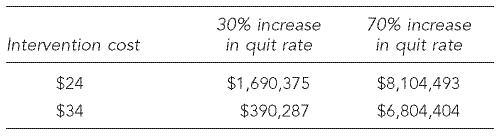SYNOPSIS
Objective
Although the rate of smoking among women giving birth in the United States has declined steadily from 19.5% in 1989 to 11.4% in 2002, it still far exceeds the Healthy People 2010 goal of 1%. The objective of this study was to estimate the costs of a recommended five-step smoking cessation counseling intervention for pregnant women.
Methods
Costs were compared across three settings: a clinical trial, a quit line, and a rural managed care organization. Cost data were collected from August 2002 to September 2003. Intervention costs were compared with potential neonatal cost savings from averted adverse outcomes using data from the Centers for Disease Control and Prevention's Maternal and Child Health Smoking-Attributable Mortality, Morbidity, and Economics Costs software.
Results
The costs of implementing the intervention ranged from $24 to $34 per pregnant smoker counseled across the three settings. Potential neonatal cost savings that could be accrued from women who quit smoking during pregnancy were estimated at $881 per maternal smoker. Assuming a 30% to 70% increase over baseline quit rates, interventions could net savings up to $8 million within the range of costs per pregnant smoker.
Conclusions
Costs may vary depending on the intensity and nature of the intervention; however, this analysis found a surprisingly narrow range across three disparate settings. Cost estimates presented here are shown to be low compared with potential cost savings that could be accrued across the quit rates that could be achieved through use of the 5A's smoking cessation counseling intervention.
Smoking during pregnancy is associated with many adverse pregnancy outcomes: low birthweight, problems with infant health and development, intrauterine growth retardation, spontaneous abortion, and sudden infant death.1 In 2001, the rate of low birthweight among babies born to mothers who smoked was nearly two-thirds higher than for nonsmoking mothers.2 Low birthweight can pose serious health problems throughout the infants' lives.
Maternal smoking also results in substantial health care costs,3 most of which are immediate, including increased use of neonatal intensive care units, and hospital stays and services.4 In 1996 alone, costs at delivery for infants with problems caused by prenatal smoking totaled about $366 million in the United States.5 Adverse outcomes are preventable and costs can be significantly reduced with effective smoking cessation intervention during pregnancy.
Little is known about the costs of implementing effective interventions within our current health care system. This study estimates costs of a recommended smoking cessation counseling intervention for pregnant women, and in turn compares these to potential cost savings from averted adverse outcomes. The five-step best practice smoking cessation counseling intervention used in this study was delivered within 5–15 minutes by a trained provider using pregnancy-specific self-help materials. This technique, known as the 5 A's, has been shown to produce quit rates that are significantly higher than those achieved through usual practice.6 Costs of implementing the 5 A's were compared across three different settings: (1) a clinical trial, (2) a national pregnant smokers quit line, and (3) a rural managed care organization (MCO).
BACKGROUND STUDIES
Although the smoking rate among women giving birth in the United States has declined steadily from 19.5% in 1989 to 11.4% in 2002 (a 42% decline),7 it still far exceeds the Healthy People 2010 goal of 1%.8 Several studies have estimated the costs attributable to this continued smoking,9–12 and others have examined costs and effects of smoking cessation interventions.13–16 Ershoff et al. evaluated a prenatal health education program within a health maintenance organization.13 This yielded a benefit-cost ratio of approximately 2:1. Ershoff et al. also conducted an economic evaluation of a self-help program consisting of printed materials and found a benefit-cost ratio of 2.8:1.14
Windsor et al. conducted a cost-effectiveness analysis of a randomized trial that assigned pregnant women to one of three groups.15 All groups received standard clinic information. In addition, the second group received a general smoking cessation manual while the third group received a pregnancy-specific smoking cessation manual. Given the end-of-pregnancy quit rates and the intervention cost per patient, the third group was the most cost-effective, achieving a higher smoking cessation rate at less than half the costs of the other two. Another early study asserted that a program costing $30 per participant would save $3 for every dollar spent.17 Finally, Hueston et al. examined the cost-effectiveness of a smoking cessation program conducted during the first trimester of pregnancy.18 The program was shown to be cost-effective for preventing low birthweight if it costs $80 or less per pregnant woman.
THE 5 A’S INTERVENTION
Although earlier studies suggest that smoking cessation interventions geared to pregnant women are cost-effective compared with usual care, many did not include pregnancy-specific self-help materials. Yet, such materials have been shown to significantly increase quit rates among pregnant women.19 We evaluated the costs of implementing a 5- to15-minute five-step cessation counseling intervention delivered by a trained health professional who also provided pregnancy-specific self-help materials. This intervention, known as the 5 A's, was based on recommendations found in the U.S. Public Health Service's Clinical Practice Guideline, Treating Tobacco Use and Dependence.20 The five components of the intervention include: Ask, Advise, Assess, Assist, and Arrange (Figure 1).
Figure 1.
Components of the 5 A's smoking cessation counseling intervention for pregnant women
SOURCE: Melvin C, Dolan Mullen P, Windsor RA, Whiteside HP, Goldenberg RL. Recommended cessation counseling for pregnant women who smoke: a review of the evidence. Tob Control 2000;9(suppl III):III80-4.
Due to concern about deception rates and the validity of self-reported smoking in intervention trials of previous studies, a multiple-choice response format was developed for the “ASK” step, assessing the pregnant woman's smoking status and improving disclosure (Figure 2).
Figure 2.
The “ASK” step of the 5 A's intervention
SOURCE: Melvin C, Dolan Mullen P, Windsor RA, Whiteside HP, Goldenberg RL. Recommended cessation counseling for pregnant women who smoke: a review of the evidence. Tob Control 2000;9(suppl III):III80-4.
The 5 A’s are currently being implemented in different settings and among diverse populations of pregnant women across the country. The intervention encourages women to quit smoking permanently, not just for the duration of the pregnancy. This study was unique in that data were collected and compared across three disparate settings: (1) a clinical trial, (2) a national pregnant smokers telephone quit line, and (3) a rural MCO. The basic characteristics of each intervention site are shown in Table 1. The clinical trial was held in a public clinic setting serving primarily low-income pregnant women on Medicaid. The study population, which comprised 46 pregnant women from six Title V prenatal care agencies in the northeastern United States, was part of a clinical trial conducted during Phase II of the Robert Wood Johnson Foundation funded Smoke-Free Families Research Project. The 5 A's intervention was integrated directly into prenatal care visits.
Table 1.
Description of basic components of the intervention across the three settings
The second setting was a national smokers telephone quit line that had added a counseling module specifically for pregnant women, integrating the 5 A's; data were obtained from seven participating states (n5408). Women who called the quit line received up to eight counseling sessions with a trained counselor, a pregnancy-specific educational booklet, and a video on pregnancy and smoking cessation. After the initial call to the quit line, the woman received her first counseling session, set a quit date, and scheduled a second session, usually for one–two days before the quit date. The third counseling session was conducted on the scheduled quit date, and the fourth through sixth sessions were scheduled from about four days to one month after the quit date.
The third setting was a rural MCO that served approximately 132,000 members in a western state. The delivering physicians, certified nurse midwives, and trained counselors in a rural county that were part of the MCO received training on the 5 A's intervention. The intervention was integrated into routine prenatal care visits at nine delivering offices and included several unique components: a neon green sticker for use on prenatal charts to remind providers to ask their patients about their smoking status at each visit, a provider training sheet, and a pregnancy-specific self-help smoking cessation booklet with a lollipop on it. Data collected from this setting were based on 419 pregnant smokers counseled.
METHODS
Before attempting to measure intervention costs, one must first determine a study's perspective, the types of costs to include/exclude (e.g.,fixed vs. operating, direct vs. indirect), and how to treat costs for training and development. For this study, the provider perspective was used in measuring intervention costs, because a basic assumption was that the intervention would be implemented as incremental changes to an ongoing clinical practice or telephone quit line and not require additional capacity. Therefore, the provider would incur the incremental costs to their practice or pay for an existing intervention. Consequently, in determining intervention costs, development costs (which are “sunk” or nonrecoverable) and research/evaluation costs were not included. Neither were direct costs (e.g., travel costs) nor indirect costs (e.g., lost time at work) incurred by the patient due to participation and not accrued to the provider. Finally, costs for the use of facilities were excluded. Thus, the key cost categories were: (1) personnel (or labor),
(2) materials and supplies, (3) incentives paid to participants, (4) use of equipment, and (5) initial training. Cost data were collected from August 2002 to September 2003.
To calculate personnel costs, we used a time-motion study to determine time spent implementing the 5 A's in each setting. After making several timed observations in each setting to estimate the average time to complete the intervention, we calculated total labor costs by multiplying the mean times recorded by the reported hourly wage and fringe benefit rate paid, based on the skill level of the participating personnel. Personnel costs varied from one setting to another as well as within settings. For example, in the MCO, average wages ranged from $9 per hour plus fringe benefits for a trained counselor to $130 per hour for a physician in a provider's office. To determine personnel costs per pregnant smoker, data were compiled on the number of counseling sessions each woman received, which varied by setting and how early in the pregnancy the participant presented to the provider.
We also calculated costs of materials, supplies, participant incentives, and use of equipment within each setting. These included $5 gift certificates for women who completed the intervention (clinical trial), videos mailed to pregnant smokers (quit line), stickers (MCO), and pregnancy-specific self-help materials (all settings). To estimate these costs, we gathered data on the per-unit costs of self-help materials distributed. Because the ultimate intent was to determine intervention costs per pregnant smoker, we also collected data on the average number of materials provided to each participant.
To estimate training costs, we gathered data on the number and type of personnel who gave and who received training along with time spent and salary plus fringe rates. These were direct costs for trainers and measures of “opportunity costs” for those trained. Average costs of instructional materials and supplies were also included. Training expenses varied widely depending on how much time was spent per training session, size of the training class, and number of pregnant women who were subsequently counseled within a given time frame. Time spent training counselors to work the quit line far exceeded the time spent training the MCO physicians, but was compensated by the lower opportunity costs of counselors vs. physicians and the greater capacity of the quit line counselors to counsel more women within a given time frame.
RESULTS
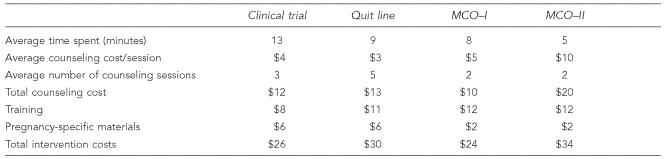
Across the three settings, smoking cessation counseling was provided by trained personnel ranging from counselors, to certified nurse midwives, to physicians. The average time spent per session across sites ranged from five minutes in the MCO to 13 minutes in the clinical trial (Table 2). The time spent counseling pregnant women also varied among MCO providers: physicians (MCO-II) generally spent less time counseling than nurse practitioners or counselors (MCO-I). Combining time spent with salary/fringe rates, the average counseling cost per session ranged from about $3 in the quit line to a high of about $10 in the MCO (Table 2). Within the MCO, the cost per counseling session ranged from $5 to $10 due to the difference in salaries of counselors vs. physicians as well as time spent per session.
Table 2.
Estimated costs of the 5 A's per pregnant smoker
MCO = managed care organization
MCO-I = MCO counselor
MCO-II = MCO physician
The number of counseling sessions each woman received also varied across the settings; for example, in the MCO, an average of two counseling sessions per person were provided vs. three sessions in the clinical trial. Based on the detailed quit line data, callers received an average of five sessions, including the intake call. Of the 408 pregnant callers in the study sample, only 16 received all eight counseling sessions that comprised the full protocol.
A large percentage of the total intervention costs were for training. These ranged from about $8 per woman in the clinical trial to $12 in the MCO (Table 2). While the time spent on training varied widely across the settings, from 45 minutes to three weeks, differences in training costs were not as varied. Physicians (who had higher opportunity costs than the counselors at the quit line) spent 45 minutes, while counselors spent three weeks in theory, practice, and mentoring-based training. However, each quit line counselor reached a significantly larger number of women than the physicians did, thus lowering the counselors' average training costs per pregnant smoker. Per-unit costs of materials provided for the intervention, also shown in Table 2, ranged from $2 in the MCO setting to $6 in both the quit line and clinical trial settings. The clinical trial provided $5 gift certificates in addition to self-help materials, while the quit line mailed out videos and the self-help materials, and the MCO gave only self-help materials.
Total costs for each intervention were derived by adding the costs of counseling, training, and materials. The estimated total costs per pregnant smoker of implementing the 5 A's intervention ranged from $24 to $34 across the three settings (Table 2). The widest variation occurred within the MCO due to the higher cost per unit of time for physician counseling.
POTENTIAL NEONATAL COST SAVINGS
One reason for estimating the cost of the interventions was to gauge their potential for actually saving money. To do this we used estimates of average costs averted from a quit, potential quit rates, and the estimated costs of the intervention. The Centers for Disease Control and Prevention's web-based software, the Smoking Attributable Morbidity, Mortality, and Economic Costs (SAMMEC),21 now includes a Maternal and Child Health module designed to assist in this calculation. Statistical analysis was used to estimate the relationship of a woman smoking during pregnancy and the probability that a child will be admitted to a neonatal intensive care unit, and the infant's length of stay. These costs include only direct medical costs for resource utilization provided to infants during their initial hospital stay. National and state-specific estimates of smoking attributable neonatal costs (SAE) per woman can be obtained from the SAMMEC software.21 The excess neonatal cost for infants of mothers who smoked was estimated at $704 per maternal smoker in 1996 dollars or $881 in 2002 dollars (adjusted for inflation using the medical care measure of the consumer price index).22
The excess neonatal costs per maternal smoker are the potential cost-savings that could be accrued from the reduced use of a neonatal intensive care unit, shorter lengths of stay, and decreased service intensity achieved from women who quit smoking during pregnancy as a result of an effective intervention. For illustrative purposes, assume that 25% of the number of estimated maternal smokers in the U.S. are reached for intervention through counseling, and the demonstrated 30% to 70% increase in the quit rate is achieved over a baseline quit rate of 14%;6,8 then about 5,460 to 12,740 additional women would quit smoking during pregnancy. Potential neonatal cost savings of $881 per maternal smoker for these incremental quits would result in approximately $5–$11 million in excess neonatal costs averted. Table 3 shows the possible range of net savings (or costs) if each pregnant smoker reached by this intervention received counseling at an estimated incremental cost range of $24–$34.
Table 3.
Potential neonatal net savings (or costs) from averted adverse outcomes given a range of quit rates
If the intervention costs $24 per pregnant smoker counseled, and the intervention achieves a 30% increase in quit rate over the baseline quit rate of 14%, there could be a net savings of about $1.7 million. However, if the intervention costs $34 per pregnant smoker counseled, there is a net savings of about $390,000. At the high end of effectiveness (i.e., a 70% increase over baseline), net savings for this illustration range from $6.8 million if the intervention costs $34 per pregnant smoker counseled to almost $8 million if the intervention costs $24 per pregnant smoker. If the mothers remain smoke-free, costs could be further reduced and outcomes improved in both the short and long term. These estimates, however, may overestimate the incremental costs of the 5 A's if practitioners already include some of this counseling in their usual practice. Of note is the finding that when the intervention effectiveness is on the lower range—for instance, the intervention is not very effective with heavy smokers (.20 cigarettes per day)—and if the costs of implementing the intervention are in the high range, the intervention may produce net costs. An earlier study estimated a break-even point for cessation programs during pregnancy at $32 per pregnant woman.23
DISCUSSION
This study has several limitations. First, cost data from only three settings were collected. Intervention costs may vary for providers in other settings, especially in those that are markedly different from the settings used. Whether those costs are higher or lower than the cost estimates presented here is hard to predict, other than taking into consideration long-standing differences across geographic areas in terms of costs-of-living and doing business. For example, costs would probably be higher in the more urbanized areas of the northeast than in other parts of the country and in more rural areas, even if the characteristics of the practice settings were similar.
In addition, the analysis was limited to point-in-time estimates. If data were collected for the analytic horizon in each setting, the number of women served would increase, which would lower per-unit costs, especially for interventions with high training costs. This is important for health care planners/providers to consider. The number of staff originally trained should be adequate to serve clientele over the long term as the intervention becomes more mature and utilization increases. Despite these limitations, the range in estimates is quite small and provides information regarding three different methods of real life implementation of the 5 A's.
The costs of the 5 A's may vary depending on the intensity and nature of the intervention, but this analysis shows a surprisingly narrow range across the three disparate settings. Cost estimates presented here varied depending on the range in time spent on counseling (which differed between physicians and trained counselors), the numbers of pregnant smokers counseled by trained staff, and the use and costs of materials within each setting. Training costs are a large portion of total costs seen in these settings; a key aspect of lowering costs is to expand the scope of the intervention in order to reduce the training costs per woman served.
Clearly, each of the settings studied approached the implementation of the 5 A's differently. Because the MCO used both counselors and physicians, who are higher cost, its per-smoker costs were the highest of the three settings. Yet, in order to encourage more women to participate in these programs, it is essential for physicians to recommend them; physicians' advice is a key factor in the patients' use of preventive services.24 Indeed, an intervention that incorporates physicians may, in the long term, prove to have lower costs per pregnant smoker as economies of scale from training are realized.
Although this article provides valuable information on the costs of a counseling-based intervention in current practice settings and compares these with estimated potential cost savings, measures are not provided on the effectiveness of the intervention in each setting. It is most likely that the somewhat different approaches taken to implement the 5A's in our study resulted in different quit rates. If they varied significantly, one approach would be more cost-effective than the other two, and it would be important to ascertain this.
Finally, both the incremental costs of a counseling-based intervention and the potential costs averted may vary with the population being served. For example, even the same smoking cessation program (e.g., the 5A's) aimed at women with multiple risk factors or women who started prenatal care in the third trimester might cost more if they need to be more intensive than those programs examined here. Thus, a complete prospective cost-effectiveness analysis should be conducted, not only across alternative settings, but also different populations that can perhaps be targeted for early and/or more intensive interventions.
REFERENCES
- 1. Department of Health and Human Services (US). Women and smoking: a report of the Surgeon General—2001. Rockville (MD): US Public Health Service; 2001.
- 2.Martin JA, Hamilton BE, Ventura SJ, Menacker F, Park MM, Sutton PD. Births:final data for 2001. Natl Vital Stat Rep. 2002;51(2):1–114. [PubMed] [Google Scholar]
- 3.Miller DP, Villa KF, Hogue SL, Sivapathasundaram D. Birth and first-year costs for mothers and infants attributable to maternal smoking. Nicotine Tob Res. 2001;3(1):25–35. doi: 10.1080/14622200020032079. [DOI] [PubMed] [Google Scholar]
- 4.Adams EK, Ayadi MO, Melvin CL, Rivera C. Smoking among Medicaid insured mothers: what are the neonatal costs? Health Care Financ Rev. 2005;26(2):105–18. [PMC free article] [PubMed] [Google Scholar]
- 5.Adams EK, Miller VP, Ernst C, Nishimura BK, Melvin C, Merritt R. Neonatal health care costs related to smoking during pregnancy. Health Econ. 2002;11:193–206. doi: 10.1002/hec.660. [DOI] [PubMed] [Google Scholar]
- 6.Melvin CL, Dolan-Mullen P, Windsor RA, Whiteside HP, Goldenberg RL. Recommended cessation counseling for pregnant women who smoke: a review of the evidence. Tob Control. 2000;9(suppl III):III80–4. doi: 10.1136/tc.9.suppl_3.iii80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martin JA, Hamilton BE, Sutton PD, Ventura SJ, Menacker F, Munson ML. Births:final data for 2002. Natl Vital Stat Rep. 2003;52(10):1–113. [PubMed] [Google Scholar]
- 8. Department of Health and Human Services (US). Healthy People 2010: understanding and improving health. 2nd edition. Rockville (MD): US Government Printing Office; 2000.
- 9.Medical-care expenditures attributable to cigarette smoking during pregnancy United States, 1995. MMWR Morb Mort Wkly Rep. 1997;46(44):1048–50. [PubMed] [Google Scholar]
- 10.Adams EK, Melvin CL. Costs of maternal conditions attributable to smoking during pregnancy. Am J Prev Med. 1998;15:212–9. doi: 10.1016/s0749-3797(98)00049-x. [DOI] [PubMed] [Google Scholar]
- 11.Oster G, Delea TE, Colditz GA. Maternal smoking during pregnancy and expenditures on neonatal health care. Am J Prev Med. 1988;4:216–9. [PubMed] [Google Scholar]
- 12.Lightwood JM, Phibbs CS, Glantz SA. Short-term health and economic benefits of smoking cessation: low birth weight. Pediatrics. 1999;104:1312–20. doi: 10.1542/peds.104.6.1312. [DOI] [PubMed] [Google Scholar]
- 13.Ershoff DH, Aaronson NK, Danaher BG, Wasserman FW. Behavioral, health, and cost outcomes of an HMO-based prenatal health education program. Public Health Rep. 1983;98:536–47. [PMC free article] [PubMed] [Google Scholar]
- 14.Ershoff DH, Quinn VP, Mullen P, Lairson DR. Pregnancy and medical cost outcomes of a self-help prenatal smoking cessation program in a HMO. Public Health Rep. 1990;105:340–7. [PMC free article] [PubMed] [Google Scholar]
- 15.Windsor RA, Warner KE, Cutter GR. A cost-effectiveness analysis of self-help smoking cessation methods for pregnant women. Public Health Rep. 1988;103(1):83–8. [PMC free article] [PubMed] [Google Scholar]
- 16.Windsor RA, Lowe JB, Perkins LL, Smith-Yoder D, Artz L, Crawford M, et al. Health education for pregnant smokers: its behavioral impact and cost benefit. Am J Public Health. 1993;83:201–6. doi: 10.2105/ajph.83.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marks JS, Koplan JP, Hogue CJ, Dalmat ME. A cost-benefit/cost-effectiveness analysis of smoking cessation for pregnant women. Am J Prev Med. 1990;6:282–9. [PubMed] [Google Scholar]
- 18.Hueston WJ, Mainous AG, Farrell JB. A cost-benefit analysis of smoking cessation programs during the first trimester of pregnancy for the prevention of low birthweight. J Fam Pract. 1994;39:353–7. [PubMed] [Google Scholar]
- 19.Windsor RA, Cutter G, Morris J, Reese Y, Manzella B, Bartlett EE, et al. The effectiveness of smoking cessation methods for smokers in public health maternity clinics: a randomized trial. Am J Public Health. 1985;75:1389–92. doi: 10.2105/ajph.75.12.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fiore MC, Bailey WC, Cohen SJ, Dorfman SF, Goldstein MG, Gritz ER, et al. Clinical Practice Guideline. Rockville MD: US Public Health Service, 2000. [cited 2002 Apr 1];Treating tobacco use and dependence. Also available from: URL: http://www.surgeongeneral.gov/tobacco/treating_tobacco_use.pdf.
- 21.Centers for Disease Control and Prevention (US) [cited 2005 Mar 10];Smoking-Attributable Mortality, Morbidity, and Economic Costs (SAMMEC): Adult SAMMEC and Maternal and Child Health (MCH) SAMMEC software, 2004. Available from: URL: http://www.cdc.gov/tobacco/sammec. [Google Scholar]
- 22.U.S. Census Bureau. Statistical Abstract of the United States 2003:475. [cited 2005 Nov 15]. Available from: URL: http://www.census.gov/prod/www/statistical-abstract-04.html.
- 23.Shipp M, Croughan-Minihane MS, Petitti DB, Washington AE. Estimation of the break-even point for smoking cessation programs in pregnancy. Am J Public Health. 1992;82:383–90. doi: 10.2105/ajph.82.3.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Floyd RL, Belodoff B, Sidhu J, Schulkin J, Ebrahim SH, Sokol RJ. A survey of obstetricians-gynecologists on their patients' use of tobacco and other drugs during pregnancy. Prenat Neonatal Med. 2001;6(4):201–7. [Google Scholar]