.
Abbreviations used:
- AP
adaptin
- CKR
chemokine receptor
- Env
envelope glycoprotein
- FBS
fetal bovine serum
- GFP
green fluorescent protein
- GPCR
G protein-coupled receptor
- HIV
human immunodeficiency virus
- IRES
internal ribosome entry site
- MHC
major histocompatibility class
- mRFP
monomeric red fluorescent protein
- PACS
phosphofurin acidic cluster sorting protein
- PTX
Bordetella pertussis toxin
- SH3
src homology-3
- SIV
simian immunodeficiency virus
- VSV-G
vesicular stomatitis virus glycoprotein.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E06-02-0117) on June 14, 2006.
 The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
REFERENCES
- Aiken C., Konner J., Landau N. R., Lenburg M. E., Trono D. Nef induces CD4 endocytosis: requirement for a critical dileucine motif in the membrane-proximal CD4 cytoplasmic domain. Cell. 1994;76:853–864. doi: 10.1016/0092-8674(94)90360-3. [DOI] [PubMed] [Google Scholar]
- Arora V. K., Fredericksen B. L., Garcia J. V. Nef: agent of cell subversion. Microbes Infect. 2002;4:189–199. doi: 10.1016/s1286-4579(01)01527-1. [DOI] [PubMed] [Google Scholar]
- Bell I., Ashman C., Maughan J., Hooker E., Cook F., Reinhart T. A. Association of simian immunodeficiency virus Nef with the T-cell receptor (TCR) zeta chain leads to TCR down-modulation. J. Gen. Virol. 1998;79:2717–2727. doi: 10.1099/0022-1317-79-11-2717. [DOI] [PubMed] [Google Scholar]
- Berger E. A., Murphy P. M., Farber J. M. Chemokine receptors as HIV-1 coreceptors: roles in viral entry, tropism, and disease. Annu. Rev. Immunol. 1999;17:657–700. doi: 10.1146/annurev.immunol.17.1.657. [DOI] [PubMed] [Google Scholar]
- Blagoveshchenskaya A. D., Thomas L., Feliciangeli S. F., Hung C. H., Thomas G. HIV-1 Nef downregulates MHC-I by a PACS-1- and PI3K-regulated ARF6 endocytic pathway. Cell. 2002;111:853–866. doi: 10.1016/s0092-8674(02)01162-5. [DOI] [PubMed] [Google Scholar]
- Bron R., et al. Promiscuous use of CC and CXC chemokine receptors in cell-to-cell fusion mediated by a human immunodeficiency virus type 2 envelope protein. J. Virol. 1997;71:8405–8415. doi: 10.1128/jvi.71.11.8405-8415.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhry A., Das S. R., Hussain A., Mayor S., George A., Bal V., Jameel S., Rath S. The Nef protein of HIV-1 induces loss of cell surface costimulatory molecules CD80 and CD86 in APCs. J. Immunol. 2005;175:4566–4574. doi: 10.4049/jimmunol.175.7.4566. [DOI] [PubMed] [Google Scholar]
- Cho S., Knox K. S., Kohli L. M., He J. J., Exley M. A., Wilson S. B., Brutkiewicz R. R. Impaired cell surface expression of human CD1d by the formation of an HIV-1 Nef/CD1d complex. Virology. 2005;337:242–252. doi: 10.1016/j.virol.2005.04.020. [DOI] [PubMed] [Google Scholar]
- Cluet D., Bertsch C., Beyer C., Gloeckler L., Erhardt M., Gut J. P., Galzi J. L., Aubertin A. M. Detection of human immunodeficiency virus type 1 Nef and CD4 physical interaction in living human cells by using bioluminescence resonance energy transfer. J. Virol. 2005;79:8629–8636. doi: 10.1128/JVI.79.13.8629-8636.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doranz B. J., Orsini M. J., Turner J. D., Hoffman T. L., Berson J. F., Hoxie J. A., Peiper S. C., Brass L. F., Doms R. W. Identification of CXCR4 domains that support coreceptor and chemokine receptor functions. J. Virol. 1999;73:2752–2761. doi: 10.1128/jvi.73.4.2752-2761.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drakesmith H., Chen N., Ledermann H., Screaton G., Townsend A., Xu X. N. HIV-1 Nef down-regulates the hemochromatosis protein HFE, manipulating cellular iron homeostasis. Proc. Natl. Acad. Sci. USA. 2005;102:11017–11022. doi: 10.1073/pnas.0504823102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emi N., Friedmann T., Yee J. K. Pseudotype formation of murine leukemia virus with the G protein of vesicular stomatitis virus. J. Virol. 1991;65:1202–1207. doi: 10.1128/jvi.65.3.1202-1207.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esche C., Stellato C., Beck L. A. Chemokines: key players in innate and adaptive immunity. J. Investig. Dermatol. 2005;125:615–628. doi: 10.1111/j.0022-202X.2005.23841.x. [DOI] [PubMed] [Google Scholar]
- Fackler O. T., Baur A. S. Live and let die: Nef functions beyond HIV replication. Immunity. 2002;16:493–497. doi: 10.1016/s1074-7613(02)00307-2. [DOI] [PubMed] [Google Scholar]
- Garcia J. V., Miller A. D. Serine phosphorylation-independent downregulation of cell-surface CD4 by nef. Nature. 1991;350:508–511. doi: 10.1038/350508a0. [DOI] [PubMed] [Google Scholar]
- Geyer M., Fackler O. T., Peterlin B. M. Structure–function relationships in HIV-1 Nef. EMBO Rep. 2001;2:580–585. doi: 10.1093/embo-reports/kve141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg M. E., Bronson S., Lock M., Neumann M., Pavlakis G. N., Skowronski J. Co-localization of HIV-1 Nef with the AP-2 adaptor protein complex correlates with Nef-induced CD4 down-regulation. EMBO J. 1997;16:6964–6976. doi: 10.1093/emboj/16.23.6964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene W. C., Peterlin B. M. Charting HIV’s remarkable voyage through the cell: basic science as a passport to future therapy. Nat. Med. 2002;8:673–680. doi: 10.1038/nm0702-673. [DOI] [PubMed] [Google Scholar]
- Haller C., Rauch S., Michel N., Hannemann S., Lehmann M. J., Keppler O. T., Fackler O. T. The HIV-1 pathogenicity factor nef interferes with maturation of stimulatory T-lymphocyte contacts by modulation of N-wasp activity. J. Biol. Chem. 2006 doi: 10.1074/jbc.M513802200. in press. [DOI] [PubMed] [Google Scholar]
- Hrecka K., Swigut T., Schindler M., Kirchhoff F., Skowronski J. Nef proteins from diverse groups of primate lentiviruses downmodulate CXCR4 to inhibit migration to the chemokine stromal derived factor 1. J. Virol. 2005;79:10650–10659. doi: 10.1128/JVI.79.16.10650-10659.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson Z., et al. Chemokine inhibition–why, when, where, which and how? Biochem. Soc. Trans. 2004;32:366–377. doi: 10.1042/bst0320366. [DOI] [PubMed] [Google Scholar]
- Kasper M. R., Collins K. L. Nef-mediated disruption of HLA-A2 transport to the cell surface in T cells. J. Virol. 2003;77:3041–3049. doi: 10.1128/JVI.77.5.3041-3049.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keppler O. T., Allespach I., Schuller L., Fenard D., Greene W. C., Fackler O. T. Rodent cells support key functions of the human immunodeficiency virus type 1 pathogenicity factor Nef. J. Virol. 2005;79:1655–1665. doi: 10.1128/JVI.79.3.1655-1665.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keppler O. T., Tibroni N., Venzke S., Rauch S., Fackler O. T. Modulation of specific surface receptors and activation sensitization in primary resting CD4+ T lymphocytes by the Nef protein of HIV-1. J. Leukoc. Biol. 2006;79:616–627. doi: 10.1189/jlb.0805461. [DOI] [PubMed] [Google Scholar]
- Keppler O. T., et al. Progress toward a human CD4/CCR5 transgenic rat model for de novo infection by human immunodeficiency virus type 1. J. Exp. Med. 2002;195:719–736. doi: 10.1084/jem.20011549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keppler O. T., Yonemoto W., Welte F. J., Patton K. S., Iacovides D., Atchison R. E., Ngo T., Hirschberg D. L., Speck R. F., Goldsmith M. A. Susceptibility of rat-derived cells to replication by human immunodeficiency virus type 1. J. Virol. 2001;75:8063–8073. doi: 10.1128/JVI.75.17.8063-8073.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraft K., Olbrich H., Majoul I., Mack M., Proudfoot A., Oppermann M. Characterization of sequence determinants within the carboxyl-terminal domain of chemokine receptor CCR5 that regulate signaling and receptor internalization. J. Biol. Chem. 2001;276:34408–34418. doi: 10.1074/jbc.M102782200. [DOI] [PubMed] [Google Scholar]
- Krautkramer E., Giese S. I., Gasteier J. E., Muranyi W., Fackler O. T. Human immunodeficiency virus type 1 Nef activates p21-activated kinase via recruitment into lipid rafts. J. Virol. 2004;78:4085–4097. doi: 10.1128/JVI.78.8.4085-4097.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupnick J. G., Benovic J. L. The role of receptor kinases and arrestins in G protein-coupled receptor regulation. Annu. Rev. Pharmacol. Toxicol. 1998;38:289–319. doi: 10.1146/annurev.pharmtox.38.1.289. [DOI] [PubMed] [Google Scholar]
- Lagane B., et al. Mutation of the DRY motif reveals different structural requirements for the CC chemokine receptor 5-mediated signaling and receptor endocytosis. Mol. Pharmacol. 2005;67:1966–1976. doi: 10.1124/mol.104.009779. [DOI] [PubMed] [Google Scholar]
- Lama J., Ware C. F. Human immunodeficiency virus type 1 Nef mediates sustained membrane expression of tumor necrosis factor and the related cytokine LIGHT on activated T cells. J. Virol. 2000;74:9396–9402. doi: 10.1128/jvi.74.20.9396-9402.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lock M., Greenberg M. E., Iafrate A. J., Swigut T., Muench J., Kirchhoff F., Shohdy N., Skowronski J. Two elements target SIV Nef to the AP-2 clathrin adaptor complex, but only one is required for the induction of CD4 endocytosis. EMBO J. 1999;18:2722–2733. doi: 10.1093/emboj/18.10.2722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madrid R., Janvier K., Hitchin D., Day J., Coleman S., Noviello C., Bouchet J., Benmerah A., Guatelli J., Benichou S. Nef-induced alteration of the early/recycling endosomal compartment correlates with enhancement of HIV-1 infectivity. J. Biol. Chem. 2005;280:5032–5044. doi: 10.1074/jbc.M401202200. [DOI] [PubMed] [Google Scholar]
- Mangasarian A., Piguet V., Wang J. K., Chen Y. L., Trono D. Nef-induced CD4 and major histocompatibility complex class I (MHC-I) down-regulation are governed by distinct determinants: N-terminal alpha helix and proline repeat of Nef selectively regulate MHC-I trafficking. J. Virol. 1999;73:1964–1973. doi: 10.1128/jvi.73.3.1964-1973.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariani M., Lang R., Binda E., Panina-Bordignon P., D’Ambrosio D. Dominance of CCL22 over CCL17 in induction of chemokine receptor CCR4 desensitization and internalization on human Th2 cells. Eur. J. Immunol. 2004;34:231–240. doi: 10.1002/eji.200324429. [DOI] [PubMed] [Google Scholar]
- Mellado M., Rodriguez-Frade J. M., Aragay A., del Real G., Martin A. M., Vila-Coro A. J., Serrano A., Mayor F., Jr., Martinez A. C. The chemokine monocyte chemotactic protein 1 triggers Janus kinase 2 activation and tyrosine phosphorylation of the CCR2B receptor. J. Immunol. 1998;161:805–813. [PubMed] [Google Scholar]
- Michel N., Allespach I., Venzke S., Fackler O. T., Keppler O. T. The Nef protein of human immunodeficiency virus establishes superinfection immunity by a dual strategy to downregulate cell-surface CCR5 and CD4. Curr. Biol. 2005;15:714–723. doi: 10.1016/j.cub.2005.02.058. [DOI] [PubMed] [Google Scholar]
- Mueller A., Kelly E., Strange P. G. Pathways for internalization and recycling of the chemokine receptor CCR5. Blood. 2002;99:785–791. doi: 10.1182/blood.v99.3.785. [DOI] [PubMed] [Google Scholar]
- Murphy P. M., Baggiolini M., Charo I. F., Hebert C. A., Horuk R., Matsushima K., Miller L. H., Oppenheim J. J., Power C. A. International union of pharmacology. XXII. Nomenclature for chemokine receptors. Pharmacol. Rev. 2000;52:145–176. [PubMed] [Google Scholar]
- Neel N. F., Schutyser E., Sai J., Fan G. H., Richmond A. Chemokine receptor internalization and intracellular trafficking. Cytokine Growth Factor Rev. 2005;16:637–658. doi: 10.1016/j.cytogfr.2005.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterlin B. M., Trono D. Hide, shield and strike back: how HIV-infected cells avoid immune eradication. Nat. Rev. Immunol. 2003;3:97–107. doi: 10.1038/nri998. [DOI] [PubMed] [Google Scholar]
- Peters P. J., Bhattacharya J., Hibbitts S., Dittmar M. T., Simmons G., Bell J., Simmonds P., Clapham P. R. Biological analysis of human immunodeficiency virus type 1 R5 envelopes amplified from brain and lymph node tissues of AIDS patients with neuropathology reveals two distinct tropism phenotypes and identifies envelopes in the brain that confer an enhanced tropism and fusigenicity for macrophages. J. Virol. 2004;78:6915–6926. doi: 10.1128/JVI.78.13.6915-6926.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piguet V., Wan L., Borel C., Mangasarian A., Demaurex N., Thomas G., Trono D. HIV-1 Nef protein binds to the cellular protein PACS-1 to downregulate class I major histocompatibility complexes. Nat. Cell Biol. 2000;2:163–167. doi: 10.1038/35004038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt E. J., Wehrly K., Kuhmann S. E., Chesebro B., Kabat D. Effects of CCR5 and CD4 cell surface concentrations on infections by macrophagetropic isolates of human immunodeficiency virus type 1. J. Virol. 1998;72:2855–2864. doi: 10.1128/jvi.72.4.2855-2864.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen S. G., Jensen A. D., Liapakis G., Ghanouni P., Javitch J. A., Gether U. Mutation of a highly conserved aspartic acid in the beta2 adrenergic receptor: constitutive activation, structural instability, and conformational rearrangement of transmembrane segment 6. Mol. Pharmacol. 1999;56:175–184. doi: 10.1124/mol.56.1.175. [DOI] [PubMed] [Google Scholar]
- Roland J., Murphy B. J., Ahr B., Robert-Hebmann V., Delauzun V., Nye K. E., Devaux C., Biard-Piechaczyk M. Role of the intracellular domains of CXCR4 in SDF-1-mediated signaling. Blood. 2003;101:399–406. doi: 10.1182/blood-2002-03-0978. [DOI] [PubMed] [Google Scholar]
- Rossi D., Zlotnik A. The biology of chemokines and their receptors. Annu. Rev. Immunol. 2000;18:217–242. doi: 10.1146/annurev.immunol.18.1.217. [DOI] [PubMed] [Google Scholar]
- Scheer A., Costa T., Fanelli F., De Benedetti P. G., Mhaouty-Kodja S., Abuin L., Nenniger-Tosato M., Cotecchia S. Mutational analysis of the highly conserved arginine within the Glu/Asp-Arg-Tyr motif of the alpha(1b)-adrenergic receptor: effects on receptor isomerization and activation. Mol. Pharmacol. 2000;57:219–231. [PubMed] [Google Scholar]
- Scheer A., Fanelli F., Costa T., De Benedetti P. G., Cotecchia S. The activation process of the alpha1B-adrenergic receptor: potential role of protonation and hydrophobicity of a highly conserved aspartate. Proc. Natl. Acad. Sci. USA. 1997;94:808–813. doi: 10.1073/pnas.94.3.808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler M., Wurfl S., Benaroch P., Greenough T. C., Daniels R., Easterbrook P., Brenner M., Munch J., Kirchhoff F. Down-modulation of mature major histocompatibility complex class II and up-regulation of invariant chain cell surface expression are well-conserved functions of human and simian immunodeficiency virus nef alleles. J. Virol. 2003;77:10548–10556. doi: 10.1128/JVI.77.19.10548-10556.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz O., Marechal V., Le Gall S., Lemonnier F., Heard J. M. Endocytosis of major histocompatibility complex class I molecules is induced by the HIV-1 Nef protein. Nat. Med. 1996;2:338–342. doi: 10.1038/nm0396-338. [DOI] [PubMed] [Google Scholar]
- Seifert R., Wenzel-Seifert K. The human formyl peptide receptor as model system for constitutively active G-protein-coupled receptors. Life Sci. 2003;73:2263–2280. doi: 10.1016/s0024-3205(03)00654-4. [DOI] [PubMed] [Google Scholar]
- Shinya E., Owaki A., Shimizu M., Takeuchi J., Kawashima T., Hidaka C., Satomi M., Watari E., Sugita M., Takahashi H. Endogenously expressed HIV-1 nef down-regulates antigen-presenting molecules, not only class I MHC but also CD1a, in immature dendritic cells. Virology. 2004;326:79–89. doi: 10.1016/j.virol.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Signoret N., Hewlett L., Wavre S., Pelchen-Matthews A., Oppermann M., Marsh M. Agonist-induced endocytosis of CC chemokine receptor 5 is clathrin dependent. Mol. Biol. Cell. 2005;16:902–917. doi: 10.1091/mbc.E04-08-0687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Signoret N., Pelchen-Matthews A., Mack M., Proudfoot A. E., Marsh M. Endocytosis and recycling of the HIV coreceptor CCR5. J. Cell Biol. 2000;151:1281–1294. doi: 10.1083/jcb.151.6.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Signoret N., Rosenkilde M. M., Klasse P. J., Schwartz T. W., Malim M. H., Hoxie J. A., Marsh M. Differential regulation of CXCR4 and CCR5 endocytosis. J. Cell Sci. 1998;111:2819–2830. doi: 10.1242/jcs.111.18.2819. [DOI] [PubMed] [Google Scholar]
- Sodhi A., Montaner S., Gutkind J. S. Viral hijacking of G-protein-coupled-receptor signalling networks. Nat. Rev. Mol. Cell Biol. 2004;5:998–1012. doi: 10.1038/nrm1529. [DOI] [PubMed] [Google Scholar]
- Sol-Foulon N., Moris A., Nobile C., Boccaccio C., Engering A., Abastado J. P., Heard J. M., van Kooyk Y., Schwartz O. HIV-1 Nef-induced upregulation of DC-SIGN in dendritic cells promotes lymphocyte clustering and viral spread. Immunity. 2002;16:145–155. doi: 10.1016/s1074-7613(02)00260-1. [DOI] [PubMed] [Google Scholar]
- Stove V., Van de Walle I., Naessens E., Coene E., Stove C., Plum J., Verhasselt B. Human immunodeficiency virus Nef induces rapid internalization of the T-cell coreceptor CD8alphabeta. J. Virol. 2005;79:11422–11433. doi: 10.1128/JVI.79.17.11422-11433.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stumptner-Cuvelette P., Morchoisne S., Dugast M., Le Gall S., Raposo G., Schwartz O., Benaroch P. HIV-1 Nef impairs MHC class II antigen presentation and surface expression. Proc. Natl. Acad. Sci. USA. 2001;98:12144–12149. doi: 10.1073/pnas.221256498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolstrup M., Ostergaard L., Laursen A. L., Pedersen S. F., Duch M. HIV/SIV escape from immune surveillance: focus on Nef. Curr. HIV Res. 2004;2:141–151. doi: 10.2174/1570162043484924. [DOI] [PubMed] [Google Scholar]
- Venkatesan S., Rose J. J., Lodge R., Murphy P. M., Foley J. F. Distinct mechanisms of agonist-induced endocytosis for human chemokine receptors CCR5 and CXCR4. Mol. Biol. Cell. 2003;14:3305–3324. doi: 10.1091/mbc.E02-11-0714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigerust D. J., Egan B. S., Shepherd V. L. HIV-1 Nef mediates post-translational down-regulation and redistribution of the mannose receptor. J. Leukoc. Biol. 2005;77:522–534. doi: 10.1189/jlb.0804454. [DOI] [PubMed] [Google Scholar]
- Williams M., Roeth J. F., Kasper M. R., Filzen T. M., Collins K. L. Human immunodeficiency virus type 1 Nef domains required for disruption of major histocompatibility complex class I trafficking are also necessary for coprecipitation of Nef with HLA-A2. J. Virol. 2005;79:632–636. doi: 10.1128/JVI.79.1.632-636.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams M., Roeth J. F., Kasper M. R., Fleis R. I., Przybycin C. G., Collins K. L. Direct binding of human immunodeficiency virus type 1 Nef to the major histocompatibility complex class I (MHC-I) cytoplasmic tail disrupts MHC-I trafficking. J. Virol. 2002;76:12173–12184. doi: 10.1128/JVI.76.23.12173-12184.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.