SYNOPSIS
Objective
The purpose of this study was to assess the effect of low birthweight on chronic renal failure among young Medicaid patients with diabetes and/or hypertension.
Methods
The study included Caucasian and African American young adults, aged 18–50, who enrolled in the Medicaid program from 1993 to 1996 in South Carolina and were diagnosed with diabetes and/or hypertension. The odds of chronic renal failure by low birthweight (<2,500 grams) was estimated using logistic regression.
Results
Of the 7,505 Medicaid patients with diabetes and/or hypertension, 179 (2.4%) were diagnosed with chronic renal failure. These patients were younger (mean age of 33.9 vs. 37.6, p=0.0024) and had a higher proportion of low birthweight (15.1% vs. 11.4%, p=0.07) compared with the 7,326 patients without renal failure. The odds ratio of chronic renal failure for low birthweight was significantly higher compared with normal birthweight (2,500–3,999 grams) (adjusted odds ratio [OR] 1.56, 95% confidence interval [95% CI] 1.0, 2.4). The association between low birthweight and chronic renal failure was stronger among the 888 patients with both diabetes and hypertension (OR 2.6, 95% CI 1.3, 5.7) than the 1,812 diabetes or the 4,805 hypertension patients.
Conclusions
The odds of chronic renal failure by low birthweight was highest in patients with both diabetes and hypertension, suggesting that the mechanism(s) involved in the disease progression to chronic renal failure may have a fetal early life origin.
Rates of end-stage renal disease (ESRD) or chronic renal failure have been increasing over the past several decades, in particular among African Americans and residents of the southeastern United States.1–4 The reasons for this epidemic have not been fully elucidated, though certainly multiple inherited and environmental factors are involved.5–7
Recently, ESRD has been proposed to have a fetal and early life origin. Low birthweight has been associated with treated ESRD in the southeastern U.S.6 and with renal disease in the Australian aboriginal population.7 This suggests that impaired kidney development in utero may have lasting effects on renal function.8 The pathophysiological mechanism of the low birthweight-ESRD association remains unclear. However, low birthweight has been hypothesized to contribute to ESRD through its association with decreased nephron number,9 functional and structural changes that modulate arterial blood pressure,10,11 and glucose intolerance.12,13 Moreover, low birthweight has been associated with an increased rate of cardiovascular disease, hypertension, and type 2 diabetes in adulthood.14–16 Studies on lower socioeconomic status (SES) (low income) as a risk factor for higher incidence of ESRD have been controversial.17–19
The objective of this study was to assess the effect of low birthweight on chronic renal failure among a high-risk population composed of young adults enrolled in the Medicaid program who had either diabetes or hypertension or both. We restricted the patient group to the Medicaid program (low income) to control for the effect of SES. We also excluded patients with diagnoses other than diabetes and/or hypertension to control for the potential confounding effect on chronic renal failure of diabetes and hypertension. This unique cohort of chronic renal failure patients was not on dialysis at the time of their enrollment in the Medicaid program and therefore they were not listed in the United States Renal Data System (USRDS). The findings from our analysis of this high-risk population extends our understanding of the risk factors associated with chronic renal failure and may shed light on prevention strategies.
METHODS
Data source
This study used a merged dataset from Medicaid records and South Carolina birth certificates. The Medicaid database provided information for all beneficiaries on date of birth, gender, race, and diagnosis of diabetes, hypertension, and chronic renal failure. Birthweight was determined from the weight reported on South Carolina birth certificates available for babies born in the state in 1950 or after. This study identified 17,499 Medicaid beneficiaries who enrolled in the Medicaid program with a diagnosis of diabetes or hypertension from 1993 to 1996 and were born after January 1, 1950. These data were matched on name and date of birth to South Carolina birth certificates using a computer process and manual search for the birth certificate.6 The Office of Vital Records successfully matched 9,747 beneficiaries (44.3% of the total; 61.2% of males and 37.9% of females). The unsuccessful matches were attributed in part to subjects who were born outside of South Carolina and to women who changed their surname at marriage. Age was calculated in completed years as of July 1, 1999. As part of subsequent data checking, 2,242 (23%) subjects were excluded because they were under age 18, data were missing, or the birthweight listed on the birth certificate was illegible.
Study population
We included 7,505 African American and Caucasian Medicaid beneficiaries who were aged 18–50 and were diagnosed with either diabetes (ICD-9-CM 250) or hypertension (ICD-9-CM 401) or both on the claim forms.20 The analysis identified the participants with diagnosis of chronic renal failure (ICD-9-CM 585) compared to those without renal failure. The study subjects with chronic renal failure would later undergo dialysis or kidney transplant, but were not in the USRDS (for dialysis patients) at the time of data collection. Birthweight was recorded in pounds and ounces and converted to grams for analysis. Subjects were categorized according to birthweight as low (<2,500 grams), normal (2,500–3,999 grams), or high (≥4,000 grams).
Statistical analyses
Differences in the patients’ demographic information were assessed for chronic renal failure patients and those with no renal failure using chi-square test and student’s t-test. The odds ratio for chronic renal failure by birthweight was estimated using logistic regression with subjects who had no renal failure as a comparison group. Additional multivariate logistic regression analyses were conducted controlling for patients’ age, gender, race, and co-morbid condition of diabetes and/or hypertension.
RESULTS
The study identified 7,505 Caucasian or African American Medicaid beneficiaries born in South Carolina whose Medicaid claim forms listed diabetes and/or hypertension. Of the Medicaid patients aged 18 to 50 in this study cohort, 5,863 (78.1%) were African American (1,491 men and 4,369 women) and 1,642 (21.9%) were Caucasian (837 men and 805 women). There were 1,812 (24.2%) patients with diabetes, 4,805 (64.0%) with hypertension, and 888 (11.8%) with both diabetes and hypertension (Table 1). Chronic renal failure was diagnosed among 179 (2.4%) patients. The mean age of the 179 chronic renal failure patients was four years younger (33.9) than the 7,326 patients without renal failure (37.6) (p=0.0024). A higher proportion of males (49.7% vs. 30.6%, p<0.0001) and low birthweight (15.1% vs. 11.4%, p=0.07) was observed among the chronic renal failure patients compared to those without renal failure. For patients with both diabetes and hypertension, the proportion of chronic renal failure was three-fold higher compared to those without renal failure (31.8% vs. 11.3%, Figure 1.)
Table 1.
Characteristics of Medicaid patients with diabetes and/or hypertension (N=7,505)
Student’s t-test or chi-square test
SE = standard error
Figure 1.

Percent of co-morbid conditions among Medicaid chronic renal failure patients (n=179) and those with no renal failure (n=7,326)
NOTE: For patients with both diabetes and hypertension, the proportion of chronic renal failure was three-fold higher compared to those without renal failure (31.8% vs. 11.3%).
Table 2 shows the odds ratios of chronic renal failure with several covariates using multivariate logistic regression. The odds ratio (OR) of chronic renal failure was significantly higher in the low birthweight group compared with the normal birthweight group (adjusted OR 1.54, 95% confidence interval [CI] 1.01, 2.34). This significant effect was not changed after adjusting for co-morbid conditions (Table 2).
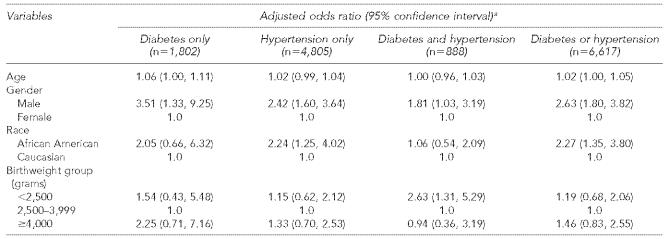
Table 2.
Adjusted odds ratios of chronic renal failure among Medicaid patients with diabetes and/or hypertension (N=7,505)
Logistic regression modeling the probability of chronic renal failure
The odds ratio of chronic renal failure for low birthweight to normal birthweight was more than two-fold for the 888 patients with both diabetes and hypertension (OR 2.6, 95% CI 1.3, 5.3), higher than the effects of the 1,812 patients with diabetes only and the 4,805 patients with hypertension only (Table 3). The effects of age, gender, and race decreased in patients with both diabetes and hypertension compared with diabetes or hypertension alone (Table 3). The effect of low birthweight, however, was greatest among patients with both diabetes and hypertension (Figure 2).
Table 3.
Adjusted odds ratios of chronic renal failure by birthweight among Medicaid patients with different co-morbid conditions
Logistic regression modeling the probability of chronic renal failure
Figure 2.

Adjusted odds ratios of chronic renal failure for low birthweight (<2,500g) compared to normal weight (2,500–3,999g): diabetes only (n=1,802), hypertension only (n=4,805), and diabetes and hypertension (n=888)
NOTE: The odds ratio of chronic renal failure for low to normal birthweight increased for patients with both diabetes and hypertension relative to patients with diabetes or hypertension alone, opposite to the effects of gender and race.
DISCUSSION
This study, focused on a high-risk population, extends previous positive findings on low birthweight/ESRD association observed in South Carolina. An earlier case-control study by Lackland et al. in the southeastern United States demonstrated the enhanced risk of low birthweight on ESRD among dialysis patients.6 In the current study, chronic renal failure patients were not included in the USRDS at the time of the data collection. A significant effect of low birthweight on chronic renal failure was observed among the Medicaid beneficiaries (with low income), after adjusting for patient age, gender, and race. The magnitude of the effect was not changed when the co-morbid conditions of diabetes and/or hypertension were considered in the logistic regression model (Table 2). The association was more striking among patients with both diabetes and hypertension. The odds ratio of chronic renal failure for low to normal birthweight increased from 1.54 for all patients (Table 2) to 2.63 for patients with both diabetes and hypertension (Figure 2). This is opposite to the effects of age, gender, and race, which decreased in the patients with both diabetes and hypertension compared with diabetes or hypertension alone (Table 3). The positive findings on the low birthweight/renal failure association suggest that low birthweight could be a risk factor for chronic renal failure among young adults with diabetes and/or hypertension in the Medicaid population.
Diabetes and hypertension are independent risk factors for ESRD.4 Diabetes is associated with an accelerated progression of vascular disease and a substantially increased risk of renal failure.21,22 Hypertensive nephrosclerosis is hypothetically the most common determinant of ESRD in African American patients.23 Epidemiological studies, however, suggest that blood pressure control in African Americans with essential hypertension does not consistently halt progression of renal dysfunction.24,25 For example, Rostand et al. reported that 15% of patients with essential hypertension had progressive deterioration in renal function despite good blood pressure control and that this occurred significantly more often in African American patients.26 The kidneys have long been thought to play a central role in the etiology of essential hypertension.27,28 Brenner and colleagues have suggested that a congenital reduction in nephron number is an unappreciated risk factor for the development of adult systemic hypertension.29 Our study suggested that low birthweight, at least in part, contributed to chronic renal failure among these high-risk patients. For patients with both diabetes and hypertension, the association between low birthweight and chronic renal failure was even stronger. This may imply that structural changes in the kidney induced by low birthweight make it more susceptible to the combined effects of diabetes and hypertension than to diabetes or hypertension in isolation. This may be a clinically significant finding because almost 50% of new patients in the U.S. have three or more co-morbid conditions when they initiate ESRD therapy.4
This study has several limitations. The age restriction to young adults was due to the fact that birthweight data have only been included on South Carolina birth certificates since 1950, which precluded older patients from our study group. However, understanding the causes of chronic renal failure that develop in a young population was a major objective. In line with this objective, we excluded children aged 18 and younger, who constitute about 51% of all Medicaid beneficiaries,30 from the study population. While being age 65 and older is a significant risk factor for chronic renal failure,4 we observed 2.4% of young adults (aged 18–50) with chronic renal failure at a mean age of 34, four years younger than the mean age of the non-renal failure patients among Medicaid diabetic and/or hypertensive patients. Another limitation in this study is the potential diagnosis bias of chronic renal failure. We speculate this limitation may introduce a random error that results in an underestimation of the true odds ratio based on the fact that the patient was not aware of the diagnosis before participating in the Medicaid program, and the patient’s birthweight was matched based on identification but not on the status of chronic renal failure. The lower match rates for birthweight for females would only bias the results if association between birthweight and chronic renal failure were different in men and women who were born outside South Carolina or between women who were married or single.
These data did not distinguish between insulin-dependent childhood onset diabetes (type 1) and non-insulin-dependent diabetes mellitus (NIDDM, type 2). For the patients with diabetes only, the effect of both low and high birthweight on chronic renal failure was observed, with a higher effect among the high birthweight group (Table 3). This may reflect a mixture of type 1 and type 2 diabetes. Studies have identified a U-shaped association between birthweight and diabetic ESRD in South Carolina,6 and birthweights and elevated urinary albumin excretion in Pima Indians with NIDDM.31 These previous studies may help to explain the effect of diabetes with high birthweight on chronic renal failure observed in our study. The effect of high birthweight among diabetes patients disappeared, however, when hypertension patients were included in the model. In contrast, the association between low birthweight and chronic renal failure after adjusting for diabetes remained strong, suggesting the effect of low birthweight on chronic renal failure was independent.
This study explored the low birthweight/chronic renal failure association among diabetes and/or hypertension patients enrolled in the Medicaid program. The results of this observational study suggest that the odds ratio of chronic renal failure was significantly associated with low birthweight among patients with both diabetes and hypertension. The combined detrimental effects of low birthweight and co-morbid conditions of diabetes and hypertension may act in concert with each other to increase the risk of chronic renal failure.
Acknowledgments
The authors thank Drs. Brent Egan, Tim Garvey, and Florence Hutchison for review of the analyses. The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy or interpretation of the Southeastern Kidney Council Inc. or the Center for Medicare & Medicaid Services.
The study was funded, in part, by grants from the Oriana Bethea Research Endowment (DTL), U.S. Department of Energy DE-FG01-92EW50625 (DTL), and the Black Pooling Project 5R01HL72377-3.
REFERENCES
- 1.Krop JS, Coresh J, Chambless LE, Shahar E, Watson RL, Szklo M, et al. A community-based study of explanatory factors for the excess risk for early renal function decline in blacks vs. whites with diabetes: the Atherosclerosis Risk in Communities study. Arch Intern Med. 1999;159:1777–1783. doi: 10.1001/archinte.159.15.1777. [DOI] [PubMed] [Google Scholar]
- 2.Martins D, Tareen N, Norris KC. The epidemiology of end-stage renal disease among African Americans. Am J Med Sci. 2002;323:65–71. doi: 10.1097/00000441-200202000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Rostand SG, Kirk KA, Rutsky EA, Pate BA. Racial differences in the incidence of treatment for end-stage renal disease. New Engl J Med. 1982;306:1276–1279. doi: 10.1056/NEJM198205273062106. [DOI] [PubMed] [Google Scholar]
- 4.Renal Data System (US), National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases. USRDS 2002 annual data report. Bethesda (MD): USRDS; 2002. [cited 2006 Jan 20]. Also available from: URL: http://www.usrds.org/adr_2002.htm. [Google Scholar]
- 5.Freedman BI. Familial aggregation of end-stage renal failure: aetiological implications. Nephrol Dial Transplant. 1999;14:295–297. doi: 10.1093/ndt/14.2.295. [DOI] [PubMed] [Google Scholar]
- 6.Lackland DT, Bendall HE, Osmond C, Egan BM, Barker DJ. Low birthweights contribute to high rates of early-onset chronic renal failure in the Southeastern United States. Arch Intern Med. 2000;160:1472–1476. doi: 10.1001/archinte.160.10.1472. [DOI] [PubMed] [Google Scholar]
- 7.Hoy WE, Rees M, Kile E, Mathews JD, Wang Z. A new dimension to the Barker hypothesis: low birthweight and susceptibility to renal disease. Kidney Int. 1999;56:1072–1077. doi: 10.1046/j.1523-1755.1999.00633.x. [DOI] [PubMed] [Google Scholar]
- 8.Brenner BM, Chertow GM. Congenital oligonephropathy: an inborn cause of adult hypertension and progressive renal injury? Curr Opin Nephrol Hypertens. 1993;2:691–695. [PubMed] [Google Scholar]
- 9.Mackenzie HS, Brenner BM. Fewer nephrons at birth: a missing link in the etiology of essential hypertension? Am J Kidney Dis. 1995;26:91–98. doi: 10.1016/0272-6386(95)90161-2. [DOI] [PubMed] [Google Scholar]
- 10.Huxley RR, Shiell AW, Law CM. The role of size at birth and postnatal catch-up growth in determining systolic blood pressure: a systematic review of the literature. J Hypertens. 2000;18:815–831. doi: 10.1097/00004872-200018070-00002. [DOI] [PubMed] [Google Scholar]
- 11.Law CM, Shiell AW. Is blood pressure inversely related to birthweight? The strength of evidence from a systematic review of the literature. J Hypertens. 1996;14:935–941. [PubMed] [Google Scholar]
- 12.Hales CN, Barker DJ, Clark PM, Cox LJ, Fall C, Osmond C, et al. Fetal and infant growth and impaired glucose tolerance at age 64. BMJ. 1991;303:1019–1022. doi: 10.1136/bmj.303.6809.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Phillips DI, Barker DJ, Hales CN, Hirst S, Osmond C. Thinness at birth and insulin resistance in adult life. Diabetologia. 1994;37:150–154. doi: 10.1007/s001250050086. [DOI] [PubMed] [Google Scholar]
- 14.Curhan GC, Willett WC, Rimm EB, Spiegelman D, Ascherio AL, Stampfer MJ. Birthweight and adult hypertension, diabetes mellitus, and obesity in US men. Circulation. 1996;94:3246–3250. doi: 10.1161/01.cir.94.12.3246. [DOI] [PubMed] [Google Scholar]
- 15.Leon DA, Koupilova I, Lithell HO, Berglund L, Mohsen R, Vagero D, et al. Failure to realise growth potential in utero and adult obesity in relation to blood pressure in 50 year old Swedish men. BMJ. 1996;312:401–406. doi: 10.1136/bmj.312.7028.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Osmond C, Barker DJ, Winter PD, Fall CH, Simmonds SJ. Early growth and death from cardiovascular disease in women. BMJ. 1993;307:1519–1524. doi: 10.1136/bmj.307.6918.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brancati SL, Whelton PK, Whittle JC, Klag MJ. Epidemiologic analysis of existing data to investigate hypertensive renal disease: an example from the Maryland End-Stage Renal Disease Registry. Am J Kidney Dis. 1993;21(4 Suppl 1):15–24. doi: 10.1016/s0272-6386(12)80857-2. [DOI] [PubMed] [Google Scholar]
- 18.Perneger TV, Whelton PK, Klag MJ. Race and end-stage renal disease. Socioeconomic status and access to health care as mediating factors. Arch Intern Med. 1995;155:1201–1208. [PubMed] [Google Scholar]
- 19.Young EW, Mauger EA, Jiang KH, Port FK, Wolfe RA. Socioeconomic status and end-stage renal disease in the United States. Kidney Int. 1994;45:907–911. doi: 10.1038/ki.1994.120. [DOI] [PubMed] [Google Scholar]
- 20.National Center for Health Statistics and Centers for Medicare & Medicaid Services (US) International classification of diseases, 9th revision, clinical modification (ICD-9-CM) Hyattsville (MD): NCHS, CMS; 1998. [Google Scholar]
- 21.The Diabetes Control and Complications (DCCT) Research Group. Effect of intensive therapy on the development and progression of diabetic nephropathy in the Diabetes Control and Complications Trial. Kidney International. 1995;47:1703–1720. doi: 10.1038/ki.1995.236. [DOI] [PubMed] [Google Scholar]
- 22.Molyneaux LM, Constantino MI, McGill M, Zilkens R, Yue DK. Better glycaemic control and risk reduction of diabetic complications in Type 2 diabetes: comparison with the DCCT. Diabetes Res Clin Pract. 1998;42:77–83. doi: 10.1016/s0168-8227(98)00095-3. [DOI] [PubMed] [Google Scholar]
- 23.Wright JT, Kusek JW, Toto RD, Lee JY, Agodoa LY, Kirk KA, et al. Design and baseline characteristics of participants in the African American Study of Kidney Disease and Hypertension (AASK) Pilot Study. Control Clin Trials. 1996;17(4 Suppl):3S–16S. doi: 10.1016/s0197-2456(96)00081-5. [DOI] [PubMed] [Google Scholar]
- 24.Bergman SM, Curtis JJ. Possible mediators in hypertension: renal factors. Semin Nephrol. 1996;16:134–139. [PubMed] [Google Scholar]
- 25.Wright JT, Agodoa L, Contreras G, Greene T, Douglas JG, Lash J, et al. African American Study of Kidney Disease and Hypertension Study Group. Successful blood pressure control in the African American Study of Kidney Disease and Hypertension. Arch Intern Med. 2002;162:1636–1643. doi: 10.1001/archinte.162.14.1636. [DOI] [PubMed] [Google Scholar]
- 26.Rostand SG, Brown G, Kirk KA, Rutsky EA, Dustan HP. Renal insufficiency in treated essential hypertension. New Engl J Med. 1989;320:684–688. doi: 10.1056/NEJM198903163201102. [DOI] [PubMed] [Google Scholar]
- 27.Dustan HP, Curtis JJ, Luke RG, Rostand SG. Systemic hypertension and the kidney in black patients. Am J Cardiol. 1987;60:73I–77I. doi: 10.1016/0002-9149(87)90464-4. [DOI] [PubMed] [Google Scholar]
- 28.Freedman BI, Iskandar SS, Appel RG. The link between hypertension and nephrosclerosis. Am J Kidney Dis. 1995;25:207–221. doi: 10.1016/0272-6386(95)90001-2. [DOI] [PubMed] [Google Scholar]
- 29.Brenner BM, Garcia DL, Anderson S. Glomeruli and blood pressure. Less of one, more the other? Am J Hypertens. 1988;1(4 Pt 1):335–347. doi: 10.1093/ajh/1.4.335. [DOI] [PubMed] [Google Scholar]
- 30.Hoffman ED, Jr, Klees BS, Curtis CA. Overview of the Medicare and Medicaid programs. Health Care Financ Rev Stat Supp. 2000;1:348. [PubMed] [Google Scholar]
- 31.Nelson RG, Morgenstern H, Bennett PH. Birthweight and renal disease in Pima Indians with type 2 diabetes mellitus. Am J Epidemiol. 1998;148:650–656. doi: 10.1093/aje/148.7.650. [DOI] [PubMed] [Google Scholar]