SYNOPSIS
Objectives
South Carolina mandates reporting of animal bites and manages distribution of biologics for rabies postexposure prophylaxis (PEP). Incidence and epidemiologic characteristics of potential human rabies exposures and preventive treatment in South Carolina from 1993 through 2002 were examined to help assess the burden of PEP in the state and determine if the incidence of rabies exposures has changed over time.
Methods
Data on animal exposure investigations and PEP administration at the state and county level were examined, and the annual incidences of potential rabies exposures and human PEP courses were calculated.
Results
The incidence of animal exposures for which investigations were initiated was 297.9 per 100,000 population per year, and the incidence of PEP was 10.6 per 100,000 population per year. At the county level, the incidence of PEP appeared inversely correlated with the population density. Most courses of PEP were administered following exposures to domestic species, although these animals accounted for only a small proportion of rabid animals in the state. The annual PEP incidence was similar throughout the study period, but it was markedly higher than estimates from 1981 (<5/100,000 population per year).
Conclusions
The incidence of PEP in South Carolina is higher than previously thought, and these findings suggest that incidence extrapolations for other states and at the national level may be underestimated. An accurate estimation of the incidence of PEP and an understanding of rabies epidemiology is important at the state level to allow for better public health planning.
Worldwide, rabies remains an important cause of fatal illness in humans, with an estimated 55,000 deaths occurring annually.1 In most developing countries, humans are at risk for rabies from dogs, which are the primary terrestrial reservoirs. In the United States, however, rabies is more often reported in wildlife species because of improved vaccination rates and decreased incidence in domestic animals.2,3 Concomitant with the decrease of canine rabies in the United States, the annual incidence of human rabies in this country has also decreased and currently averages fewer than five cases annually.2,4 Most human rabies is due to infection with rabies virus variants associated with insectivorous bats or to exposures to dogs in countries where canine rabies is still prevalent.4
Although rabies infection is invariably fatal, the disease is preventable with timely treatment and administration of appropriate biologics following an exposure to a rabid animal. The recommended regimen for postexposure prophylaxis (PEP) consists of three components: wound treatment, administration of rabies immune globulin to provide immediate passive immunity, and administration of rabies vaccine to stimulate an active immune response. Human rabies immune globulin (RIG) is infiltrated into and around a wound, with any remaining volume injected intramuscularly at a site other than where the vaccine was administered. Rabies vaccine is administered intramuscularly as soon as possible after exposure (day 0), and then on days 3, 7, 14, and 28. In cases of prior rabies immunization, an abbreviated vaccine course is recommended (two booster doses on days 0 and 3), and no RIG is administered.5
Although relatively few human rabies deaths are recognized each year in the United States, rabies continues to pose an important public health concern as evidenced by the considerable annual financial expenditure on PEP following exposure to rabid or potentially rabid animals. An estimated 16,000 to 39,000 total PEP treatments are administered each year in the United States, with an annual cost for PEP biologics ranging from $26.5 million to $65.3 million.6 Because of the broad range of these estimates, there is a need for a more accurate determination of the incidence of PEP in the United States.
In the eastern United States, the primary terrestrial rabies virus variant that is present circulates among raccoons, with occasional spillover to other wildlife species and domestic animals.2 Raccoons often live in geographic proximity to humans and may cause human exposures through direct contact as well as spillover rabies transmission to domestic animals, such as dogs and cats. The raccoon rabies virus variant has been recognized for over four decades in South Carolina. Bites from animals to humans are a reportable condition in South Carolina, and the South Carolina Department of Health and Environmental Control (SCDHEC) maintains thorough records of animal bites and of subsequent PEP events. Unlike most states, South Carolina ensures the availability of human antirabies vaccine and immune globulin products through the SCDHEC for individuals exposed to animals suspected to be affected by rabies. This system provides an opportunity to determine a highly accurate incidence of PEP in South Carolina for 1993 through 2002.
METHODS
The SCDHEC Bureau of Environmental Health, Division of General Sanitation, provided information on all animals tested for rabies in the SCDHEC laboratory. They tested brain specimens from suspect animals by direct fluorescent antibody tests and reported results as positive or negative based on observed fluorescence. State totals for the annual number of animal exposure investigations initiated at the county level were also provided. “Exposure” was defined as contact with an animal that was considered a possible risk for exposure to rabies; in most cases, the exposure scenario was a bite but may have also included non-bite contact, such as a scratch or splash of fluids onto mucous membranes.
We analyzed data for rabies PEP administered in South Carolina from 1993 through 2002. These data were collected at the county level and consolidated at the SCDHEC central office. Data were available from all 46 counties within South Carolina during the study period. Available information for cases included date of rabies exposure, sex and age of exposed human, exposing animal type, and the county in which treatment occurred. Not all data were available for every case, so specific denominators are presented where appropriate. PEP was initiated according to the 1991 and 1999 Advisory Committee on Immunization Practices (ACIP) guidelines for human rabies prevention in the United States.5,7 According to the SCDHEC protocol, rabies PEP is recommended following an exposure from a known rabid animal or an animal otherwise unavailable for testing or observation. We calculated the annual incidences of animal exposures and human courses of PEP by using state and county U.S. Census Bureau population estimates for each year.8–10
RESULTS
From 1993 through 2002, the SCDHEC laboratory examined 23,335 specimens for rabies (mean = 2,334 per year). Of those tested, 1,552 (6.7%) were positive. The percent of animals that tested positive ranged from a low of 4.3% in 1996 to a high of 7.8% in 1994. Raccoons accounted for 991 (63.9%) of rabies-positive animals in the state; dogs accounted for only 21 (1.4%) and cats for only 79 (5.1%). The relative proportions of other rabid animals included 226 foxes (14.6%), 132 skunks (8.5%), 69 bats (4.4%), 26 other wild animals (1.7%), and 8 other domestic animals (0.5%).
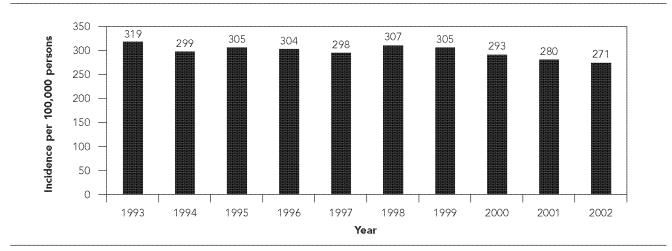
From 1993 through 2002, the SCDHEC investigated a total of 114,488 animal exposures (mean = 11,449 per year). Animal exposure was believed to most commonly have been a bite, but in some cases may have included a non-bite exposure. The mean animal exposure incidence was 297.9 (range: 271.0–319.4) exposures per 100,000 population per year (Figure 1). Ninety-two percent of exposures (mean = 10,412 per year) were to animals classified as pets. Approximately 74% of investigations for pet exposure incidents were handled by a 10-day observation period for the animal; if the exposing animal remained healthy during this time, it was not considered to represent a threat for rabies at the time of the exposure. The remaining 26% of pet exposure investigations involved situations where the pet was unavailable for observation or was tested for rabies.
Figure 1.
Annual incidence of animal exposures investigated per 100,000 population, South Carolina, 1993–2002
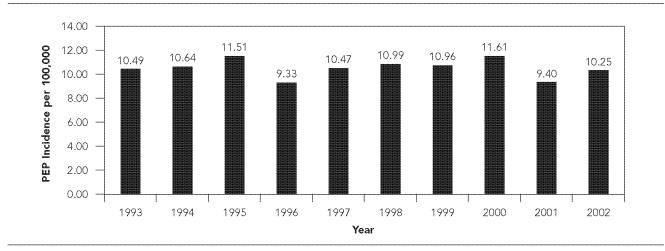
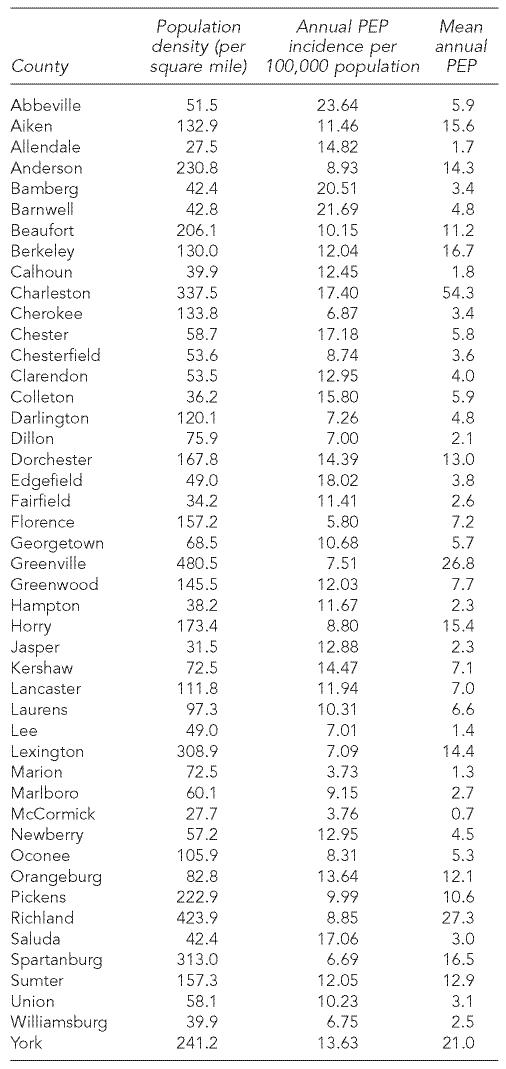
From 1993 through 2002, 4,061 courses of rabies PEP were reported, yielding an annual mean of 406 PEP courses (range: 349–467) and an incidence of 10.6 (range: 9.3–11.6) per 100,000 population per year (Figure 2). PEP was administered in 3.5% of the animal exposures investigated. On a county level, PEP incidence ranged from 3.8–23.6 PEP courses per 100,000 population per year (Table).
Figure 2.
Annual rabies postexposure prophylaxis (PEP) incidence per 100,000 population, South Carolina, 1993–2002
Table.
Rabies postexposure prophylaxis (PEP) in South Carolina counties, 1993–2002
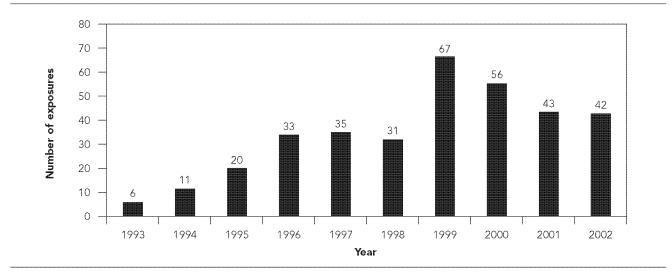
PEP administration peaked during summer months, and 41% (1,656/4,008) of PEP courses were administered from May through August. Males accounted for 53% (2,160/4,060) of all PEP courses. Overall, PEP administration by age was not dissimilar from the age distribution of South Carolina residents. Exposures to domestic species accounted for the majority (74%, 2,989/4,061) of PEP courses. Thirty-six percent (1,481/4,061) of all PEP courses were attributed to exposures to dogs, 35% (1,422/4,061) to cats, 11% (443/4,061) to raccoons, and 8% (344/4,061) to bats. PEP was also administered for exposure to several other domestic species, including goats (9/4,061), ferrets (8/4,061), cows (6/4,061), and pigs (3/4,061). PEP administration was also required for exposure to some wildlife species, including fox (160/4,061); squirrel (38/4,061); skunk (30/4,061); opossum (17/4,061); bobcat (15/4,061); rat (5/4,061); monkey (4/4,061); otter and coyote (3/4,061 each); bear, beaver, boar, mouse (2/4,061 each); chipmunk, groundhog, mink, mountain lion, ocelot, weasel, wolf-hybrid (1/4,061 each); and unknown (21/4,061). One PEP course was administered following a rabies exposure in a laboratory setting. Bat-associated PEP peaked in 1999 at 67 reported bat exposures and remained elevated through 2002 (Figure 3).
Figure 3.
Annual number of rabies postexposure prophylaxis (PEP) courses administered following exposures to bats, South Carolina, 1993–2002
DISCUSSION
Although human and animal rabies deaths are reportable in the United States, national reporting of PEP administration to prevent rabies infection is not required. The determination of when PEP should be administered varies greatly from state to state and even from county to county on the basis of legal statutes within each state. Some states leave the decision to administer PEP to primary healthcare providers, while other states may play a more active role in the assessment of exposures and provision of support for decisions regarding healthcare management of the exposure.11–15 The act of consolidating data on treatments provided by multiple health care providers, including emergency room physicians, primary care physicians, and health departments, makes accurate assessments of PEP incidence difficult or impossible for states in which PEP administration is not closely monitored.
Despite these difficulties, periodic assessments of PEP administration are important because they provide a way to compare changes in incidence over time. These assessments also contribute to the determination of PEP costs relative to the total budget of rabies prevention. The number of people receiving rabies PEP each year in the United States is unknown but is presumably higher than a prior minimum estimate of 20,000, when vaccine distribution was more tightly regulated.15 The most recent estimates of rabies PEP administration in the United States range from 16,000–39,000 courses annually (or an estimated incidence of 6.4–15.7 per 100,000 population).6
In this review, animal bite reports and PEP administration for South Carolina were reviewed for 1993 through 2002. Because South Carolina has mandatory animal-bite reporting and controls the release of PEP biologics following review of exposures and determination of risk assessments, the state has an unusually robust system to evaluate PEP incidence and epidemiology. Our estimate of the incidence of PEP during the study period (10.6 per 100,000 population) is substantially higher than previously published estimates for South Carolina.6,16 In previous assessments derived from similar methods conducted from 1979 through 1981 in South Carolina, fewer than five PEP courses per 100,000 population were estimated to have been administered annually.6,15 These new data illustrate that prior estimates may no longer reflect the current status of PEP administration, and they reaffirm the need to interpret previous state and national estimates with caution.
In South Carolina, most rabies PEP courses were administered for exposures to domestic species (predominantly dogs and cats that could not be quarantined and observed), despite the fact that these animals accounted for only 6.4% of rabies-positive animals within the state. These findings are consistent with reports from South Carolina for 1979 through 1981, which indicated that dog and cat exposures accounted for the majority of rabies PEP; they likely reflect the frequency with which humans interact with domestic animals compared with wild species.16 Of interest, PEP incidence in South Carolina counties appears inversely correlated with human population density. Of eight counties with a high PEP incidence (≥15 per 100,000 population), seven counties were classified as having a low population density (<60 individuals per square mile). This may reflect differences in actual rabies exposures or differences in management of exposing animals between rural and urban areas.
The ACIP serves as a comprehensive resource for PEP providers across the nation. These guidelines were originally published in 1991, and were updated in 1999.5,7 Unlike the 1991 recommendations, which advised that bites by wild carnivores and bats be considered possible exposures to rabies,7 the 1999 recommendations included revisions that defined bat exposures more specifically.5 The role of bats as important wildlife reservoirs separate from wild carnivores is highlighted, and consideration of rabies PEP is recommended when direct contact between a human and a bat cannot be ruled out.5 These new recommendations resulted from evidence that unrecognized or seemingly minor bites from bats can cause transmission of rabies virus.5 In this study, the observed increase in PEP due to bat exposures in 1999 provides a state-level example of the impact of elaborated information on bat exposures included in the 1999 ACIP recommendations.
Because of South Carolina's centralized procedures regarding PEP administration, an accurate assessment of PEP events from 1993–2002 was possible. However, one limitation of this surveillance system is that PEP incidence may still be underestimated, as physicians may in some cases obtain biologics through the manufacturer directly. The availability of no-cost biologics through the state health department likely ensures that these events remain uncommon in South Carolina. Continued monitoring of PEP administration in South Carolina will be an important factor for ongoing assessment of this public health issue.
Acknowledgments
The authors thank Carolyn Morgan for assistance in compiling data at the SCDHEC, staff at the SCDHEC laboratory for review of how rabies tests were performed, and Robert Holman, MS, Charles Rupprecht, VMD, Cathleen Hanlon, VMD, and David Swerdlow, MD, for expert advice and critical review.
REFERENCES
- 1.World Health Organization. Human and animal rabies. [cited 2004 July 27]. Available from: URL: http://www.who.int/rabies/en/
- 2.Krebs JW, Mandel EJ, Swerdlow DL, Rupprecht CE. Rabies surveillance in the United States during 2003. J Am Vet Med Assoc. 2004;225:1837–49. doi: 10.2460/javma.2004.225.1837. [DOI] [PubMed] [Google Scholar]
- 3.Rupprecht CE, Smith JS, Fekadu M, Childs JE. The ascension of wildlife rabies: a cause for public health concern or intervention? Emerg Infect Dis. 1995;1:107–14. doi: 10.3201/eid0104.950401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Noah DL, Drenzek CL, Smith JS, Krebs JW, Orciari L, Shaddock J, et al. Epidemiology of human rabies in the United States, 1980 to 1996. Ann Intern Med. 1998;128:922–30. doi: 10.7326/0003-4819-128-11-199806010-00012. [DOI] [PubMed] [Google Scholar]
- 5.Human rabies prevention—United States, 1999: Recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 1999;48(RR-1):1–21. [PubMed] [Google Scholar]
- 6.Krebs JW, Long-Marin SC, Childs JE. Causes, costs, and estimates of rabies postexposure prophylaxis treatment in the United States. J Public Health Manag Practice. 1998;4(5):56–62. doi: 10.1097/00124784-199809000-00009. [DOI] [PubMed] [Google Scholar]
- 7.Rabies prevention—United States, 1991 Recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 1991;40(RR-03):1–19. [PubMed] [Google Scholar]
- 8.Census Bureau (US) Annual estimates of the population for counties of South Carolina: April 1, 2000 to July 1, 2003. [cited 2004 Nov 10]. Available from: URL: http://www.census.gov/popest/counties/tables/CO-EST2003-01-45.pdf.
- 9.Census Bureau (US) Time series of intercensal state populations estimates: April 1, 1990 to April 1, 2000. [cited 2004 Nov 10]. Available from: URL: http://www.census.gov/popest/archives/2000s/vintage_2001/CO-EST2001-12/CO-EST2001-12-00.html.
- 10.Census Bureau (US) Time series of South Carolina intercensal population estimates by county: April 1, 1990 to April 1, 2000. [cited 2004 Nov 10]. Available from: URL: http://www.census.gov/popest/archives/2000s/vintage_2001/CO-EST2001-12/CO-EST2001-12-45.html.
- 11.Moore DA, Sischo WM, Hunter A, Miles T. Animal bite epidemiology and surveillance for rabies postexposure prophylaxis. J Am Vet Med Assoc. 2000;217:190–4. doi: 10.2460/javma.2000.217.190. [DOI] [PubMed] [Google Scholar]
- 12.Kreindel SM, McGuill M, Meltzer M, Rupprecht C, Demaria A., Jr The cost of rabies postexposure prophylaxis: one state's experience. Public Health Rep. 1998;113:247–51. [PMC free article] [PubMed] [Google Scholar]
- 13.Rabies postexposure prophylaxis—Connecticut, 1990–1994. MMWR Morb Mortal Wkly Rep. 1996;45(11):232–4. [PubMed] [Google Scholar]
- 14.Auslander M, Kaelin C. Rabies postexposure prophylaxis survey—Kentucky, 1994. Emerg Infect Dis. 1997;3:199–202. doi: 10.3201/eid0302.970216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Helmick CG. The epidemiology of human rabies postexposure prophylaxis, 1980–1981. JAMA. 1983;250:1990–6. [PubMed] [Google Scholar]
- 16.Reynolds KL, Parker RL. Characterization of rabies consultations in South Carolina, 1979-1981. J S C Med Assoc. 1983;79:683–6. [PubMed] [Google Scholar]
- 17.Childs JE, Colby L, Krebs JW, Strine T, Feller M, Noah D, et al. Surveillance and spatiotemporal associations of rabies in rodents and lagomorphs in the United States, 1985–1994. J Wildl Dis. 1997;33:20–7. doi: 10.7589/0090-3558-33.1.20. [DOI] [PubMed] [Google Scholar]
- 18.Krebs JW, Smith JS, Rupprecht CE, Childs JE. Rabies surveillance in the United States during 1997. J Am Vet Med Assoc. 1998;213:1713–28. [PubMed] [Google Scholar]
- 19.Krebs JW, Smith JS, Rupprecht CE, Childs JE. Rabies surveillance in the United States during 1996. J Am Vet Med Assoc. 1997;211:1525–39. [PubMed] [Google Scholar]