Abstract
The frequency of mutation to rifampin resistance of 200 clinical Streptococcus pneumoniae isolates was examined. Two peaks were observed in the distribution, with mode frequencies of 2.5 × 10−7 (20% of isolates) and 2.5 × 10−8. The hexA and hexB gene entire sequences were analyzed in 13 isolates. Sequences from both hypermutable and “normomutable” strains were conserved relative to that of the R6 S. pneumoniae control strain. The phenotypic Hex system proficiency, in terms of transforming efficiency, was also maintained irrespective of the variations in mutation frequency values.
Streptococcus pneumoniae resistance to antibiotics commonly used in therapy, such as beta-lactam agents, fluoroquinolones, cotrimoxazole, macrolides (to a limited extent), and, as is expected, oxazolidinones, is ultimately of mutational origin (16). S. pneumoniae is frequently challenged by adverse ecological changes, including frequent exposure to antibiotics and the host immune system (3, 11), and it is known that bacterial stress promotes the emergence and selection of hypermutable variants (7, 12). Hypermutation in S. pneumoniae has been described to be the result of inactivation of the hexA and hexB genes in the Hex repair system, which is responsible for mutation avoidance through the correction of errors resulting from nucleotide misincorporation during replication (4, 8). Even though the increase in mutation frequency, on average, rarely exceeds 10-fold that of the wild-type proficient control strain (4, 8, 10), the hex-deficient variants have a clear advantage to acquire adaptive mutations leading to antibiotic resistance (10).
In this study, the alleged presence of hypermutable variants of S. pneumoniae was examined by determining the proportion of rifampin-resistant colonies with regard to the total viable cell count (overall rifampin mutation frequency) in a 5-year (1995 to 1999) Spanish collection of 200 clinical isolates from different geographical origins. With this aim, three to five colonies from an overnight culture in 5% sheep blood agar plates (Oxoid Ltd., Basingstoke, United Kingdom) were resuspended in 10 ml of brain heart infusion broth (Oxoid) and incubated for 6 to 8 h at 35°C. A sample of 100 μl was diluted for total viable count determination on antibiotic-free medium. The remaining culture was centrifuged for 10 min at 2,500 × g. The pellet was resuspended in 500 μl of brain heart infusion broth and seeded on 5% sheep blood agar plates containing 2 μg of rifampin/ml (Sigma Chemical Co., St Louis, Mo.). Mutation frequency values are reported as the number of rifampin-resistant colonies (detected after 48 h of incubation in a 5% CO2 atmosphere) as a proportion of total viable count. The results corresponded to the mean value obtained in duplicate experiments, each with duplicate colony counts.
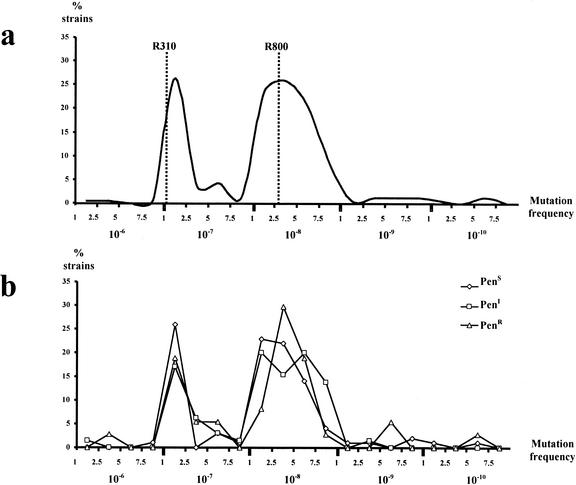
The results indicated that there was a considerable range of variation in frequencies of mutation to rifampin resistance among the studied population, although these frequencies rarely exceeded 10-fold that of the Hex-proficient control strain. Previously reported results (10), confirmed in this study, show that mutation frequencies to rifampin resistance for the R800 (DNA repair-proficient R6 derivative [ami+ aliA+ aliB+ hex+]) and R310 (DNA repair-deficient R800 hexA::syn spc derivative) control strains (1) are 2.6 × 10−8 and 1.06 × 10−7, respectively (fourfold difference). A bimodal distribution emerged when mutation frequencies were plotted versus the percentage of strains presenting a given mutation frequency (Fig. 1a). The two main peaks corresponded to mutation frequencies of 2.5 × 10−7 and 2.5 × 10−8 (10-fold difference). Of the tested strains, 21.5% corresponded to the first peak and 56.5% to the second one. The mutation frequency of 8.5% of the strains was higher than 2.5 × 10−7. Values lower than 10−8 were infrequent. Among the most divergent strains, although representing a low number, the overall mutation frequencies differed by nearly 1,000-fold, ranging from 1 × 10−6 to 6 × 10−10. Additionally, mutation frequencies for rifampin resistance did not seem to correlate with the penicillin susceptibility status, as identical bimodal distribution was observed when strains were split into penicillin-susceptible, -intermediate, and -resistant groups (Fig. 1b).
FIG. 1.
Percentage of strains exhibiting a given mutation frequency to rifampin resistance among all 200 S. pneumoniae clinical isolates studied (a) and among penicillin-susceptible (PenS), -intermediate (PenI), and -resistant (PenR) subgroups of the isolates studied. The dotted lines in panel a show mutation frequencies of R800 (hexA-proficient) and R310 (hexA-deficient) S. pneumoniae control strains.
A sample of 13 isolates characterized by macrorestriction patterns by pulsed-field gel electrophoresis analysis (15) (Table 1) was selected for further studies. Eight strains belonged to the group with a mean mutation frequency of ≥2 × 10−7 (putative hypermutable), and five strains were chosen among those exhibiting a mean mutation frequency of ≤6 × 10−8 (Table 1). hexA and hexB genes were PCR amplified by using sets of primers (Table 2) that comprised internal fragments covering the whole genes. Purified PCR products (QIAquick PCR purification kit; Qiagen GmbH, Hilden, Germany) were then submitted for entire sequencing of both strands.
TABLE 1.
Representative data of the 13 selected S. pneumoniae strains
| Sample | Range of mutation frequency | Penicillin MIC (μg/ml) | PFGE typea | Sero- type | Sample type |
|---|---|---|---|---|---|
| 1 | 1.5-1.6 (× 10−8) | 0.12 | A | 18 F | Blood |
| 2 | 3.4-3.5 (× 10−8) | 0.25 | B | 19 | Blood |
| 3 | 6.0-6.3 (× 10−10) | 2 | C | 14 | Nasal |
| 4 | 5.9-6.1 (× 10−8) | ≤0.01 | D | 24 | Ocular |
| 5 | 4.4-4.8 (× 10−8) | 0.5 | E | 23 F | Sputum |
| 6 | 2.1-2.5 (× 10−7) | 1 | E2 | 23 F | Nasal |
| 7 | 4.2-4.6 (× 10−7) | 0.12 | E3 | 23 F | BALF |
| 8 | 3.0-3.3 (× 10−7) | 1 | B1 | 19 | Blood |
| 9 | 4.7-5.0 (× 10−7) | 0.5 | C1 | 14 | Blood |
| 10 | 4.3-4.7 (× 10−7) | ≤0.01 | G | 18 C | BALF |
| 11 | 3.5-3.8 (× 10−7) | 0.06 | G1 | 23 A | Nasal |
| 12 | 5.0-5.3 (× 10−7) | ≤0.01 | H | 3 | Blood |
| 13 | 1.0-1.2 (× 10−6) | 1 | E1 | 23 F | CSFb |
PFGE, pulsed-field gel electrophoresis. Data from reference 15.
CSF, cerebrospinal fluid.
TABLE 2.
Primers used for PCR amplification of hexA and hexB genes
| Primer | Sequence (5′ → 3′) |
|---|---|
| hexA primers | |
| A1 (bp 880-902) | CCGGAATTCATGGAAGATCGTTTGCTAGAT |
| A2 (bp 1984-1963) | CAAGCGCTCAATGTCATAAACA |
| A3 (bp 1437-1456) | TGGATTTCACGCTGGTTTGT |
| A4 (bp 2637-2616) | CGAATCAAATGCTGGGTTTCA |
| A5 (bp 2408-2428) | GCGACGCTGAAAAACTCAGAA |
| A6 (bp 3594-3573) | CCGGAATTCTGTTTGTGTGATGTTATTCTA |
| hexB primers | |
| B1 (bp 403-420) | CCGGAATTCGGCTCCTATGTGGTGGAG |
| B2 (bp 1574-1554) | TCTTTGAGTGGGAGAATAGTT |
| B3 (bp 1407-1427) | GCAAGAGGTGCGGATTTCCAA |
| B4 (bp 2520-2499) | CCGGAATTCTCAAGGTTTTTGAACACCTGAT |
The analysis of the hexA sequence of each of the 13 selected strains revealed changes in 21 sites among the 2,534 nucleotides of the gene relative to those of the S. pneumoniae R6 control strain (Table 3). Nucleotide changes implied 12 global amino acid replacements in the 13 strains (range, 1 to 4 changes per strain); the rest of the changes were synonymous transitions or transversions. The influence, if any, of replaced amino acids on HexA protein structure or function is unknown. The polymorphic sites resulting from the nucleotide changes observed gave rise to nine polymorphic patterns. However, taken as a whole, the hexA sequence is highly conserved among strains with no deletions or insertions.
TABLE 3.
Sequence analysis of hexA and hexB genes of the 13 selected S. pneumoniae strains
| DNA sequence | Sequence positiona | Amino acid change | Presence of base changes in clinical strain no.b:
|
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R6 | Clinical strainc | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | ||
| hexA | ||||||||||||||||
| CTC | CTT | 78 | —d | * | ||||||||||||
| GAT | AAT | 280 | Asp→Asn | * | * | |||||||||||
| CCA | CCC | 336 | — | * | * | |||||||||||
| TCC | GCC | 388 | Ser→Ala | * | * | * | ||||||||||
| GAA | CAA | 514 | Glu→Gln | * | ||||||||||||
| GCA | GGA | 656 | Ala→Gly | * | ||||||||||||
| CAG | CGG | 674 | Gln→Arg | * | * | |||||||||||
| CGC | CGA | 729 | — | * | * | |||||||||||
| GGC | GGT | 1035 | — | * | * | * | * | * | * | * | ||||||
| ACT | GCT | 1126 | Thr→Ala | * | * | * | ||||||||||
| CTA | TTA | 1129 | — | * | ||||||||||||
| GCA | GAA | 1145 | Ala→Glu | * | ||||||||||||
| CTG | CTA | 1407 | — | * | ||||||||||||
| GGC | GGT | 1569 | — | * | ||||||||||||
| CGA | CAA | 1667 | Arg→Gln | * | ||||||||||||
| GTC | GTT | 2232 | — | * | ||||||||||||
| ATC | ATA | 2283 | — | * | ||||||||||||
| TTG | TTT | 2307 | Leu→Phe | * | ||||||||||||
| GCA | ACA | 2323 | Ala→Thr | * | * | |||||||||||
| CAA | AAA | 2389 | Gln→Lys | * | ||||||||||||
| CAG | CAT | 2412 | Gln→His | * | ||||||||||||
| hexB | ||||||||||||||||
| GCA | GCG | 48 | — | * | * | * | ||||||||||
| GGA | GGG | 54 | — | * | * | * | * | * | * | |||||||
| TTG | CTG | 613 | — | * | * | |||||||||||
| TCC | TCT | 714 | — | * | * | * | * | * | * | * | * | |||||
| TTG | TTA | 717 | — | * | * | * | * | * | * | * | * | |||||
| ATC | ATT | 777 | — | * | * | * | ||||||||||
| TTT | TTC | 815 | — | * | * | * | * | * | * | * | * | * | ||||
| GAG | GAA | 1053 | — | * | * | |||||||||||
| TTC | TTT | 1338 | — | * | * | |||||||||||
| AGC | AGT | 1485 | — | * | * | * | * | * | ||||||||
Position numbering is from the first nucleotide of the structural gene.
*, base change is present.
Boldface indicates a base change with respect to the R6 sequence.
—, no change.
In the case of hexB (Table 3), proportionally fewer changes were found in its sequence. Ten polymorphic sites among 1,949 nucleotides generated eight polymorphic patterns. For this gene, strikingly, not a single amino acid change was found, and all nucleotide changes represented synonymous transitions. Once again, neither deletions nor insertions were found. Significantly, certain polymorphic patterns found in hexB are shared by many of the studied strains. An explanation could be supported by the possibility that if subsequent recombination events have occurred, a putative long-term shuffling process could be responsible for such remarkable conserved sequences. The presence of different hexA and hexB alleles among clinical pneumococcal strains by sequencing internal fragments of the genes has been previously reported (9).
Differences in transformation frequencies permit the characterization of Hex proficiency status in recipient strains. Evaluation of the Hex phenotype in 8 of the 13 selected strains (four strains each with mean mutation frequencies on the order of 10−8 and 10−7) was performed. Hex phenotype was established through the ratio of novobiocin-resistant (Novr) to streptomycin-resistant (Strr) transformants obtained in independent cultures, roughly following previously reported procedures (2). Chromosomal DNA used as the donor was obtained from the S. pneumoniae RF46SKNE strain (5) carrying novobiocin (low-efficiency) and streptomycin (high-efficiency) resistance markers. The procedure for transformation experiments was essentially as described previously (13). Selection of transformants was carried out on plates containing either 2.5 μg of novobiocin/ml or 100 μg of streptomycin/ml. All experiments were done in duplicate, and S. pneumoniae control strains R800 and R310 were also tested.
Efficiency of transformation, expressed as the ratio of Novr to Strr transformants, was similar for the eight clinical strains studied, with a range of values from 0.015 to 0.022, as expected for Hex-proficient cells (2). This means that hex genes' functionality did not seem to be affected, irrespective of the different mutation frequencies to rifampin resistance exhibited by these isolates. Novr/Strr ratios for control strains were 0.012 for R800 (Hex-proficient) and 1.3 for R310 (hexA-deficient).
Although in the bimodal distribution of mutation frequency to rifampin resistance, the peak mutation frequency of 2.5 × 10−7 was similar to that found for the R310 Hex-deficient strain (1.06 × 10−7) (10), the role of a presumptive deficiency in hexA and/or hexB mismatch repair genes was not apparent in the hypermutator isolates. Moreover, these isolates exhibited neither substantial alterations in the integrity of the genetic background of the hex genes nor changes in Hex-modulated transforming efficiencies.
However, certain polymorphic clusters or even unique polymorphic sites, particularly along the hexB sequences, were shared by most of the strains analyzed irrespective of their mutation frequency grouping. Nevertheless, due to the few and clustered variable sites found and the limited sample size of the studied strains, evidence of recombination processes leading to such polymorphic variants cannot be inferred. The presence of other runs of similarity in other genes or even the presence of more variability in other parts of hexB could strengthen the hypothesis of either intergenic or intragenic recombination. In the case of hexA, a similar approach can be made, although polymorphic changes among the 13 sequences were rather more dispersed, and many of them resulted in amino acid replacements.
Hex-independent repair genes mutX (6) and mutY (14) were not characterized in the present study, and therefore their involvement in the hypermutable phenotype cannot be ruled out. In any case, a substantial proportion of the clinical isolates examined in this work displayed a mutation frequency similar to that found for the Hex-defective laboratory strain. It has been previously reported (10) that this increased mutation frequency may be sufficient to assure that most of the mutations leading to antibiotic resistance will occur among hypermutable S. pneumoniae populations that are expected to be coselected by hitchhiking with antibiotic resistance. We cannot discard the possibility that once the mutational adaptation occurs, the wild-type hexA or hexB allelic sequences could be reacquired by previously defective S. pneumoniae strains, thus minimizing the biological cost associated with a hypermutable state as soon as the antibiotic-selective bottleneck has been successfully crossed.
Acknowledgments
The excellent technical assistance of Felisa Almaraz is greatly appreciated. Capsular types were determined at the Spanish Pneumococcal Reference Laboratory, Instituto de Salud Carlos III, Majadahonda, Spain by Asunción Fenoll. S. pneumoniae strains R800 and R310 were kindly provided by J. P. Claverys (Toulouse, France). S. pneumoniae strain RF46SKNE was kindly provided by Paloma López García (Centro de Investigaciones Biológicas, CSIC, Madrid, Spain).
This work was supported by the grants BIO4-CT98-0424 and QLK 2-CT-2001-873 from the European Commission. M. R. Baquero was the recipient of a postdoctoral fellowship (02/0067/1998) from the Consejería de Educación de la Comunidad de Madrid. Juan Carlos Galán was the recipient of a fellowship (BEFI-98/9060) from the Fondo de Investigaciones Sanitarias. R. del Campo is the recipient of a postdoctoral fellowship (09/447639.6/00) from the Comunidad de Madrid.
REFERENCES
- 1.Alloing, G., B. Martin, C. Granadel, and J. P. Claverys. 1998. Development of competence in Streptococcus pneumoniae: pheromone autoinduction and control of quorum sensing by the oligopeptide permease. Mol. Microbiol. 29:75-83. [DOI] [PubMed] [Google Scholar]
- 2.Balganesh, T. S., and S. A. Lacks. 1985. Heteroduplex DNA mismatch repair system of Streptococcus pneumoniae: cloning and expression of the hexA gene. J. Bacteriol. 162:979-984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Claverys, J. P., M. Prudhomme, I. Mortier-Barrière, and B. Martin. 2000. Adaptation to the environment: Streptococcus pneumoniae, a paradigm for recombination-mediated genetic plasticity? Mol. Microbiol. 35:251-259. [DOI] [PubMed] [Google Scholar]
- 4.Humbert, O., M. Prudhomme, R. Hakenbeck, C. G. Dowson, and J. P. Claverys. 1995. Homeologous recombination and mismatch repair during transformation in Streptococcus pneumoniae: saturation of the Hex mismatch repair system. Proc. Natl. Acad. Sci. USA 92:9052-9056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.López, P., M. Espinosa, and S. A. Lacks. 1984. Physical structure and genetic expression of the sulfonamide-resistance plasmid pLS80 and its derivatives in Streptococcus pneumoniae and Bacillus subtilis. Mol. Gen. Genet. 195:402-410. [DOI] [PubMed] [Google Scholar]
- 6.Méjean, V., C. Salles, L. C. Bullions, M. J. Bessman, and J. P. Claverys. 1994. Characterization of the mutX gene of Streptococcus pneumoniae as a homologue of Escherichia coli mutT, and tentative definition of a catalytic domain of the dGTP pyrophosphohydrolases. Mol. Microbiol. 11:323-330. [DOI] [PubMed] [Google Scholar]
- 7.Miller, K., A. J. O'Neill, and I. Chopra. 2002. Response of Escherichia coli hypermutators to selection pressure with antimicrobial agents from different classes. J. Antimicrob. Chemother. 49:925-934. [DOI] [PubMed] [Google Scholar]
- 8.Mortier-Barrière, I., O. Humbert, B. Martin, M. Prudhomme, and J. P. Claverys. 1997. Control of recombination rate during transformation of Streptococcus pneumoniae: an overview. Microb. Drug Resist. 3:233-242. [DOI] [PubMed] [Google Scholar]
- 9.Müller-Graf, C. D. M., A. M. Whatmore, S. J. King, K. Trzcinski, A. P. Pickerill, N. Doherty, J. Paul, D. Griffiths, D. Crook, and C. G. Dowson. 1999. Population biology of Streptococcus pneumoniae isolated from oropharyngeal carriage and invasive disease. Microbiology 145:3283-3293. [DOI] [PubMed] [Google Scholar]
- 10.Negri, M. C., M. I. Morosini, M. R. Baquero, R. del Campo, J. Blázquez, and F. Baquero. 2002. Very low cefotaxime concentrations select for hypermutable Streptococcus pneumoniae populations. Antimicrob. Agents Chemother. 46:528-530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nesin, M., M. Ramírez, and A. Tomasz. 1998. Capsular transformation of a multidrug-resistant Streptococcus pneumoniae in vivo. J. Infect. Dis. 177:707-713. [DOI] [PubMed] [Google Scholar]
- 12.Oliver, A., R. Cantón, P. Campo, F. Baquero, and J. Blázquez. 2000. High frequency of hypermutable Pseudomonas aeruginosa in cystic fibrosis lung infection. Science 288:1251-1253. [DOI] [PubMed] [Google Scholar]
- 13.Pozzi, G., L. Masala, F. Iannelli, R. Manganelli, L. S. Havarstein, L. Piccoli, D. Simon, and D. A. Morrison. 1996. Competence for genetic transformation in encapsulated strains of Streptococcus pneumoniae: two allelic variants of the peptide pheromone. J. Bacteriol. 178:6087-6090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Samrakandi, M. M., and F. Pasta. 2000. Hyperrecombination in Streptococcus pneumoniae depends on an atypical mutY homologue. J. Bacteriol. 182:3353-3360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tenover, F. C., R. D. Arbeit, R. V. Goering, P. A. Mickelsen, B. E. Murray, D. H. Persing, and B. Swaminathan. 1995. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J. Clin. Microbiol. 33:2233-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Widdowson, C. A., and K. P. Klugman. 1999. Molecular mechanisms of resistance to commonly used non-betalactam drugs in Streptococcus pneumoniae. Semin. Respir. Infect. 14:255-268. [PubMed] [Google Scholar]