Abstract
The characteristics of in vitro micafungin (FK463) antifungal activity against six species of dimorphic fungi were investigated in accordance with the NCCLS M27-A microdilution methods. MICs of micafungin, amphotericin B, itraconazole, and fluconazole for Histoplasma capsulatum var. capsulatum, Blastomyces dermatitidis, Paracoccidioides brasiliensis, Penicillium marneffei, and Sporothrix schenckii were determined both for the yeast-like form and mycelial form. Coccidioides immitis was tested only in its mycelial form. We have clearly demonstrated that the in vitro activity of micafungin depends considerably on the growth form of dimorphic fungi. Micafungin exhibited potent activity against the mycelial forms of H. capsulatum, B. dermatitidis, and C. immitis (MIC range, 0.0078 to 0.0625 μg/ml), while it was very weakly active against their yeast-like forms (MIC range, 32 to >64 μg/ml). Micafungin was also more active against the mycelial forms than the yeast-like forms of Paracoccidioides brasiliensis, Penicillium marneffei, and S. schenckii. The MICs of amphotericin B were 2 to 5 dilutions lower for the mycelial forms than for the yeast-like forms of B. dermatitidis and Paracoccidioides brasiliensis. There was no apparent difference in the activity of itraconazole between the two forms. The MICs of fluconazole for the yeast-like forms were generally lower than those for the mycelial forms, and considerably so for B. dermatitidis. These results suggest that the growth form employed in antifungal susceptibility testing of dimorphic fungi can considerably influence the interpretation of results. At present, it cannot be judged whether micafungin has clinical usefulness for dimorphic fungus infections, since for most fungi it remains uncertain which growth form correlates better with therapeutic outcome. However, the results of this study warrant further investigations of micafungin as a therapeutic agent for infections caused by dimorphic fungi.
Endemic mycoses caused by certain dimorphic fungi remain a major problem for public health in several countries. In particular, AIDS-associated histoplasmosis and coccidioidomycosis are becoming increasingly frequent with the spread of human immunodeficiency virus infection and usually present as disseminated infections (1). The mortality rate for acute disseminated disease is high, even in nonimmunocompromised patients (8, 28). Amphotericin B (AMB) is still the first-choice drug for these life-threatening endemic mycoses, regardless of the immunological condition of the patient (15). However, its strong toxicity makes the effective management of severe infections difficult. Although itraconazole (ITC) and ketoconazole are effective for mild or moderate disease, a long duration of therapy and sometimes a lifelong secondary prophylaxis are required, especially in AIDS patients with a high relapse incidence (15). Therefore, a more effective drug which also has a better safety profile than the currently available drugs would greatly contribute to improved treatment of dimorphic fungus infections.
Candins are a novel class of antifungal agent that inhibit 1,3-β-d-glucan synthesis in the fungal cell wall (7). The in vitro and in vivo antifungal activity of caspofungin, the first commercial candin, against Histoplasma capsulatum has been previously reported (6, 9, 14); however, the results of these studies are inconsistent with each other, and no studies to date have clearly demonstrated the efficacy of candins against dimorphic fungi. Micafungin (MCFG; formerly FK463) is also an echinocandin-like compound and has been reported to show potent activity both in vitro and in vivo against a variety of pathogenic fungi including the most common pathogens, Candida and Aspergillus spp. (10, 16, 17, 29). It has been also reported that MCFG has no in vitro activity against Cryptococcus neoformans, Fusarium solani, and zygomycetes and only moderate activity against dematiaceous fungi (20, 29). Further evaluation is required to ascertain whether MCFG possesses clinical usefulness as a therapeutic agent for dimorphic fungus infections. In this study, we evaluated the in vitro antifungal activity of MCFG against six species of dimorphic fungi in accordance with the standardized M27-A method approved by the NCCLS, in order to determine the potential clinical usefulness of this drug. At the beginning of the experiment, we serendipitously found that the antifungal activity of MCFG was influenced by the growth forms of dimorphic fungi. Therefore, we compared the levels of activity against the different growth forms and characterized the difference in activity between MCFG and other commercially available antifungal agents.
MATERIALS AND METHODS
Test organism.
Type strains and clinical isolates of six species of dimorphic fungi, H. capsulatum (var. capuslatum), Blastomyces dermatitidis, Paracoccidioides brasiliensis, Penicillium marneffei, Sporothrix schenckii, and Coccidioides immitis, were evaluated in this study. All isolates were maintained by the Research Center for Pathogenic Fungi and Microbial Toxicoses, Chiba University, Chiba, Japan.
MIC assays.
MICs for the yeast-like form (Y form) and mycelial form (M form) were evaluated independently, except for C. immitis, for which MICs against only the M form were evaluated. Antifungal susceptibility assays were performed by the broth microdilution method according to M27-A guidelines recommended by the NCCLS (21). The methods were slightly modified to be suitable for each organism tested and each growth condition. RPMI 1640 medium with l-glutamine, without sodium bicarbonate, and buffered with 165 mM morpholinepropanesulfonic acid (pH 7.0) was used as a test medium. RPMI medium containing 1% glucose was used for yeast-like growth (Y growth) of S. schenckii.
Antifungal agents.
MCFG was synthesized in the Medicinal Chemistry Research Laboratories, Fujisawa Pharmaceutical Co., Ltd., Osaka, Japan. AMB, ITC, and fluconazole (FLC) were purchased from Bristol-Myers Squibb (Tokyo, Japan), Janssen-Kyowa (Tokyo, Japan), and Pfizer (Tokyo, Japan), respectively. Stock solutions were made with sterile distilled water (MCFG and AMB) or 100% dimethyl sulfoxide (ITC). The commercial solution of FLC was used. Stock solutions were diluted in RPMI medium and then further serially diluted twofold, yielding two times the final strength required for the test. The final concentration ranges of the antifungal agents tested were 0.0039 to 64 μg/ml (MCFG), 0.0039 to 8 μg/ml (AMB and ITC), and 0.0313 to 64 μg/ml (FLC).
Inoculum preparation.
Experiments for both forms of H. capsulatum, B. dermatitidis, and Paracoccidioides brasiliensis were performed simultaneously by using the yeast-like cells (Y cells) as the inocula. Y cells were also used as inocula for the Y growth of Penicillium marneffei and S. schenckii. To obtain the Y cells, isolates were cultured on brain heart infusion agar containing 1% glucose (BHID) at 37°C and maintained by continuous weekly passages. Y cells of S. schenckii were obtained by culture on BHID at 35°C in a 5% CO2 atmosphere (30). The cells were harvested 4 to 6 days after the last passage and squashed by a sterile cotton swab with a small volume of sterile saline. The cells were then suspended in saline and thoroughly vortexed. If large aggregates existed, they were allowed to settle for several minutes, and the supernatants were collected. The number of viable Y cells was estimated by counting with a hemacytometer after staining with lactophenol cotton blue. The suspensions were then diluted in RPMI medium to obtain a final inoculum size suitable for each form of each organism; this size was in the range of approximately 104 to 105 cells/ml. Inocula for the mycelial growth (M growth) of Penicillium marneffei and S. schenckii were composed of conidia, which were harvested from cultures on potato dextrose agar slants at 30°C for 9 days. Conidial suspensions of 107cells/ml were prepared by a hemacytometric procedure and then diluted in RPMI medium to obtain a final inoculum size of 104 cells/ml. These inoculum sizes were larger than that described in the M27-A guidelines because dimorphic fungi generally grow slowly. The experiment for C. immitis was performed by using arthroconidia, which were harvested from cultures on BHID at 37°C for 15 days, at a final inoculum size of 103 cells/ml.
Microdilution test.
Inoculated microplates were incubated at 37 and 25°C for Y growth and M growth, respectively, except for the microplate used for C. immitis M growth, which was incubated at 37°C, and that used for S. schenckii Y growth, which was incubated at 35°C with shaking in a 5% CO2 atmosphere. Readings were taken when adequate growth in the growth control wells was observed. With the aid of a reading mirror, growth in the control wells was compared with that in each test well. The MICs of all compounds tested were defined as the lowest concentrations at which there was prominent growth inhibition compared with the growth control.
Quality control.
Quality control strains Candida parapsilosis ATCC 22019 and Candida krusei ATCC 6258, recommended by NCCLS standard M27-A, were evaluated each time as well. MICs of the reference drugs for these strains were read as described in the standard. MICs of MCFG were defined as the lowest concentrations at which no visible growth was observed.
RESULTS
The MIC ranges of AMB, ITC, and FLC for the two quality control strains were within the expected range for each strain. In addition, the MIC of MCFG was generally constant among experiments (MIC ranges, 1 to 2 μg/ml for Candida parapsilosis ATCC 22019 and 0.125 to 0.25 μg/ml for Candida krusei ATCC 6258).
The in vitro antifungal activities of MCFG and the other reference compounds against Y and M forms of five species (only the M form of C. immitis was tested) of dimorphic fungi were independently evaluated (Table 1). Representative photomicrographs of the Y and M forms of B. dermatitidis in the MIC test are shown in Fig. 1. MICs of MCFG for the M form were lower than those for the Y form for all species tested. Surprisingly, a big difference in activity against the two forms of H. capsulatum and B. dermatitidis was observed: MCFG exhibited potent activity against the M forms of H. capsulatum and B. dermatitidis (MIC range, 0.0078 to 0.0625 μg/ml), while it was very weakly active against their Y forms (MIC range, 32 to >64 μg/ml). MCFG inhibited strongly, sometimes visually completely, the M growth of H. capsulatum, B. dermatitidis, and C. immitis (Fig. 2), but it was not fungicidal (Fig. 3). In contrast, MCFG inhibited only slightly the Y growth, even at concentrations up to 64 μg/ml (Fig. 2). Difference in levels of MCFG activity against the Y and M forms of Paracoccidioides brasiliensis, Penicillium marneffei, and S. schenckii were also observed but were not as distinct as those for H. capsulatum and B. dermatitidis. The MIC ranges of MCFG in the M and Y forms were, respectively, 4 to 16 μg/ml and >64 μg/ml for Paracoccidioides brasiliensis, 0.0313 to 2 μg/ml and 4 to 16 μg/ml for Penicillium marneffei, and 0.5 to 1 μg/ml and 16 to >64 μg/ml for S. schenckii. MCFG also showed potent activity against the M form of C. immitis (MIC, 0.0156 μg/ml). The MICs of AMB for the Y and M forms of H. capsulatum, Penicillium marneffei, and S. schenckii were comparable, but those for the M forms of B. dermatitidis and Paracoccidioides brasiliensis (0.0156 and 0.0078 to 0.0625 μg/ml, respectively) were 2 to 5 dilutions lower than those for the Y forms (0.125 and 0.0625 to 0.25 μg/ml, respectively). MICs of ITC against the growth forms of all strains tested were comparable. ITC was strongly active against H. capsulatum, B. dermatitidis, Paracoccidioides brasiliensis, Penicillium marneffei, and C. immitis (MIC range, ≤0.0039 to 0.0313 μg/ml), while S. schenckii was relatively resistant (MIC range, 1 to 2 μg/ml). The MICs of FLC for the Y form were generally lower than those for the M form, and the FLC MIC for the Y form of B. dermatitidis (1 to 4 μg/ml) was quite different from that for the M form (16 to 32 μg/ml).
TABLE 1.
Independently determined MICs for the Y and M forms of dimorphic fungi
| Organism | MIC (μg/ml)a
|
|||
|---|---|---|---|---|
| MCFG | AMB | ITC | FLC | |
| H. capsulatum | ||||
| IFM41612 | >64/0.0625 | 0.25/0.125 | 0.0156/0.0156 | 2/4 |
| IFM41329 | >64/0.0313 | 0.25/0.0625 | 0.0078/0.0313 | 2/16 |
| IFM41330 | >64/0.0313 | 0.125/0.125 | ≤0.0039/0.0313 | 4/16 |
| G217B | >64/NTb | 0.5/NT | 0.0156/NT | 1/NT |
| B. dermatitidis | ||||
| ATCC 26197 | >64/0.0078 | 0.125/0.0156 | ≤0.0039/0.0078 | 2/16 |
| ATCC 26198 | >64/0.0156 | 0.125/0.0156 | 0.0156/0.0078 | 4/32 |
| ATCC 26199 | 32/0.0313 | 0.125/0.0156 | 0.0078/0.0313 | 1/32 |
| IFM40753 | NT/0.0156 | NT/0.0156 | NT/0.0156 | NT/32 |
| IFM40754 | 32/0.0156 | 0.125/0.0156 | ≤0.0039/0.0313 | 4/32 |
| IFM41634 | 64/0.0078 | 0.125/0.0156 | ≤0.0039/0.0078 | 1/32 |
| P. brasiliensis | ||||
| IFM41620 | >64/16 | 0.25/0.0156 | ≤0.0039/≤0.0039 | 0.25/0.5 |
| IFM41621 | >64/16 | 0.25/0.0078 | ≤0.0039/≤0.0039 | 0.5/0.5 |
| IFM41626 | >64/8 | 0.0625/0.0156 | ≤0.0039/≤0.0039 | 0.25/1 |
| IFM41632 | >64/16 | 0.125/0.0313 | ≤0.0039/≤0.0039 | 0.125/0.25 |
| IFM41633 | >64/8 | 0.125/0.0313 | ≤0.0039/≤0.0039 | 0.125/1 |
| IFM46215 | >64/4 | 0.25/0.0156 | ≤0.0039/0.0078 | 0.5/1 |
| IFM46930 | >64/4 | 0.25/0.0625 | ≤0.0039/≤0.0039 | 0.25/1 |
| P. marneffei | ||||
| IFM41707 | 16/NT | 0.25/NT | 0.0156/NT | 2/NT |
| IFM41708 | 16/0.0313 | 0.25/0.25 | 0.0313/0.0625 | 2/4 |
| IFM47279 | 16/2 | 0.5/0.25 | 0.0313/0.0156 | 2/2 |
| IFM47280 | 8/2 | 0.25/0.125 | 0.0156/0.0078 | 1/2 |
| IFM47281 | 4/2 | 0.25/0.25 | 0.0156/0.0156 | 1/4 |
| S. schenckii | ||||
| IFM46011 | >64/NT | 0.5/NT | 1/NT | 16/NT |
| IFM46605 | 64/NT | 1/NT | 2/NT | >64/NT |
| IFM46927 | 16/1 | 1/1 | 2/0.5 | >64/>64 |
| IFM47068 | >64/0.5 | 1/1 | 2/1 | >64/>64 |
| IFM46989 | NT/1 | NT/2 | NT/1 | NT/>64 |
| IFM47069 | NT/1 | NT/2 | NT/1 | NT/>64 |
| IFM46990 | NT/1 | NT/1 | NT/1 | NT/>64 |
| C. immitis | ||||
| IFM4935 | NT/0.0156 | NT/0.25 | NT/0.0625 | NT/4 |
| IFM4945 | NT/0.0156 | NT/0.25 | NT/0.125 | NT/4 |
| IFM45816 | NT/0.0156 | NT/0.0625 | NT/0.0625 | NT/4 |
| IFM45868 | NT/0.0156 | NT/0.25 | NT/0.125 | NT/4 |
MICs were determined as minimum drug concentrations resulting in prominent growth inhibition compared with the growth control.
NT, not tested.
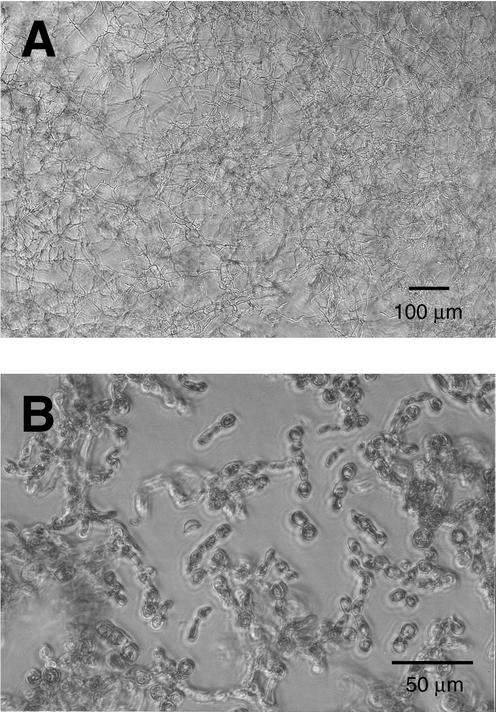
FIG. 1.
M (A) and Y (B) growth of B. dermatitidis in the antifungal susceptibility testing.
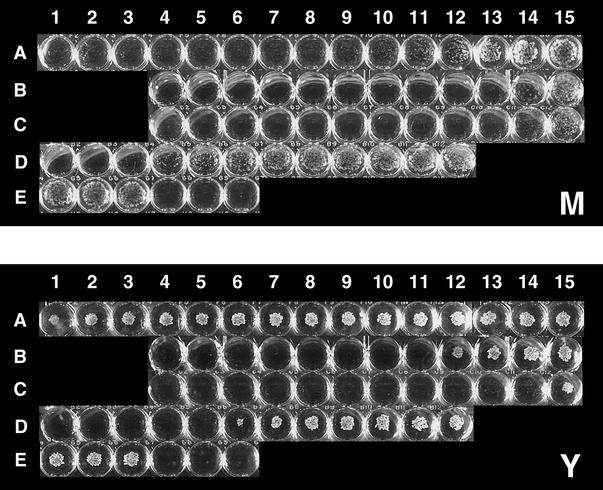
FIG. 2.
Visual observation of MIC plates for the Y and M forms of B. dermatitidis. Test concentrations ranged from 64 (column 1) to 0.0039 μg/ml (column 15). Rows: A, MCFG at 64 to 0.0039 μg/ml; B, AMB at 8 to 0.0039 μg/ml; C, ITC at 8 to 0.0039 μg/ml; D, FLC at 64 to 0.0313 μg/ml; E, growth control (left 3 wells) and medium control (right 3 wells). MCFG showed strong inhibition to B. dermatitidis M growth (A1 to A11), whereas the Y growth was inhibited only slightly.
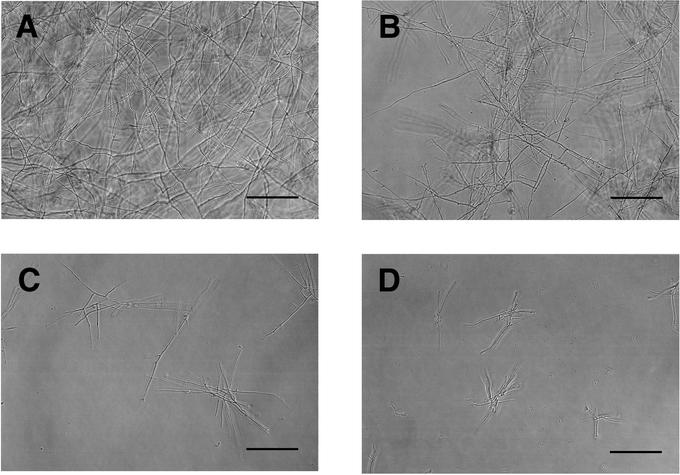
FIG. 3.
Microscopic observations of antifungal activity of MCFG against the M form of H. capsulatum. (A) Growth control; (B) MCFG at the MIC; (C) MCFG at 4 times the MIC; (D) MCFG at 32 times the MIC. MCFG strongly inhibited the growth of mycelia at or above the MIC, but it was not fungicidal. Bars, 100 μm.
DISCUSSION
The only currently available approved standard for antifungal susceptibility testing is NCCLS M27-A, which has been adopted for yeasts. This methodology has been recently modified, resulting in standard M38-P, which is proposed to be suitable for conidium-forming filamentous fungi; however, no examination to develop a standardized method for dimorphic fungi is currently being conducted (22).
The final goal of this study was to predict the clinical value of MCFG for treatment of dimorphic fungus infections by evaluation of in vitro antifungal activity. We first evaluated the in vitro activity of MCFG against the Y form according to M27-A guidelines, because it has been reported that the parasitic form of dimorphic fungi is the Y form (2, 4, 18, 19). When the Y cells were inoculated and incubated at 35°C, as described in the guidelines, some isolates grew in an incomplete Y form (mycelia germinated from Y cells increasing in number). We found that MCFG was ineffective against the complete Y growth but strongly inhibited the M growth. This finding led us to make a comparison of the in vitro activities of antifungal drugs against the Y and M forms of dimorphic fungi. As a result, we clearly demonstrated that the antifungal activity of MCFG considerably depended on the growth form. This difference may be related to the mechanism of action of MCFG. It has been reported that the 1,3-β-d-glucan content in the fungal cell wall, the target of the candin class of compounds, differs with growth form for dimorphic fungi (3, 11, 12, 25). These studies indicate that the cell walls of the Y forms of H. capsulatum, B. dermatitidis, and Paracoccidioides brasiliensis contain predominantly α-glucan and only small amounts of β-glucan, whereas β-glucan is abundant in the M form. It is thus reasonable that MCFG is inactive against the Y form of these species. This speculation is partly supported by the observation that MICs of caspofungin against the M form of H. capsulatum were also lower than those against the Y form (6, 9, 14). However, since little is known about the cell wall components of the other species tested, it is difficult at present to unequivocally explain the relationship between activity and β-glucan content. On the other hand, AMB and azole agents act on the ergosterol component of the cell membrane. There are no reports on different ergosterol contents depending on growth form, suggesting that the relatively similar MICs of the reference compounds for the growth forms should be theoretically acceptable. The large growth-form-dependent difference in the susceptibility of B. dermatitidis to FLC and other minor differences for the other species and compounds might due to other unknown factors, for instance, drug permeability across the cell wall.
The differences in the activity of MCFG depending on the growth form were distinct, particularly for H. capsulatum and B. dermatitidis. Thus, it is very important to know whether the activity of MCFG against the Y form or the M form reflects its therapeutic potency. If activity against the Y form reflects clinical potency, MCFG would generally be unlikely to achieve a therapeutic response to infections caused by dimorphic fungi because it is relatively inactive against the Y form. If activity against the M form reflects potency, MCFG might achieve a therapeutic response to infections caused by H. capsulatum, B. dermatitidis, and C. immitis, whose M forms were highly susceptible to MCFG. The MICs for the M forms of these fungi were as low as those for Candida albicans and Aspergillus fumigatus, against which MCFG is effective in vivo (29). We also demonstrated that commercially available drugs had different levels of activity against the different growth forms. Although the small number of isolates tested in this study prevents drawing the conclusion that such differences in activity are broadly applicable, it can be proposed that variation in methodology may cause misinterpretation of the results. A few studies have compared the two forms in in vitro susceptibility testing, but the number of species tested was limited (26, 27). No studies have investigated against which form of dimorphic fungi in vitro activity correlates better with treatment outcome. Most investigators used the Y form in animal studies and simultaneous in vitro susceptibility testing of the infecting strains, probably because the Y form has been described as being the parasitic form (2, 4, 18, 19). In contrast, simple in vitro susceptibility studies usually used the M form, probably because conidial inocula can be easily prepared and the modified standard method for filamentous fungi can be applied. It is considered that this variation in methodology among investigators or in the purposes of studies is due to the lack of a sufficient understanding of or concern about the existence of some differences in susceptibility between the growth forms of dimorphic fungi. It is reasonable to consider that activity against the parasitic Y form correlates better with treatment outcome. However, some disagreements between in vitro and in vivo activities have been reported in the studies using the Y form. Kobayashi et al. reported that, in a mouse model, FLC was more effective against histoplasmosis caused by a highly FLC-resistant strain (MIC for the Y form, >1,000 μg/ml) than against that caused by a susceptible strain (MIC for the Y form, 0.78 μg/ml) (13). Pappagianis et al. demonstrated that three azole drugs had comparable efficacies against a murine pulmonary infection by C. immitis for which the MICs (for either spherules or endospores) of the three drugs were considerably different (0.25, 1.0, and >64 μg/ml) and concluded that the results of the in vitro susceptibility tests for this fungus were not predictive of therapeutic outcome (23). In addition, notwithstanding that the Y forms of H. capsulatum and B. dermatitidis showed relatively high susceptibility to FLC in this study (Candida isolates for which the MIC of FLC is 8 μg/ml or lower are categorized as susceptible), FLC is described as having poor clinical efficacy against histoplasmosis and blastomycosis (15). However, no evidence that in vitro activity against the M form is predictive of treatment outcome has been found. As for AMB and ITC, which are the standard drugs of choice for the treatment of dimorphic fungus infections, the differences in activity between the forms observed in this study were minimal; even where differences were evident, both MICs were at or below the clinically relevant susceptibility levels for Candida infections (5, 24). Therefore, it may not be very important for these drugs which form correlates better with clinical outcome. However, for estimation of whether new antifungal agents are useful for treatment of dimorphic fungus infection, animal and clinical studies in which it is clear which form used in susceptibility testing correlates better with therapeutic outcome are required. On the basis of such a demonstration, a standardized method for in vitro susceptibility testing of dimorphic fungi is also required.
With candins, there are two reports on the efficacy of caspofungin in mouse models of histoplasmosis (9, 14). Graybill et al. reported that caspofungin at ≥0.05 mg/kg of body weight/day prolonged survival and reduced fungal burden in a model of hematogenous infection induced by a strain (tested in the M form) for which the MIC of caspofungin was 0.25 μg/ml (9). In contrast, Kohler et al. reported that caspofungin, even administered at 10 mg/kg twice daily, produced only a slight reduction of fungal burden in a model of pulmonary infection induced by a strain (tested in the Y form) for which the MIC was 8 μg/ml (14). They also found that the MICs of caspofungin for the Y form of 20 H. capsulatum isolates ranged from 8 to 32 μg/ml and suggested that, based on a comparison of these data and those of Graybill et al., there might be a difference in activity of caspofungin between the growth forms of H. capsulatum (14). Kohler et al. seemed to clearly demonstrate that caspofungin was not effective against H. capsulatum in vivo, reflecting to its poor in vitro activity. At present, however, conclusions on the efficacy of caspofungin against H. capsulatum cannot be made from the two studies, neither of which compared MICs for the growth forms of the strains used in the animal studies.
In the work described herein, we evaluated the in vitro antifungal activity of MCFG against dimorphic fungi and clearly demonstrated that its activity considerably depended on growth form. However, we have not yet reached the final goal of this study, which is to decide whether MCFG has clinical value as a therapeutic agent for dimorphic fungus infections. Further investigations are required to evaluate the in vivo efficacy of MCFG.
Acknowledgments
We are grateful to David Barrett for kind help and advice.
REFERENCES
- 1.Ampel, N. M. 1996. Emerging disease issues and fungal pathogens associated with HIV infection. Emerg. Infect. Dis. 2:109-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chandlar, F. W., M. M. McNeil, and L. Kaufman. 1998. Emerging fungal infection: histoplasmosis, phaeohyphomycosis, and sporotricosis, p. 115-144. In A. M. Nelson and C. R. Horsburgh, Jr. (ed.), Pathology of emerging infection 2. American Society for Microbiology, Washington, D.C.
- 3.Domer, J. E. 1971. Monosaccharide and chitin content of cell walls of Histoplasma capsulatum and Blastomyces dermatitidis. J. Bacteriol. 107:870-877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Drutz, D. J., and M. Huppert. 1983. Coccidioidomycosis: factors affecting the host-parasite interaction. J. Infect. Dis. 147:372-390. [DOI] [PubMed] [Google Scholar]
- 5.Espinel-Ingroff, A. 1997. Clinical relevance of antifungal resistance. Infect. Dis. Clin. N. Am. 11:929-944. [DOI] [PubMed] [Google Scholar]
- 6.Espinel-Ingroff, A. 1998. Comparison of in vitro activities of the new triazole SCH56592 and the echinocandins MK-0991 (L-743,872) and LY303366 against opportunistic filamentous and dimorphic fungi and yeasts. J. Clin. Microbiol. 36:2950-2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Georgopapadakou, N. H. 2001. Update on antifungals targeted to the cell walls: focus on β-1,3-glucan synthase inhibitors. Exp. Opin. Investig. Drugs 10:269-280. [DOI] [PubMed] [Google Scholar]
- 8.Goldman, M. 1994. Histoplasmosis: a treatment update. Curr. Opin. Infect. Dis. 7:667-670. [Google Scholar]
- 9.Graybill, J. R., L. K. Najvar, E. M. Montalbo, F. J. Barchiesi, M. F. Luther, and M. G. Rinaldi. 1998. Treatment of histoplasmosis with MK-0991 (L-743,872). Antimicrob. Agents Chemother. 42:151-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ikeda, F., Y. Wakai, S. Matsumoto, K. Maki, E. Watabe, S. Tawara, T. Goto, Y. Watanabe, F. Matsumoto, and S. Kuwahara. 2000. Efficacy of FK463, a new lipopeptide antifungal agent, in mouse models of disseminated candididasis and aspergillosis. Antimicrob. Agents Chemother. 44:614-618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kanetsuna, F., and L. M. Carbonell. 1971. Cell wall composition of the yeastlike and mycelial forms of Blastomyces dermatitidis. J. Bacteriol. 106:946-948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kanetsuna, F., L. M. Carbonell, I. Azuma, and Y. Yamamura. 1972. Biochemical studies on the thermal dimorphism of Paracoccidioides brasiliensis. J. Bacteriol. 110:208-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kobayashi, G. S., S. J. Travis, M. G. Rinaldi, and G. Medoff. 1990. In vitro and in vivo activities of Sch 39304, fluconazole, and amphotericin B against Histoplasma capsulatum. Antimicrob. Agents Chemother. 34:524-528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kohler, S., L. J. Wheat, P. Connolly, C. Schnizlein-bick, M. Durkin, M. Smedema, J. Goldberg, and E. Brizendine. 2000. Comparison of the echinocandin caspofungin with amphotericin B for treatment of histoplasmosis following pulmonary challenge in a murine model. Antimicrob. Agents Chemother. 44:1850-1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lorthlary, O., D. W. Denning, and B. Dupont. 1999. Endemic mycosis: a treatment update. J. Antimicrob. Chemother. 43:321-331. [DOI] [PubMed] [Google Scholar]
- 16.Maesaki, S., M. A. Hossain, Y. Miyazaki, K. Tomono, T. Tashiro, and S. Kohno. 2000. Efficacy of FK463, a (1,3)-β-d-glucan synthase inhibitor, in disseminated azole-resistant Candida albicans infection in mice. Antimicrob. Agents Chemother. 44:1728-1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matsumoto, S., Y. Wakai, T. Nakai, K. Hatano, T. Ushitani, F. Ikeda, S. Tawara, T. Goto, F. Matsumoto, and S. Kuwahara. 2000. Efficacy of FK463, a new lipopeptide antifungal agent, in mouse models of pulmonary aspergillosis. Antimicrob. Agents Chemother. 44:619-621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miyaji, M., and K. Nishimura. 1977. Investigation on dimorphism of Blastomyces dermatitidis by agar-implantation method. Mycopathologia 60:73-78. [DOI] [PubMed] [Google Scholar]
- 19.Miyaji, M., and K. Nishimura. 1983. Granuloma formation and killing function of granuloma in congenitally athymic nude mice infected with Blastomyces dermatitidis and Paracoccidioides brasiliensis. Mycopathologia 82:129-141. [DOI] [PubMed] [Google Scholar]
- 20.Nakai, T., J. Uno, K. Otomo, F. Ikeda, S. Tawara, T. Goto, K. Nishimura, and M. Miyaji. 2002. In vitro activity of FK463, a novel lipopeptide antifungal agent, against a variety of clinically important molds. Chemotherapy 48:78-81. [DOI] [PubMed] [Google Scholar]
- 21.National Committee for Clinical Laboratory Standards. 1997. Reference method for broth dilution antifungal susceptibility testing of yeasts. Approved standard M27-A. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 22.National Committee for Clinical Laboratory Standards. 1998. Reference method for broth dilution antifungal susceptibility testing of conidium-forming filamentous fungi. Proposed standard M38-P. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 23.Pappagianis, D., B. L. Zimmer, G. Theodoropoulos, M. Plempel, and R. F. Hector. 1990. Therapeutic effect of the triazole Bay R 3783 in mouse models of coccidioidomycosis, blastomycosis, and histoplasmosis. Antimicrob. Agents Chemother. 34:1132-1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rex, J. H., M. A. Pfaller, J. N. Galgiani, M. S. Bartlett, A. Espinel-Ingroff, M. A. Ghannoum, M. Lancaster, F. C. Odds, M. G. Rinaldi, T. J. Walsh, and A. L. Barry. 1997. Development of interpretive breakpoints for antifungal susceptibility testing: conceptual framework and analysis of in vitro-in vivo correlation data for fluconazole, itraconazole, and Candida infections. Clin. Infect. Dis. 24:235-247. [DOI] [PubMed] [Google Scholar]
- 25.San-Blas, G. 1982. The cell wall of fungal human pathogens: its possible role in host-parasite relationships. Mycopathologia 79:159-184. [DOI] [PubMed] [Google Scholar]
- 26.San-Blas, G., A. M. Calcagno, and F. San-Blas. 1993. A preliminary study of in vitro antibiotic activity of saperconazole and other azoles on Paracoccidioides brasiliensis. J. Med. Vet. Mycol. 31:169-174. [PubMed] [Google Scholar]
- 27.Sekhon, A. S., A. K. Garg, A. A. Padhye, and Z. Hamir. 1993. In vitro susceptibility of mycelial and yeast forms of Penicillium marneffei to amphotericin B, fluconazole, 5-fluorocytosine and itraconazole. Eur. J. Epidemiol. 9:553-558. [DOI] [PubMed] [Google Scholar]
- 28.Stevens, D. A. 1995. Coccidioidomycosis. N. Engl. J. Med. 332:1077-1082. [DOI] [PubMed] [Google Scholar]
- 29.Tawara, S., F. Ikeda, K. Maki, Y. Morishita, K. Otomo, N. Teratani, T. Goto, M. Tomishima, H. Ohki, A. Yamada, K. Kawabata, H. Takasugi, K. Sakane, H. Tanaka, F. Matsumoto, and S. Kuwahara. 2000. In vitro activities of a new lipopeptide antifungal agent, FK463, against a variety of clinically important fungi. Antimicrob. Agents Chemother. 44:57-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Valle, N. R., M. Rosario, and G. Torres-Blasini. 1983. Effects of pH, temperature, aeration and carbon source on the development of the mycelial or yeast forms of Sporothrix schenckii from conidia. Mycopathologia 82:83-88. [DOI] [PubMed] [Google Scholar]